Abstract
Objective
The efficacy of aprotinin in reducing blood loss after CPB is well established although its neuroprotective potential is less well known. Furthermore, there is controversy regarding optimal dosing and possible renal complications.
Methods
54 piglets were randomized to one of three CPB groups designed to carry risk of post-op cerebral and renal dysfunction: circulatory arrest at 25°C, ultra-low flow (10 ml/kg/min) at either 25°C or 34°C. Animals were randomized to: control (no aprotinin), low dose (30,000 KIU/kg into prime only), standard full dose (30,000 KIU/kg bolus IV into prime plus 10,000 KIU/kg infusion), and double full dose. Tissue oxygenation index (TOI) was monitored by near-infrared spectroscopy (NIRS). Neurologic functional and histological scores, creatinine and blood urea nitrogen (BUN) were outcomes of interest.
Results
Aprotinin significantly improved neurological scores on postoperative day 1 after ultra-low flow bypass at 25°C or 34°C (P<.01), but not after HCA (P=.57). Linear regression indicated a strong dose-response relationship with higher aprotinin doses having the best neurological scores. During LF, a higher TOI was correlated with a higher aprotinin dose (P<.05). Aprotinin dose had no significant effect on creatinine or BUN on day 1. Low body weight was the only predictor of high BUN (r = −0.39, P<.01).
Conclusion
Aprotinin significantly improves neurologic recovery without compromising renal function in the young piglet.
Introduction
Aprotinin is a broad spectrum serine protease inhibitor isolated from bovine lung. It has anti-inflammatory effects and reduces postoperative blood loss after cardiopulmonary bypass by blocking complement activation and reduction of fibrinolysis through inhibition of trypsin, plasmin and kallikrein. [1 Westaby, 2 Levy]. However the optimal dose of aprotinin has been controversial. Levy reported that both low and high dose reduced postoperative blood loss. Kawasuji reported that low-dose aprotinin could reduce postoperative blood loss [3 Kawasuji]. In general the dose used for adults in contrast to pediatric dosing has not varied according to body surface area and the blood level has not been monitored. Nuttall, et al. reported that the dose of aprotinin adjusted by body weight was helpful in obtaining optimal plasma concentration of aprotinin and to avoid overdosing [4 Nuttall].
For many years there have been intermittent reports of possible adverse effects of aprotinin such as myocardial infarction or renal dysfunction [5 Cosgrove, 6 Sundt, 7 Feindt]. In 2006 Mangano, et al. reported in an influential article in the New England Journal of Medicine that aprotinin increased the risk of renal failure requiring dialysis, myocardial infarction and stroke [8 Mangano]. Preliminary results of a prospective study in adults suggesting a possibly higher mortality because of bleeding led the FDA to recommend that aprotinin not be marketed for any application including pediatric cardiopulmonary bypass.
In pediatric cardiac surgery major neurological injury such as choreoathetosis as well as loss of developmental potential remain feared complications. Although the role of genetic factors, prenatal cerebral blood flow and socioeconomic factors as well as postoperative ICU management have been reported as influencing developmental outcome, nevertheless 2 carefully designed prospective studies have implicated intraoperative factors as important determinants of developmental outcome after pediatric cardiac surgery. Aprotinin however has never been implicated as a cause of neurological injury in children despite its widespread use in pediatric cardiac surgery. In fact several investigators have demonstrated the potential of aprotinin for neuroprotection. Kamiya reported that aprotinin improved preservation of cerebral ATP and reduced cerebral water content after ischemia [9 Kamiya]. Aoki et al used a piglet model and magnetic resonance spectroscopy to show that aprotinin improved acute recovery of cerebral energy metabolism after DHCA possibly through preservation of endothelial function in the cerebral microvasculature [10 Aoki]. Antilla used cerebral intravital microscopy to demonstrate that aprotinin reduced inflammation and improved neurologic functional outcome after DHCA using a piglet survival model and the full pediatric Hammersmith dose regimen, although it did not improve histological score [11 Antilla].
Reports in the neuroscience literature have suggested that serine protease inhibitors protect neurons from ischemic injury [12 Buisson, 13 Vivien, 14 Yepes]. Nicole et al described reduced excitotoxic neuronal injury with exposure to the serpin PAI-1 and Lebeurrier reported that neuroserpin, also protects neurons from glutamatergic excitotoxic neuronal cell death [15 Nicole, 16 Lebeurrier]. Using a similar neuronal cell culture model we previously reported that aprotinin also has a direct and dose-dependent neuroprotective effect against neuronal cell death caused by N-methyl-D-aspartate (NMDA) [17 Iwata].
The purpose of this piglet survival study was to investigate the optimal dose of aprotinin for neuroprotection and the impact of aprotinin on renal function.
Materials and Methods
Animals
Fifty-four young (27 ± 5 days) Yorkshire piglets weighting 10.4 ± 1.4 kg were studied. All animals received humane care in accordance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, revised in 1996. This study was approved by the Institutional Animal Care and Use Committee of the Children’s National Medical Center.
Surgical Preparation
After premedication with an intramuscular injection of ketamine (20 mg/kg) and xylazine (4 mg/kg), the piglets were intubated with 5-mm cuffed endotracheal tubes and ventilated with 21% oxygen at a respiratory rate between 12 to 16 breaths/min. to achieve an arterial PCO2 of 40 mm Hg by pressure cycle ventilator (Sechrist IV-100B Ventilator, Sechrist Industries, Inc., Anaheim, CA). After induction with an intravenous bolus injection of fentanyl (50 μg/kg) and pancuronium (0.5 mg/kg), anesthesia was maintained with continuous infusion of fentanyl (25 μg · kg−1 · h−1), midazolam (0.2 mg · kg−1 · h−1), and pancuronium (0.2 mg · kg−1 · h−1) throughout the entire experiment. Temperature probes were placed into the esophagus and rectum. Optodes for NIRS were placed over the frontal lobes, with an interoptode distance of 4.0 cm. The receiving optode incorporates 3 detectors. The piglets were in the supine position and all surgical procedures were performed under sterile condition. For pressure monitoring, blood sampling and drug infusion, a catheter (19G Intracath; Becton Dickinson, Sandy, Utah) was inserted into the abdominal aorta via the left superficial femoral artery and another catheter was introduced through the right femoral vein into the inferior vena cava. After systemic heparinization (300 IU/kg), a right anterolateral thoracotomy in the third intercostal space, an 8F arterial cannula (Bio-Medicus; Medtronic Inc, Eden Prairie, Minn) was inserted into the abdominal aorta through the right femoral artery, and a 28F cannula (Harvey; Bard, Tewksbury, Mass) was inserted for venous drainage via the right atrial appendage.
