DESPITE RECENT improvements in operative techniques,1,2 immunosuppression,1,3,4 and organ procurement,5–7 failure of a hepatic allograft remains an important risk to liver recipients. In the absence of any effective method of extracorporeal support, the only alternative to death for these patients is retransplantation (RETX). The need for RETX can occur in one of three settings: emergent, acute, and elective. Proper timing of elective RETX requires balancing the risk of further attempts to rescue the primary graft against the risks of reoperation. Successful use of RETX in the emergent and acute settings requires an effective organ procurement system and optimization of all available donors.
The present study involves a review of the experience obtained from the sequential transplantation of two or more hepatic grafts into 70 patients over a 21-year period.
MATERIALS AND METHODS
Data Analysis
From March 1, 1963, to Feb 3, 1980, of the 170 liver recipients transplanted at the University of Colorado under conventional immunosuppression with azathioprine and prednisone (AZA-P), 21 required RETX. Survival data are provided for this group to allow a comparison with similar data obtained from 199 patients treated at Colorado (14 patients) and at the University of Pittsburgh Health Center (185 patients) with cyclosporine and prednisone (CYCLO-P), among whom 49 required RETX.
The major causes for RETX were reviewed and designated as either primary nonfunction of a graft (PNF), rejection, or technical causes (TECs).
A diagnosis of PNF was made if a graft never demonstrated evidence of initial function following transplantation. Such evidence would include not only frank signs of total hepatic failure (eg, profound hypoglycemia, uncorrectable coagulopathy, stage IV coma, renal failure, acidosis, and cardiodynamic shock), but also other signs of irreparable damage to the organ (ie, massive rises in transaminases along with unrelenting daily rises in bilirubin, persistent renal insufficiency, mental confusion and persistent coagulopathy).
Any secondary failure of a graft, following an initial period of acceptable function, was assigned to one of the other two categories. Rejection was diagnosed mainly on clinical grounds. The histopathology of the grafts removed was reviewed as well. A TEC classification was assigned to all grafts failing from vascular thromboses, complications of biliary tract reconstruction, or other errors in operative technique.
Survival data are calculated using life table methods. All CYCLO-P patients have been observed for at least six months. All AZA-P patients have died.
Blood usage data were collected for all patients transplanted from Jan 1, 1983, to Aug 1, 1984. Paired t tests were used to compare the means of operative blood loss during the first transplant to those during the second. Median data was compared using a rank sum test.
The presence of cytotoxic T-warm antibodies in both groups of patients undergoing one liver transplant, and those requiring more than one, was examined using methods previously described8 and compared using chisquare analysis.
RESULTS
Incidence of Retransplantation
RETX under AZA-P therapy was used infrequently, with only 21 of 170 patients (12.4%) during a 19-year period receiving second hepatic grafts. The increasing importance of RETX since the introduction of cyclosporine as an immunosuppressant is illustrated graphically in Fig 1. Over a five-year period ending Aug 1, 1984, 69 of 323 total operations (21.4%) have been RETX's. Of these 69 reoperations, 60 were second transplants and nine were third transplants. No RETXs occurred in 1980, although one patient receiving a first graft in that year received retransplants twice in 1982. During 1983, the retransplant rate increased to 26.9%, the highest in any year thus far.
Fig 1.
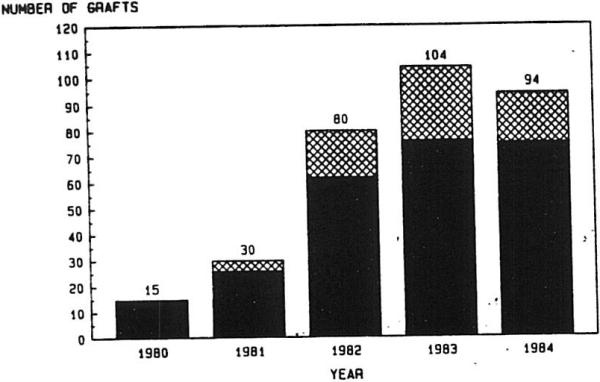
Liver transplants per calendar year. ▩, Retransplants; ■, first grafts.