Experimental Protocol
The study protocol is depicted in Figure 1. Details of the survival piglet model have been described elsewhere [18 Forbes, 19 Sakamoto, 20 Hagino]. The fifty-four piglets were each randomly assigned to one of 12 settings (3 bypass conditions and 4 aprotinin dosings) with 4 piglets for each setting. Animals were weaned from the ventilator and extubated next early morning. Neurological evaluation was performed every day postoperatively. Animals were euthanized at postoperative day 4 for histological assessment.
Figure 1.
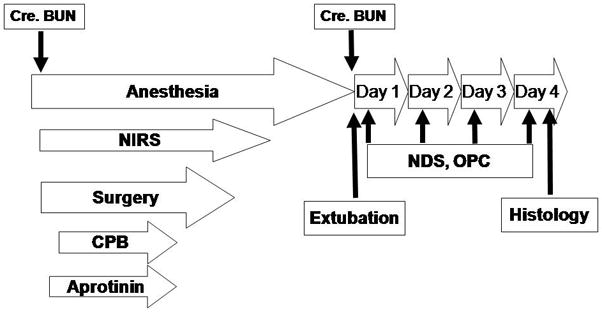
Experimental protocol. CPB, cardiopulmonary bypass; NIRS, near-infrared spectroscopy; NDS, neurologic deficit score; OPC, overall performance category; Cre, creatinine; BUN, blood urea nitrogen.
Experimental Groups
Brain damage according to bypass conditions
Brain damage according to the bypass protocols has been defined in this model in our previous studies, namely 10 mL · kg−1 · min.−1 at 25°C mild injury, 10 mL · kg−1 · min.−1 at 34°C moderate injury or circulatory arrest at 25°C severe injury.. The duration of ULF or HCA period was 60 minutes in each setting.
Aprotinin dosing schedules
No aprotinin (Trasylol; Bayer, West Haven, CT) was administered in control group (Group C). Aprotinin 30,000 KIU/kg (kallikrein inhibitor units) was added to the pump prime in low dose group (Group L). An intravenous bolus infusion of aprotinin of 30,000 KIU/kg was given as the loading dose as well as in the pump prime, followed by maintenance infusion of aprotinin of 10,000 KIU · kg−1 · h−1 in the standard full dose group (Group SF). This dosing regimen was doubled in the double full dose group (Group DF). For all dosing regimens aprotinin was stopped during HCA and at 5 minutes after weaning from CPB.
CPB Technique
The CPB circuit consisted of a roller pump, membrane oxygenator (Minimax; Medtronic Inc, Anaheim, CA), and sterile tubing with a 40-μm arterial filter. Fresh whole blood from a donor pig was transfused into the prime to adjust the hematocrit level to 30%. Methylprednisolone (30 mg/kg), furosemide (0.25 mg/kg), sodium bicarbonate 7.4% (10 mL), cephazolin sodium (25 mg/kg), fentanyl (50 μg/kg) and pancuronium (0.5 mg/kg) were added to the prime. The pH-stat strategy was used (sweep gas 95% O2/5% CO2). The gas flow was fixed at 3 l/min. to obtain an arterial PCO2 of 40 to 45 mm Hg (corrected to esophageal temperature). After baseline recordings, CPB with a flow rate of 100 mL · kg−1 · min−1 was started, and the animals were perfused for 10 minutes at normothermia (esophageal temperature, 37°C). Ventilation was stopped after the establishment of CPB. The piglets underwent 40 minutes of cooling on CPB to an esophageal temperature of 25°C or 34°C. After cooling, HCA or ULF perfusion at a flow rate of 10 mL · kgv1 · min−1 was initiated for 60 minutes. Before rewarming, sodium bicarbonate 7.4% (10 mL), methylprednisolone (30 mg/kg), furosemide (0.25 mg/kg), and mannitol (0.5 g/kg) were administered into the pump. During 40 minutes of rewarming, animals were warmed to 37°C with a flow rate of 100 mL · kg−1 · min−1. The heart was defibrillated if necessary. Ventilation (100% oxygen) was started 10 minutes before weaning from CPB. Also, dopamine was administered by continuous drip infusion if necessary. Protamine (5 mg/kg) was administered intravenously after the animal was hemodynamically stable. The wounds were closed in a sterile fashion.
Postoperative management
Animals remained sedated and paralyzed with a continuous infusion of fentanyl (50 μg · kg−1 · h−1), midazolam (0.2 mg · kg−1 · h−1), and pancuronium (0.2 mg · kg−1 · h−1) and were mechanically ventilated with a gradually decreasing oxygen fraction. They were monitored continuously for 12 hours after CPB. The chest tubes were removed, and the animals were weaned from ventilation and extubated.
On postoperative day 4, all surviving piglets were sedated using intramuscular induction with ketamine (20 mg/kg) and xylazine (4 mg/kg) and anesthetized with intravenous fentanyl (50 μg/kg). After a midline sternotomy, heparin (300 IU/kg) was administered, and a cannula was inserted into the bovine trunk. 1 L of Plasmalyte solution (Baxter, Deerfield, IL) was infused through the bovine trunk. Blood was suctioned from the superior vena cava until the perfusate was clear of blood. Then 3 L of 10% formalin solution was perfused through the brain in the same manner to accomplish perfusion fixation. The entire head of the piglet was immersed in 10% formalin for a week, and the brain was harvested and fixed with 10% formalin solution for the histologic assessment [11 Vesa, 18 Forbess, 19 Sakamoto, 20 Hagino].
Data collection
Arterial pressure was monitored continuously throughout each experiment and was recorded every 10 minutes.
Blood gas analyses
Blood gas including hematocrit were measured at base line, end of normothermic bypass, 20 and 40 minutes during cooling, every 15 minutes during low flow, 5, 15, 30 and 40 minutes after beginning rewarming, and after the procedure as needed with a blood gas analyzer (Bayer 860, Bayer healthcare, West Haven, CT).
Near-Infrared Spectroscopy
A pair of fiberoptic optodes was attached to the head of the animal with a probe holder after induction of anesthesia. The spacing of optodes was 4.0 cm in the coronal plane. These 2 optodes, a transmitter and a receiver of laser light of near-infrared wavelength, were connected to the NIRS device (NIRO-300; Hamamatsu Photonics KK, Hamamatsu City, Japan). This device calculated the tissue oxygen index (TOI) from the relative concentration changes in oxyhemoglobin (HbO2) and deoxyhemoglobin (HHb) concentrations and with calculation of the optical pathlength[19 Sakamoto, 21 Shin’oka]. Data were recorded every 10 seconds after the induction of anesthesia and for 3 hours after weaning from CPB. Average TOI which correlates with minimum TOI and TOI at 15 minutes after the onset of ULF or HCA was calculated [20 Hagino].
Renal function
Serum creatinine and BUN levels were assessed at base line and on postoperative day 1.
Aprotinin concentration
Aprotinin concentration was measured at baseline, end of cooling, end of rewarming, 3 hours and 12 hours after bypass using Uni-test™ (Unicorn Diagnostics Ltd., London, UK) [22 Cardigan]. The blood samples were immediately centrifuged, and the plasma was frozen and stored at −80°C until measurement.
Neurologic and behavioral evaluations
After the operation, neurologic and behavioral evaluations were performed at 24-hour intervals beginning on postoperative day 1, as described previously [18 Forbes, 19 Skamoto, 20 Hagino, 21 Shin’oka]. Neurologic deficit score (NDS; 500 = brain death, 0 = normal) and overall performance category (OPC; 5 = brain death, 1 = normal) were performed by an animal care technician under the supervision of the senior veterinarian blinded to the experimental protocol.
Histologic assessment
The preparation of the cerebral specimens and the details of histological analyses have been described previously [18 Forbess, 19 Sakamoto, 20 Hagino, 21 Shin’oka]. Twenty-four areas, including the neocortex, hippocampus, dentate gyrus, caudate nucleus, thalamus, and cerebellum were examined. Histologic damage was scored by using the following criteria: 5 = cavitated lesions with necrosis; 4 = significant damage to neurons; 3 = large clusters of injured neurons; 2 = small clusters of damaged neurons; 1 = isolated neuronal damage; 0 = normal, and scores were summed to determine the total histologic score (total HS; range, 0–120). A single neuropathologist examined all specimens in a blinded fashion.
Statistical Analysis
Physiological variables were compared between the three bypass conditions before and after CPB using one-way analysis of variance with a two-tailed α-level to protect against committing Type I errors (i.e., false positives)[23 Cabral]. Mean changes in aprotinin and tissue oxygenation index (% TOI) were evaluated across different time points using repeated-measures ANOVA with Greenhouse-Geisser F-tests to assess the effects of dose and bypass condition[24 Wallenstein] Neurologic deficit scores (NDS) and total histologic scores (HS) were analyzed using two-way ANOVA with Bonferroni adjustment to determine whether these scores were related to differences in aprotinin dose and bypass condition. The linear relationship between NDS and aprotinin concentration was measured for the 33 piglets in the ultra-low flow bypass groups using a Pearson correlation and linear regression model to derive a prediction equation of the form y = β0 + β1x, where β0 denotes the intercept and β1 represents the slope coefficient for aprotinin concentration at the end of cooling. [25 Harrell] Renal function was evaluated at baseline and postoperative day 1 whereby changes in creatinine and blood urea nitrogen (BUN) were assessed using paired t tests and differences in the Δ BUN between aprotinin dose groups compared by repeated-measures ANOVA. Linear regression analysis was applied to determine whether body weight was associated with the Δ BUN and whether this relationship was dependent on aprotinin dose and bypass condition. Statistical analysis was performed using the SPSS software package (version 16.0, SPSS Inc., Chicago, IL). Power analysis was conducted a priori and indicated that a minimum of 12 animals were needed in each aprotinin dose and the control group and randomized equally to the bypass conditions (n = 4) to achieve 80% power for detecting significant dose-response effects with respect to % TOI, NDS, and total HS based on ANOVA (version 7.0, nQuery Advisor, Statistical Solutions, Saugus, MA).
Results
Forty-eight of the 54 animals survived the entire protocol and were euthanized on POD 4 for histological assessment. 4 piglets subjected to 10 mL · kg−1 · min−1 at 34°C receiving low dose of aprotinin (n=2) and/or double full dose of aprotinin (n=2) died after weaning from CPB with sudden ventricular fibrillation. Data from these animals were excluded for analysis. 2 piglets subjected to HCA at 25°C or ULF at 34°C receiving no aprotinin died on POD 1 with sudden death. Data from these piglets were included for analysis except histological analysis.
Physiological parameters and bypass related variables
There were no significant differences at base line for body weight, mean blood pressure, body temperature, blood gases and hematocrit values between bypass conditions. Also, no significant differences were observed for any parameters at the end of cooling, rewarming 5 min, end of bypass, and 6 hours post-CPB (one-way ANOVA with two-tailed P < .01 as the criterion for statistical significance to protect against Type I errors due to multiple comparisons).(Table 1).
TABLE 1.
Physiological variables during and after cardiopulmonary bypass*
| Ultra-Low Flow | Bypass Groups | HCA Group | |
|---|---|---|---|
| Variable | 25°C, 10 ml· kg−1· min.−1 (n = 16) | 34°C, 10 ml· kg−1· min.−1 (n = 17) | 25°C, Circulatory arrest (n = 17) |
| Baseline | |||
| Temperature (°C) | 36.1 ± 1.0 | 35.9 ± 0.7 | 36.1 ± 0.8 |
| MAP (mmHg) | 69.1 ± 13.9 | 68.9 ± 11.5 | 71.5 ± 13.0 |
| pH | 7.51 ± 0.05 | 7.51 ± 0.05 | 7.52 ± 0.03 |
| PaO2 (mmHg) | 83.1 ± 6.2 | 74.5 ± 15.0 | 79.7 ± 9.8 |
| PaCO2 (mmHg) | 39.1 ± 4.4 | 40.4 ± 4.3 | 39.6 ± 2.6 |
| Hematocrit (%) | 29.1 ± 3.3 | 29.7 ± 3.0 | 28.3 ± 2.2 |
| SvO2 (%) | 74.6 ± 6.2 | 72.7 ± 8.3 | 72.3 ± 6.2 |
| 12 hours after CPB (Fio2 = 0.25) | |||
| Temperature (°C) | 37.2 ± 0.5 | 37.1 ± 0.8 | 37.0 ± 0.4 |
| MAP (mmHg) | 90.9 ± 15.8 | 91.4 ± 15.0 | 89.7 ± 14.4 |
| pH | 7.49 ± 0.06 | 7.51 ± 0.05 | 7.51 ± 0.07 |
| PaO2 (mmHg) | 96.4 ± 15.2 | 96.4 ± 24.4 | 90.3 ± 19.5 |
| PaCO2 (mmHg) | 41.7 ± 3.5 | 40.5 ± 3.7 | 41.4 ± 4.8 |
| Hematocrit (%) | 30.7 ± 2.9 | 30.4 ± 2.3 | 30.1 ± 1.9 |
| SvO2 (%) | 74.8 ± 16.4 | 74.2 ± 10.4 | 80.5 ± 10.0 |
HCA, hypothermic circulatory arrest; MAP, mean arterial pressure; SvO2, oxygen saturation of venous blood; CPB, cardiopulmonary bypass. Data are presented as mean ± standard deviation.