The primary disease categories and the proportion of patients receiving RETX for each are shown in Fig 2. No significant difference in incidence of RETX was found among the disease categories, except that none of the 12 primary hepatic tumor patients required RETX. Furthermore, Fig 3 shows that the causes of RETX occurred at a frequency that was not significantly different among the disease entities, except that technical failures did not occur in the primary biliary cirrhosis or sclerosing cholangitis groups.
Fig 2.
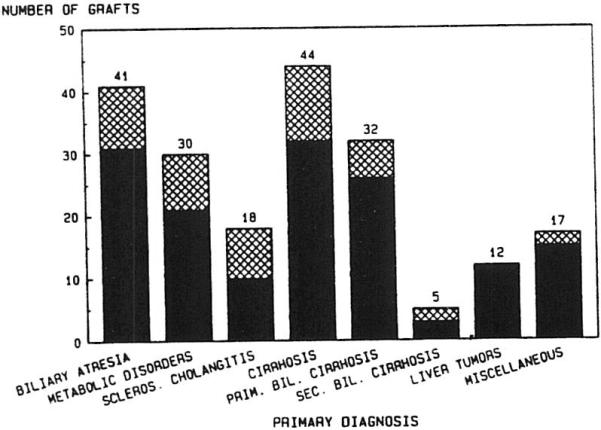
Liver retransplantation with cyclosporine and prednisone: primary disease categories. ▩, Retransplants; ■, first grafts.
Fig 3.
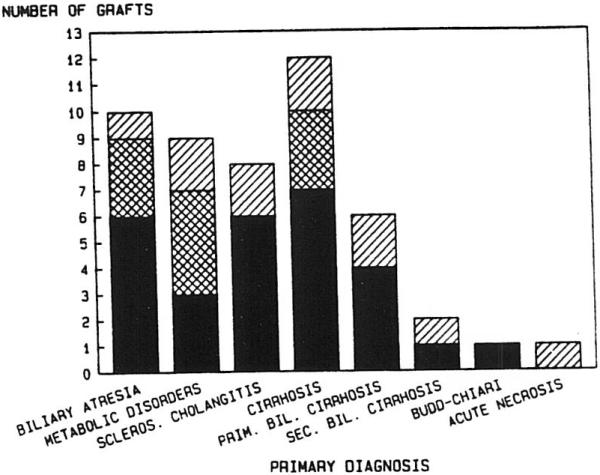
Indications for liver retranplantation: cyclosporine era. ▨, Primary nonfunction, n = 11; ▩, technical failure, n = 10; ■, rejection, n = 28.
Of the three major causes of hepatic graft loss, rejection was by far the most common, occurring in 28 of the 49 patients—15 of 24 adult patients and 13 of 25 pediatric patients (Fig 4). TECs occurred with greater frequency in children than in adults (P < .05).
Fig 4.
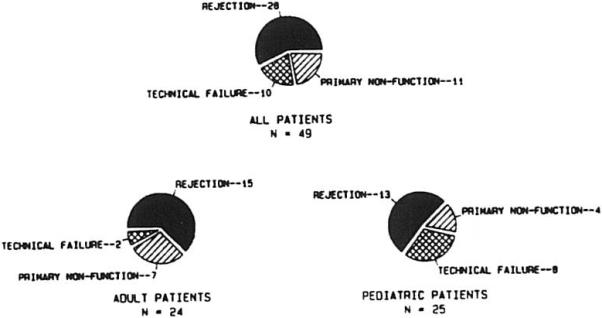
Indications for retransplantation.
Timing of Retransplantation
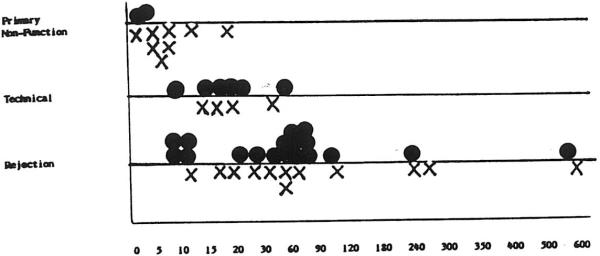
The interval between the first and second transplant operations compared with both the causes for RETX and subsequent survival for six months or more is illustrated in Fig 5. TEC failures and those secondary to PNF required early rather than late reoperation, whereas rejection appeared to occur throughout the time course, with a noticeable cluster of patients at the one-week and the 60-day intervals. Seven patients received retransplants more than 90 days after primary grafting. Two subsequently died of sepsis related to overimmunosuppression, and two others died of technical failures. Three of these patients have survived, two for more than two years and one for 13 months.
Fig 5.
Timing of retransplantation. •, Alive at >6 months; X, dead before six months
Major Causes of Death
Figure 6 plots the causes of death against the primary cause for RETX. The chart emphasizes the dominant role that sepsis with multiple-organ failure played in the death of these patients. This is particularly evident when examining patients receiving retransplants for chronic rejection or in whom the first grafts failed to provide adequate function. The technical causes of death include one patient with both portal vein and arterial thrombosis in the second graft and two patients with ruptured mycotic aneurysms of the donor hepatic arterial supply. Three patients died of irreparable brain injury following RETX. All three were returned to the operating room in deep coma secondary to profound hepatic allograft failure, two within seven days of primary grafting and one after 16 days. Three patients died of predominant hepatic failure.
Fig 6.
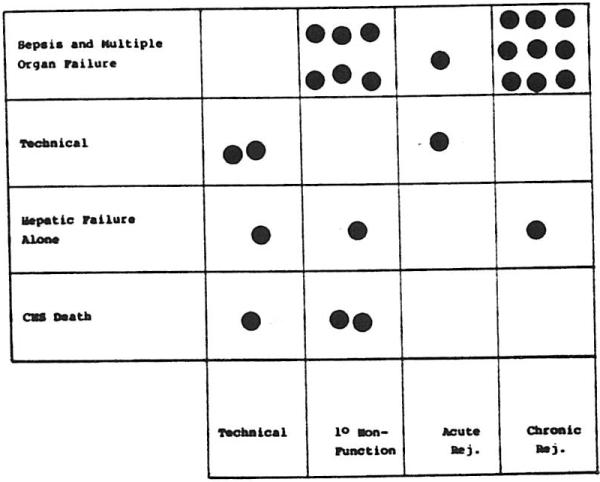
Major cause of death v cause for retransplantation
Survival Data
Patients under CYCLO-P therapy had a markedly better one-year survival rate than those under AZA-P (49% v 14%; P < .001) (Fig 7). A breakdown of overall survival of all patients, comparing survival of primary v secondary grafting and adults v children is shown in Fig 8. All patients who required only primary grafting fared better at one year than those who required RETX, with 68.5% of the former and 49% of the latter surviving at least one year (P = .014). This relationship holds true when primary and secondary grafting are compared in adults (63.5% v 42.3%; P = .026) and children (76.8% v 56.5%; P = .042) at one year. Children obtained better one-year survival than adults in the primary group (P = .038), but not in the retransplant group (P = .159).
Fig 7.