No significant differences were observed for any variable. Groups were also compared at the end of cooling, re-warming 5 min, end of bypass, and 6 hours post-CPB and no differences were found (one-way ANOVA with two-tailed P < .01 as the criterion for statistical significance to protect against Type I errors due to multiple comparisons).
Aprotinin concentration
As anticipated Group DF had significantly higher aprotinin concentration than other groups (P < .01). Also Group SF had higher concentration than group L and C (P < .02). Group L did not reach significant difference against group C. Aprotinin concentration reached a peak value at the end of cooling, namely 289.8 ± 72.1 in group DF, 150.6 ± 103.2 in group SF and 64.5 ± 61.7 KIU/ml in group L, and decreased gradually in all dosing schedules (Figure 2).
Figure 2.
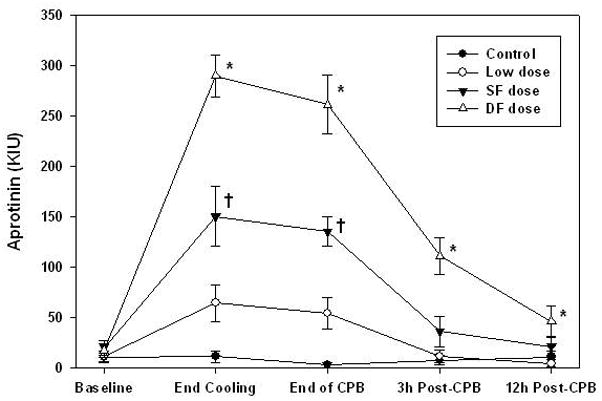
Aprotinin concentration was significantly higher (denoted by *) in the double full dose group compared to the standard full dose, low dose, and control at the end of cooling, end of bypass, 3 hours post-CPB (all P < .001), and 12 hours post-CPB (all P < .05). Standard full dose group was significantly higher (denoted by †) than low dose and control group at end of cooling and end of bypass (all P < .01). Low dose group was not significantly different from control group at any time point (all P > .25). Analysis was determined using repeated-measures ANOVA with Bonferroni-adjusted group comparisons.
Tissue oxygenation index
TOI increased during cooling and decreased rapidly from the onset of ULF or HCA, and reached a nadir in all animals. During HCA at 25°C aprotinin did not influence TOI even in group DF. However, during ULF at 25°C or 34°C, average TOI was 51.9 ± 4.4, 49.1 ± 4.1, 48.6 ± 3.7 and 46.1 ± 4.9 in group DF, SF, L and control, respectively. Group DF was significantly higher than control (Figure 3, P < .05).
Figure 3.
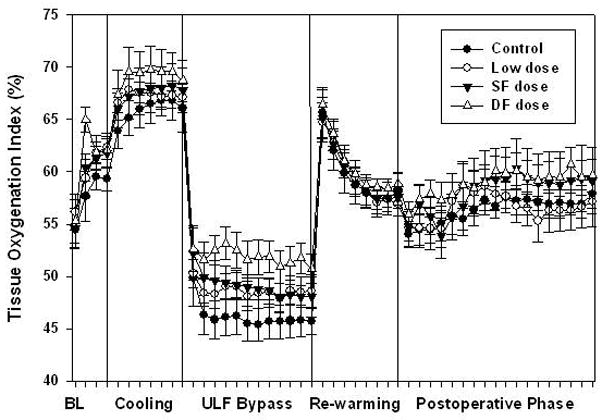
Average tissue oxygenation index during ultra-low flow (ULF) bypass at 25°C or 34°C in double full (DF) dose group was significantly higher than control (P < .05, ANOVA with group comparisons using the Tukey method). Aprotinin improved oxygenation during ULF bypass independent of bypass temperature. Error bars denote standard errors.
Neurologic and behavioral evaluations
NDS and OPC showed relatively rapid recovery in all surviving animals. Aprotinin was associated with significantly improved neurologic scores on postoperative day 1 after ULF at 25°C or 34°C (P < .05), but not after HCA (P = .57) (Figure 4). Linear regression indicated a strong dose-response relationship with higher aprotinin doses having the best neurological scores after ULF, where dose are 0; none, 1; low dose, 2; standard full dose, and 3; double full dose (Figure 5, r = −.73, P < .01). Also, higher aprotinin concentration at the end of cooling was correlated to lower NDS (Figure 6, r = −.57, P < .01).
Figure 4.
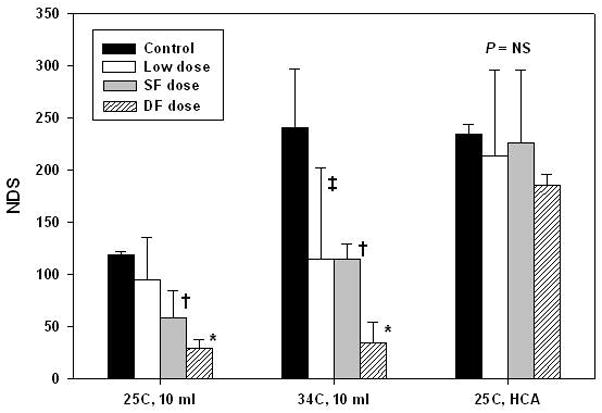
Neurological deficit scores on postoperative day 1 according to aprotinin dose for the three bypass conditions. NDS were significantly lower (ie improved)with aprotinin after ultra-low flow bypass at 25°C or 34°C, but not after HCA (P = .57). After ULF at 25°C, NDS were significantly lower with DF dose compared to control (*P < .001) and SF dose (P = .02). NDS was significantly lower with SF dose than control (†, P = .03). After ULF at 34°C, NDS was significantly lower with aprotinin DF dose compared to control (P < .001) and with SF dose (†), and low dose (‡) compared to control (both P = .02). Analysis was performed using two-way ANOVA with Bonferroni adjustment for multiple comparisons. Error bars denote standard deviations. NS = not significant.
Figure 5.
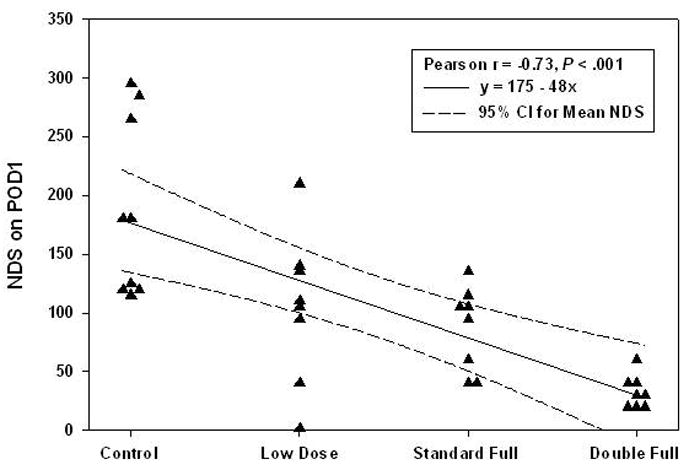
Linear relationship between aprotinin dose and neurological scores for the combined group of 33 piglets undergoing ULF bypass (n = 16 at 25°C, n = 17 at 34°C). Linear regression analysis indicated a strong dose-response relationship with higher aprotinin doses having significantly lower neurological scores (Pearson r = −0.73, P < .001). The linear equation specifies aprotinin dosing schedule as none = 0, low dose = 1, standard full dose = 2, and double full dose = 3. The model fit was judged to be very good judging from the coefficient of determination (adjusted R2 = 0.51) indicating that over 50% of the variability in neurological scores can be accounted for by aprotinin dosing. The 95% confidence interval for the mean NDS shows three piglets in the control group (no aprotinin) with very high scores. No linear relationship was observed in the HCA group (P = .22, n = 17).
Figure 6.
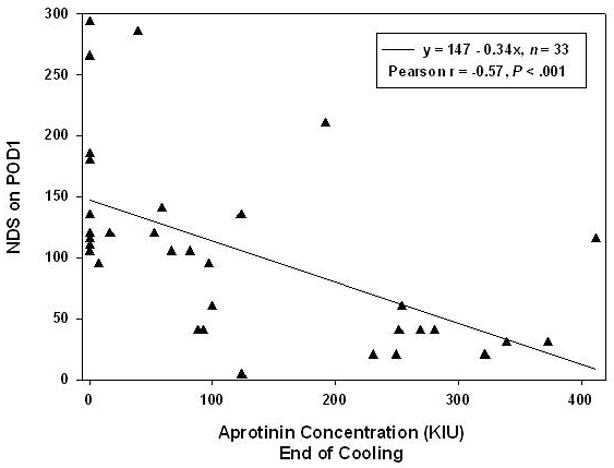
Linear regression analysis depicts moderately strong inverse relationship between aprotinin concentration at the end of cooling and neurologic deficit score on postoperative day 1 (Pearson r = −0.57, P < .001). Regression line predicts a lower NDS with higher aprotinin concentrations (solid line). Analysis is based on piglets undergoing ULF bypass (n = 33) since the HCA group did not show a relationship between aprotinin concentration and NDS. Multiple regression revealed that, although NDS tend to be higher for 34°C and 10 mL than for 25°C and 10 mL, the significance of aprotinin concentration as a predictor of NDS stands for both conditions.
Histological assessment
Histologic damage was found predominantly in the neocortex, hippocampus and caudate nucleus. 3 animals assigned to ULF at 25°C with no aprotinin or standard full dose, or ULF at 34°C with standard full dose of aprotinin had no brain damage. In control animals, as anticipated there were significant differences in total HS between bypass conditions (P < .01). They were mild injury (ULF at 25°C) 0.8 ± 0.5, moderate injury (ULF at 34°C) 9.8 ± 7.2, and severe injury (HCA at 25°C) 14.8 ± 4.2 (Figure 7). Aprotinin did not influence histological outcomes in any bypass conditions, although scores after the condition of ULF at 25°C were significantly lower than ULF at 34°C or HCA at 25°C (Figure 7).
Figure 7.
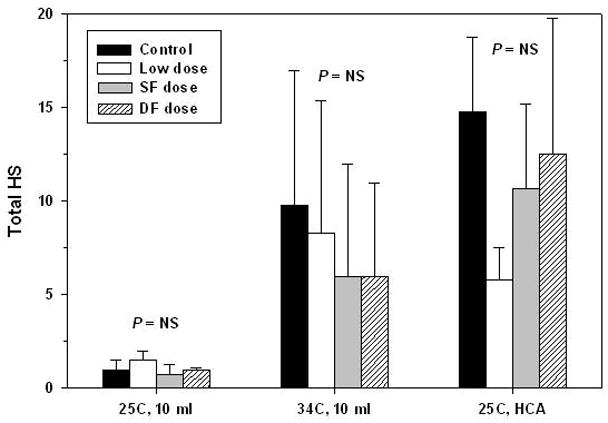
Total histologic scores according to aprotinin dose for the three bypass conditions. ANOVA with Bonferroni comparisons revealed no significant differences in total HS between the four groups (control and aprotinin doses) for any of the bypass conditions (25°C, 10 mL, P = .12; 34°C, 10 mL, P = .82; HCA, P = .10). Error bars denote standard deviations. NS = not significant.
Renal function
Overall creatinine and BUN significantly increased at POD 1 from 0.8 ± 0.3 to 1.3 ± 0.8 mg/dl and from 8.9 ± 3.8 to 30.1 ± 10.9 mg/dL, respectively. Multivariate analysis showed that bypass conditions and aprotinin dosing schedules had no influence on renal function (Figure 8a, b). Also, the peak aprotinin concentration did not correlate with renal outcomes. Lower body weight was the only predictor of higher BUN on POD 1 (Figure 9, r = −0.39, P < .01).