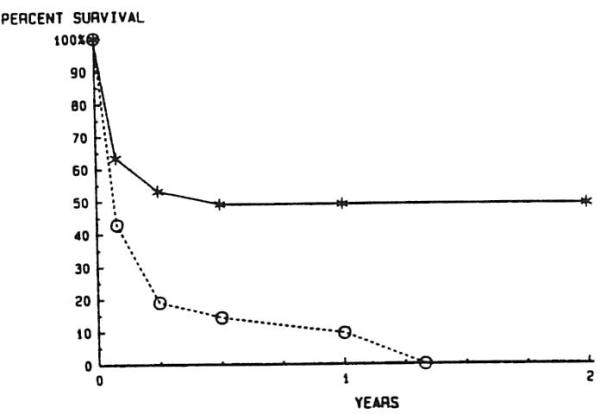
Actuarial survival after liver retransplantation, before and after the introduction of cyclosporineprednisone therapy. —*—, CYCLO-P, n = 49; --∘--, AZA-P, n = 21
Fig 8.
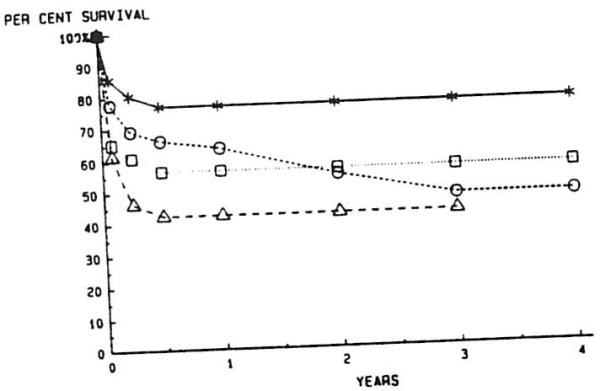
Actuarial survival after liver retransplantation with cyclosporine and prednisone in pediatric and adult patients. —*—, One graft, pediatric; --∘--, one graft, adult; ··□··, retransplanted, pediatric; --▵--, retransplanted, adult
A further breakdown of the survival for retransplanted patients based upon primary disease categories is shown in Fig 9. All curves are virtually flat after six months, since no deaths have occurred later than six months in any retransplant group. Survival at six months and beyond for each group is not significantly different from the overall survival for the entire population of retransplanted patients. Patients with biliary atresia had the best overall survival, and those with sclerosing cholangitis the worst, following RETX, but these differences are not significant (P = .074).
Fig 9.
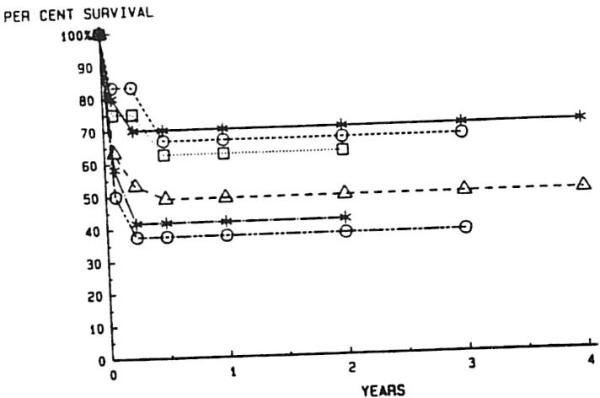
Actuarial survival after liver retransplantation with cyclosporine and prednisone: primary diagnostic categories. —*—, Biliary atresia: --∘--, primary biliary cirrhosis; ··□··, inborn errors of metabolism; --▵--, all patients; —·*·—; cirrhosis; —·∘·—, sclerosing cholangitis
Figure 10 illustrates the survival of patients dependent upon the cause of RETX. Both rejection and TEC patients had better survival after six months than PNF patients (P = .029 for rejection v PNF; P = .015 for TECs v PNF).
Fig 10.