Figure 8.
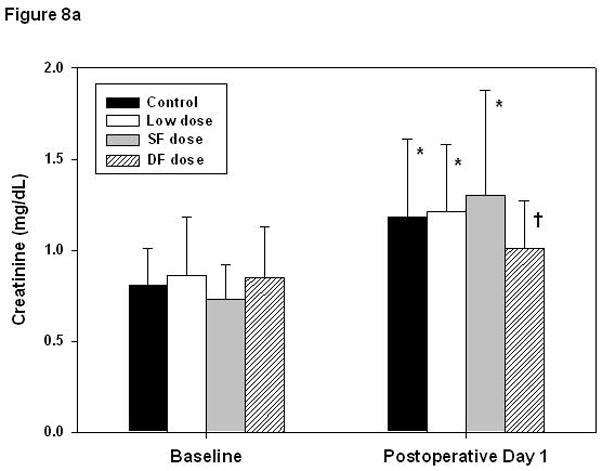
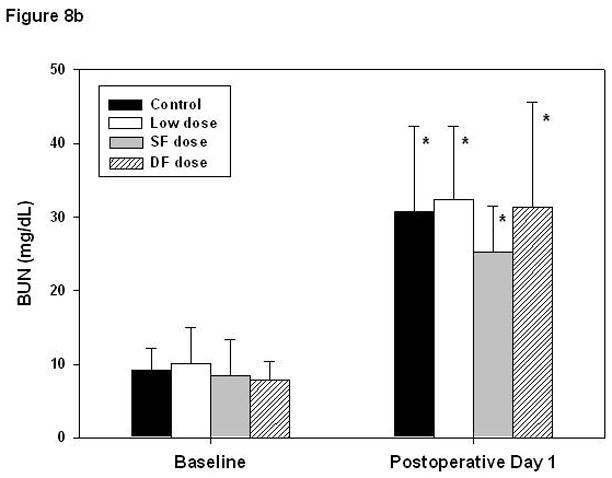
Renal function evaluated by changes in creatinine level and blood urea nitrogen (BUN). Creatinine levels (A) increased on postoperative day 1 (* P < .01, † P < .05, paired t tests), though the amount of change did not differ with respect to aprotinin dose (P = .08, ANOVA). BUN (B) increased significantly in all groups, approximately 3-fold (all P < .01). Error bars denote standard deviations. Additional statistical analysis using repeated-measures ANOVA indicated that changes in creatinine were not significantly related to aprotinin dose (P =.79) or specific bypass conditions (P = .18) and changes in BUN were not dependent on aprotinin dose (P = .36) or bypass conditions (P = .13).
Figure 9.
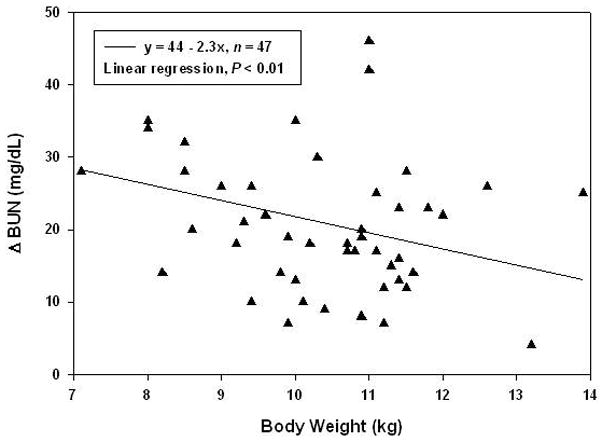
Inverse relationship between body weight and change in BUN (r = −0.39, n = 47). Linear regression analysis indicates that lower body weight was predictive of a greater increase in BUN at POD1 (P < .01) and that this relationship is independent of aprotinin dose and bypass conditions. For example, a piglet weighing 9 kg is expected on average to increase in BUN by 24 mg/dL, whereas a 13 kg piglet is expected to increase by 14 mg/dL. The solid line represents the fitted regression line, where x is body weight in kg.
Discussion
This study demonstrates that aprotinin improves functional neurological recovery in animals with mild or moderate brain injury after ultra-low flow cardiopulmonary bypass. The beneficial effects of aprotinin are dose dependent over the dosage regimens applied in this study. The beneficial effect of aprotinin was not seen in animals with severe brain injury after circulatory arrest. This result suggests that aprotinin may have influenced blood flow under ultra-low flow states and is consistent with our previous studies which suggest that vascular preservation is an important mechanism of action of aprotinin. On the other hand, Antilla reported that aprotinin improved NDS even after deep hypothermic circulatory arrest at 15°C (DHCA) [11 Antilla]. This difference may be explained by the severity of brain injury which was moderate in Anttila’s study while it was severe in ours.
In our study aprotinin did not improve histological score in any bypass condition including those in which there was an improvement of NDS. Antilla also reported that aprotinin did not improve histological scores after DHCA [11 Antilla]. We have reported previously that NDS at POD1 correlates with total HS [26 Sakamoto]. In this study, NDS at POD1 after ULF did not correlate with total HS. This discrepancy may result from our histological evaluation method which focused on necrotic neuronal cell death. Kurth reported that apoptosis plays a key role in neuronal cell death after DHCA [27 Kurth]. It is known that aprotinin reduces apoptosis. For example Eser reported that aprotinin decreased apoptotic cell death after brain ischemia [28 Eser]. We also have confirmed that aprotinin reduces apoptotic cell death caused by serum deprivation at the concentration of 100 KIU/mL using a cell culture model (data not shown). Thus reduction of apoptotic neuronal cell death by aprotinin may contribute to improve neurological outcome.
Renal function
Aprotinin did not influence renal function with any dosing regimen in this study although creatinine and BUN increased at POD1 in all conditions. Lower body weight but not aprotinin use was predictive of higher BUN at POD1 by multivariate analysis.