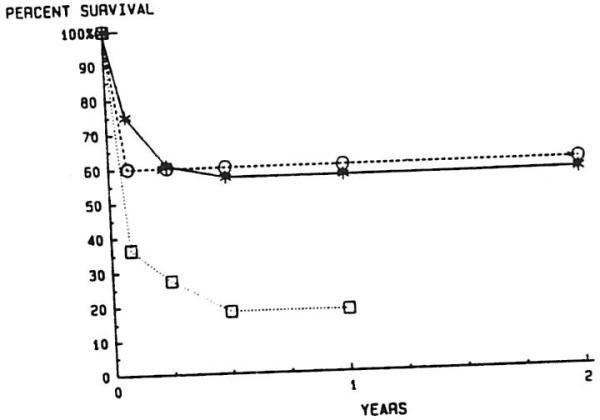
Actuarial survival for liver transplantation, based on indications for graft replacement. —*—, Rejection, n = 28; --∘--, technical failure, n = 10; ··□··, primary nonfunction, n = 11
Finally, the overall effect of RETX on survival is demonstrated in Fig 11. The lower curve assumes that all RETX patients not retransplanted would have died on the date of RETX. As a comparison, one can look at the causes of death among seven patients for whom appropriate donors could not be located in time (Table 1). The technical PNF, and acute rejection causes of graft failure created emergent situations in which the availability of appropriate donors could have been life-saving. The two cases of death following chronic rejections occurred in patients in whom the over-use of immunosuppression led to eventual sepsis, subsequent multiple-organ failure, and death. Both were pediatric recipients in whom the decision to retransplant was made early, but for whom appropriate donors were not made available before sepsis developed.
Fig 11.
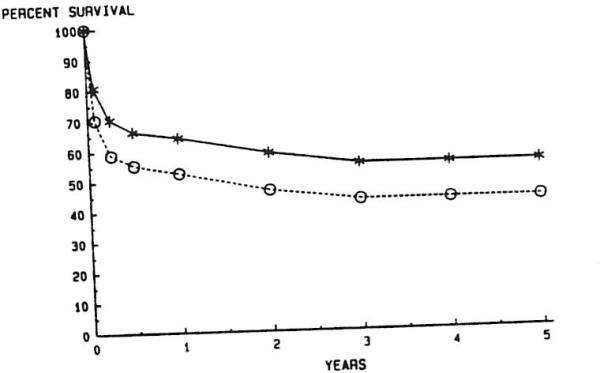
Effective liver retransplantation of actuarial survival. —*—. With retransplantation; --∘--, without retransplantation
Table 1.
Seven Patients for Whom Donors Were Not Available in Time
| Cause of Graft Failure | Cause of Death |
|---|---|
| Technical | Hepatic gangrene |
| Technical | Hemorrhage |
| Primary nonfunction | Hepatic failure |
| Acute rejection | Hepatic failure |
| Acute rejection | Hepatic failure |
| Chronic rejection | Sepsis |
| Chronic rejection | Sepsis |
Blood Usage
As seen in Table 2, the mean operative blood loss in patients undergoing primary grafting is significantly greater than in those with RETX. This is true in both adults and children, although in the latter group, in order to see a difference, one must eliminate from consideration the three cases of arterial thrombosis (see Discussion). These data were obtained only for 1983 and 1984, since venous bypass was introduced in all adult patients beginning in early 1983. The use of venous bypass has resulted in a significant reduction in operative blood use.2
Table 2.
Operative Blood Use: First Versus Second Hepatic Transplantation 1983 to 1984
| First Graft | Second Graft | P Value | |
|---|---|---|---|
| Adults (n = 20) | |||
| Mean | 37.9 ± 37.2 | 16.5 ± 12.9 | <.05 |
| Median | 29 | 16 | <.02 |
| Range | 4–157 | 1–56 | |
| Children (n = 16) | |||
| Mean | 10.9 ± 7.0 | 5.5 ± 2.5 (9.7 ± 10.6)* | <.05 |
| Median | 10 | 5 | <.01 |
| Range | 3–25 | 2–11 (2–36)* |
Values expressed are units of packed RBCs.
If cases of arterial thrombosis are included (see Discussion).
Crossmatch
Information regarding the presence or absence of donor-specific cytotoxic T cell antibodies in the serum of liver recipients was available in 148 patients but was not available in another 92 (Table 3).