Aprotinin has a high affinity for renal tissue and is rapidly eliminated from the circulation necessitating a continuous infusion after a bolus dose to maintain a targeted concentration of aprotinin [29 Rustom]. The proximal tubular cells absorb and metabolize aprotinin. Feindt demonstrated in adults that renal dysfunction could result from tubular damage caused by aprotinin, which increases α2-microglobulin and total protein in the urine although they did not find relevant changes in serum creatinine levels. They concluded that patients with normal renal function preoperatively were able to compensate for both the perioperative renal dysfunction caused by the CPB and the additional tubular workload imposed by aprotinin [7 Feindt]. Our data confirm that animals with normal renal function are able to compensate for the stress of surgery, cardiopulmonary bypass and aprotinin even at double full dose regimen. This finding is consistent with clinical reports which have looked at renal function in children with aprotinin use. Although there has been a trend to higher creatinine levels postoperatively with aprotinin use, this has not been clinically significant [30 Backer].
Aprotinin concentration
In this study, aprotinin concentration reached a peak at the end of cooling and was 150KIU/ml in group SF and 280KIU/mL in group DF by functional assay [22 Cardigan]. Aprotinin inhibits plasmin at a concentration of 50 KIU/ml (hemostatic effect) and kallikrein (anti-inflammatory) at 200 KIU/mL in vitro.. In vivo it is thought that a large molar excess of aprotinin up to 125 and 250–500 KIU/mL, respectively is required to inhibit plasmin and kallikrein, [31 Fritz]. The original high-dose Hammersmith hospital regimen aimed to achieve at least 200 KIU/mL by immunological assay [32 Royston, 33 Bidstrup]. The results of our current study support the notion that a dose as high as double the current standard pediatric dose may be more effective in improving functional outcome while not impairing renal function
Brain oxygenation
We have reported previously that TOI is a useful monitor of brain oxygenation during CPB and that average TOI correlates with brain damage [19 Sakamoto, 20 Hagino, 21 Shin’oka, 26 Sakamoto ]. In the present study double full dose aprotinin significantly increased TOI during ULF but did not influence the decline in TOI during HCA. This result is consistent with our previous finding that aprotinin improves cerebrovascular protection.
Direct neuronal protection and role of the blood brain barrier
Although our previous cell culture studies have demonstrated that aprotinin, like other serine protease inhibitors, can reduce excitotoxic brain injury, the present study does not shed light as to how important that mechanism might be in improving functional outcome as observed in this study. Improved vascular protection of the brain and generalized anti-inflammatory effects alone might explain the fact that animals recovered more rapidly on the first day after surgery when aprotinin was used. Furthermore the ability of aprotinin to cross the blood brain barrier so as to be able to have a direct neuronal impact is not assessed in the present study.
In conclusion, our data suggest that aprotinin improves neurologic recovery without compromising renal function in the piglet. Well-designed aprotinin dose-response studies are needed to assess the safety versus efficacy of aprotinin in the clinical setting, particularly a double-full dose strategy. Pediatric surgeons will continue to explore pharmacologic strategies to reduce the hemorrhagic and inflammatory consequences of cardiopulmonary bypass which undoubtedly delay recovery from congenital cardiac procedures, especially in the very young.
Acknowledgments
This study was supported by NIH R01HL060922
This study was supported by National Institutes of Health grant R01HL060922. We are grateful to Inger Hogan for technical support and animal care. We also thank Laura Young for preparation of the manuscript.
References
- 1.Westaby S. Aprotinin in perspective. Ann Thorac Surg. 1993;55(4):1033–41. doi: 10.1016/0003-4975(93)90149-c. [DOI] [PubMed] [Google Scholar]
- 2.Levy JH, Pifarre R, Schaff HV, Horrow JC, Albus R, Spiess B, Rosengart TK, Murray J, Clark RE, Smith P. A multicenter, double-blind, placebo-controlled trial of aprotinin for reducing blood loss and the requirement for donor-blood transfusion in patients undergoing repeat coronary artery bypass grafting. Circulation. 1995;92(8):2236–44. doi: 10.1161/01.cir.92.8.2236. [DOI] [PubMed] [Google Scholar]
- 3.Kawasuji M, Ueyama K, Sakakibara N, Tedoriya T, Matsunaga Y, Misaki T, Watanabe Y. Effect of low-dose aprotinin on coagulation and fibrinolysis in cardiopulmonary bypass. Ann Thorac Surg. 1993;55(5):1205–9. doi: 10.1016/0003-4975(93)90035-g. [DOI] [PubMed] [Google Scholar]
- 4.Nuttall GA, Fass DN, Oyen LJ, Oliver WC, Jr, Ereth MH. A study of a weight-adjusted aprotinin dosing schedule during cardiac surgery. Anesth Analg. 2002;94(2):283–9. doi: 10.1097/00000539-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove DM, 3rd, Heric B, Lytle BW, Taylor PC, Novoa R, Golding LA, Stewart RW, McCarthy PM, Loop FD. Aprotinin therapy for reoperative myocardial revascularization: a placebo-controlled study. Ann Thorac Surg. 1992;54(6):1031–6. doi: 10.1016/0003-4975(92)90066-d. [DOI] [PubMed] [Google Scholar]
- 6.Sundt TM, 3rd, Kouchoukos NT, Saffitz JE, Murphy SF, Wareing TH, Stahl DJ. Renal dysfunction and intravascular coagulation with aprotinin and hypothermic circulatory arrest. Ann Thorac Surg. 1993;55(6):1418–24. doi: 10.1016/0003-4975(93)91082-x. [DOI] [PubMed] [Google Scholar]
- 7.Feindt PR, Walcher S, Volkmer I, Keller HE, Straub U, Huwer H, Seyfert UT, Petzold T, Gams E. Effects of high-dose aprotinin on renal function in aortocoronary bypass grafting. Ann Thorac Surg. 1995;60(4):1076–80. doi: 10.1016/0003-4975(95)00525-p. [DOI] [PubMed] [Google Scholar]