Table 3.
Hepatic Retransplantation: Influence of Donor-Specific Cytotoxic T Cell Antibodies
| Negative Crossmatch | Positive Crossmatch | Not Done | |
|---|---|---|---|
| No retransplant | 94 | 21 | 76 |
| All retransplants | 27 | 6 | 16 |
| Rejected retransplants | 16 | 4 | 8 |
RETX did not occur with a greater frequency in those patients with a positive crossmatch than in those with a negative crossmatch.
Similarly, the incidence of a positive crossmatch was no greater among the RETX population than it was among those requiring only one graft. More importantly, the frequency of a positive crossmatch was no greater in patients given RETX for rejection than it was in PNF or TECs.
As reported previously, the incidence of a positive crossmatch at the time of the first transplant increased from approximately 25% to 50% at the time of RETX and to 75% at the time of tertiary RETX.8
Tertiary Transplants
A subgroup of nine patients has been given three sequential hepatic allografts (Table 4). Four are dead. One developed chronic rejection and required a second graft 572 days after primary grafting. The second graft failed to function, and a third transplant was performed seven days later. Death occurred 14 days later in the face of candida septicemia and irreversible brain injury. The second patient had severe arteriovenous shunting secondary to chronic liver disease. The first liver failed due to PNF and was replaced four days later. The second graft eventually failed because of persistent severe hypoxia in the recipient, as did a third, transplanted 57 days later. Massive intracranial hemorrhage led to the patient's death 46 days following the final transplant. Two other patients received third grafts when their second livers were lost to a form of relentless rejection similar to that which destroyed their first grafts. In both cases, death occured ten days after tertiary grafting, predominantly the result of liver failure and sepsis. Both of these latter patients were in stage III or IV hepatic coma with renal failure requiring dialysis, cardiodynamic instability, and severe coagulopathy at the time of the third transplantation.
Table 4.
Tertiary Hepatic Transplantation as of Aug 1, 1984
| Dead | Alive | |
|---|---|---|
| Interval from 2nd to 3rd transplant | 7, 10, 19, 57 d | 13, 28, 41, 120, 264 d |
| Survival after 3rd transplant | 9, 10, 14, 43 d | 7, 9, 11, 17, 18 mo |
Five patients are alive after tertiary liver transplantations. They have survived for 7, 9, 11, 17, and 18 months. Three are male children between the ages of 2 and 4 years. All three are at home and doing well. The two adults include a 26-year-old man and a 45-year-old woman, both of whom remain well and quite active.
DISCUSSION
Modifications of Recipient and Donor Procedures
A few minor changes in technique useful when performing an hepatic RETX are worth noting.
The utility of procuring vascular grafts in concert with the donor liver has been emphasized before.9 This precaution is even more important in the setting of RETX, particularly if a vascular thrombosis is the cause of graft failure, since an alternative method of arterial or portal venous reconstruction may be required.10,11
The recipient operation may vary significantly, depending upon the time interval since the first transplant operation. Early reoperations, as one might expect, require very little dissection to allow for removal of the graft. On the other hand, if other than thin filmy adhesions are encountered, care must be taken to avoid overly aggressive blunt dissection, since the vessel walls of the donor organ may prove to be the weakest point in the area being stressed. Sharp dissection along tissue planes, along with judicious use of electrocautery, may minimize blood loss.
In three cases of hepatic artery thrombosis in children, collateral arterialization of the graft through adhesions of the liver to the daiphragm, bowel, and omentum occurred. In all three, blood loss the second time exceeded that during the first transplant by a factor of three or more (Table 2).