- 8.Mangano DT, Tudor IC, Dietzel C, Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354(4):353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 9.Kamiya T, Katayama Y, Kashiwagi F, Terashi A. The role of bradykinin in mediating ischemic brain edema in rats. Stroke. 1993;24(4):571–5. doi: 10.1161/01.str.24.4.571. discussion 575–6. [DOI] [PubMed] [Google Scholar]
- 10.Aoki M, Jonas RA, Nomura F, Stromski ME, Tsuji MK, Hickey PR, Holtzman DH. Effects of aprotinin on acute recovery of cerebral metabolism in piglets after hypothermic circulatory arrest. Ann Thorac Surg. 1994;58(1):146–53. doi: 10.1016/0003-4975(94)91089-8. [DOI] [PubMed] [Google Scholar]
- 11.Anttila V, Hagino I, Iwata Y, Mettler BA, Lidov HG, Zurakowski D, Jonas RA. Aprotinin improves cerebral protection: evidence from a survival porcine model. J Thorac Cardiovasc Surg. 2006;132(4):948–53. doi: 10.1016/j.jtcvs.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Buisson A, Nicole O, Docagne F, Sartelet H, Mackenzie ET, Vivien D. Up-regulation of a serine protease inhibitor in astrocytes mediates the neuroprotective activity of transforming growth factor beta1. FASEB J. 1998;12(15):1683–91. [PubMed] [Google Scholar]
- 13.Vivien D, Buisson A. Serine protease inhibitors: novel therapeutic targets for stroke? J Cereb Blood Flow Metab. 2000;20(5):755–64. doi: 10.1097/00004647-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, Lawrence DA. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96(2):569–76. [PubMed] [Google Scholar]
- 15.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7(1):59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 16.Lebeurrier N, Liot G, Lopez-Atalaya JP, Orset C, Fernandez-Monreal M, Sonderegger P, Ali C, Vivien D. The brain-specific tissue-type plasminogen activator inhibitor, neuroserpin, protects neurons against excitotoxicity both in vitro and in vivo. Mol Cell Neurosci. 2005;30(4):552–8. doi: 10.1016/j.mcn.2005.09.005. Epub 2005 Oct 4. [DOI] [PubMed] [Google Scholar]
- 17.Iwata Y, Nicole O, Okamura T, Zurakowski D, Jonas RA. Aprotinin confers neuroprotection by reducing excitotoxic cell death. J Thorac Cardiovasc Surg. 2008;135(3):573–8. doi: 10.1016/j.jtcvs.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 18.Forbess JM, Ibla JC, Lidov HG, Cioffi MA, Hiramatsu T, Laussen P, Miura T, Jonas RA. University of Wisconsin cerebroplegia in a piglet survival model of circulatory arrest. Ann Thorac Surg. 1995;60(6 Suppl):S494–500. doi: 10.1016/0003-4975(95)00876-4. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto T, Hatsuoka S, Stock UA, Duebener LF, Lidov HG, Holmes GL, Sperling JS, Munakata M, Laussen PC, Jonas RA. Prediction of safe duration of hypothermic circulatory arrest by near-infrared spectroscopy. J Thorac Cardiovasc Surg. 2001;122(2):339–50. doi: 10.1067/mtc.2001.115242. [DOI] [PubMed] [Google Scholar]
- 20.Hagino I, Anttila V, Zurakowski D, Duebener LF, Lidov HG, Jonas RA. Tissue oxygenation index is a useful monitor of histologic and neurologic outcome after cardiopulmonary bypass in piglets. J Thorac Cardiovasc Surg. 2005;130(2):384–92. doi: 10.1016/j.jtcvs.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 21.Shin’oka T, Shum-Tim D, Jonas RA, Lidov HG, Laussen PC, Miura T, du Plessis A. Higher hematocrit improves cerebral outcome after deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1996;112(6):1610–20. doi: 10.1016/S0022-5223(96)70020-X. discussion 1620–1. [DOI] [PubMed] [Google Scholar]
- 22.Cardigan RA, Mackie IJ, Gippner-Steppert C, Jochum M, Royston D, Gallimore MJ. Determination of plasma aprotinin levels by functional and immunologic assays. Blood Coagul Fibrinolysis. 2001;12(1):37–42. doi: 10.1097/00001721-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Cabral HJ. Multiple comparison procedures. Circulation. 2008;117:698–701. doi: 10.1161/CIRCULATIONAHA.107.700971. [DOI] [PubMed] [Google Scholar]
- 24.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE., Jr . With applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. Regression modeling strategies; pp. 11–40. [Google Scholar]
- 26.Sakamoto T, Zurakowski D, Duebener LF, Lidov HG, Holmes GL, Hurley RJ, Laussen PC, Jonas RA. Interaction of temperature with hematocrit level and pH determines safe duration of hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2004;128(2):220–32. doi: 10.1016/j.jtcvs.2003.11.070. [DOI] [PubMed] [Google Scholar]
- 27.Kurth CD, Priestley M, Golden J, McCann J, Raghupathi R. Regional patterns of neuronal death after deep hypothermic circulatory arrest in newborn pigs. J Thorac Cardiovasc Surg. 1999;118(6):1068–77. doi: 10.1016/S0022-5223(99)70103-0. [DOI] [PubMed] [Google Scholar]
- 28.Eser O, Kalkan E, Cosar M, Buyukbas S, Avunduk MC, Aslan A, Kocabas V. The effect of aprotinin on brain ischemic-reperfusion injury after hemorrhagic shock in rats: an experimental study. J Trauma. 2007;63(2):373–8. doi: 10.1097/01.ta.0000236054.42254.b7. [DOI] [PubMed] [Google Scholar]
- 29.Rustom R, Grime JS, Maltby P, Stockdale HR, Critchley M, Bone JM. Observations on the early renal uptake and later tubular metabolism of radiolabelled aprotinin (Trasylol) in man: theoretical and practical considerations. Clin Sci (Lond) 1993;84(2):231–5. doi: 10.1042/cs0840231. [DOI] [PubMed] [Google Scholar]
- 30.Backer CL, Kelle AM, Stewart RD, Suresh SC, Ali FN, Cohn RA, Seshadri R, Mavroudis C. Aprotinin is safe in pediatric patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2007;134(6):1421–6. doi: 10.1016/j.jtcvs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Fritz H, Wunderer G. Biochemistry and applications of aprotinin, the kallikrein inhibitor from bovine organs. Arzneimittelforschung. 1983;33(4):479–94. [PubMed] [Google Scholar]
- 32.Royston D, Bidstrup BP, Taylor KM, Sapsford RN. Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet. 1987;2(8571):1289–91. doi: 10.1016/s0140-6736(87)91190-1. [DOI] [PubMed] [Google Scholar]
- 33.Bidstrup BP, Royston D, Sapsford RN, Taylor KM. Reduction in blood loss and blood use after cardiopulmonary bypass with high dose aprotinin (Trasylol) J Thorac Cardiovasc Surg. 1989;97(3):364–72. [PubMed] [Google Scholar]