When removing the failed hepatic graft, the upper vena caval anastomosis is left intact, and a short cuff of vein from the liver being removed is left in place to allow enough length for anastomosis with the new organ (Fig 12). Portions of the first liver's lower vena cava and portal vein may also be reused if extra length is needed. Portions of graft hepatic artery, on the other hand, are never reused, since in three cases, necrosis in the vessel wall associated with a mononuclear infiltrate in the media of the artery has resulted in arterial thrombosis or rupture.
Fig 12.
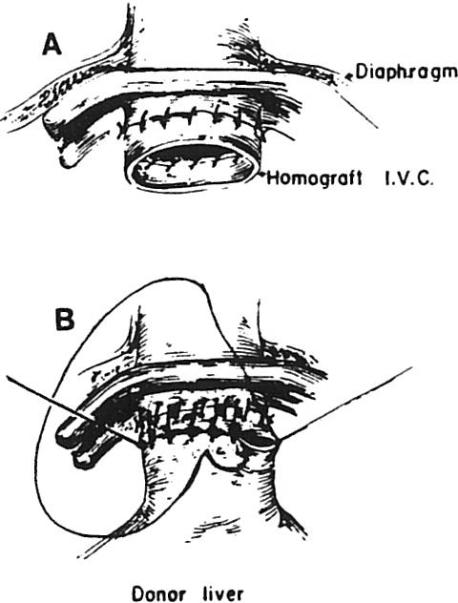
Intact upper vena caval anastomosis with short cuff of vein from the liver being removed, which is left in place for anastomosis with the new organ
Biliary reconstruction in RETX requires careful assessment of the viability of the recipient duct tissue and removal of all duct tissue from the first donor if a duct-to-duct anastomosis is to be redone. Construction of a Rouxen-Y jejunal loop may be necessary if the recipient bile duct is of inadequate length or insufficient quality. Reuse of a jejunal loop requires closure of the first anastomostic site and choledochoenterostomy at another point along the limb.
Clinical Setting of Retransplantation
Massive hepatic necrosis secondary to rejection or hepatic gangrene from arterial thrombosis occurs infrequently but requires emergency RETX. Although only three of the 49 patients in this series fall into this category, three others did die before a donor could be found. Early RETX is also required for PNF, but seldom on an emergent basis. Acute and chronic rejection caused graft failure predominantly at two intervals: seven to ten days and six to eight weeks. In the former cases, the decision to retransplant was seldom difficult in the face of liver failure unresponsive to all therapy. The latter cases occurred on a more elective basis, although urgency was present on those occasions where marked clinical deterioration began to occur before donors became available.
Developing clinical criteria that accurately determined a need for RETX in the elective setting is impractical with only 49 patients. However, some useful general guidelines can be set forth. These guidelines are based on the observation in the present series: (1) that survival after RETX approaches that of primary grafting; (2) the major cause of failure after RETX is sepsis; (3) the RETX operation is generally less difficult than primary grafting, for both the surgeon and the recipient; and (4) failure of a second transplant does not preclude survival after a third, since five of nine such patients are alive and well.
In the first month after grafting, all patients with a persistent elevation of bilirubin above 10 mg/dL (with or without elevation of transaminase), which is unresponsive to two full courses of antirejection therapy,1 and those without evidence of correctable lesions should be considered for RETX. The same holds for any patient, regardless of serum bilirubin levels, whose liver function deteriorates rapidly when steroid doses are reduced to acceptable maintenance levels (approximately 20 to 30 mg daily in adults and 10 to 20 mg daily in children), or who is receiving frequent intravenous bolus injections of steroids in an attempt to control rising liver function tests. Liver biopsy may help to confirm the diagnosis of rejection, but absence of classical signs does not rule out rejection. The presence of viral inclusion particles may allow appropriate antiviral therapy to be instituted, but this rarely results in significant long-term improvement since rejection is almost always a predominant accompanying feature.
Patients who develop rejection after several months of normal liver function seldom respond to manipulations of immunosuppression. A biopsy that shows signs of chronic rejection (arteriolar thickening, extensive periportal fibrosis, and disappearance of bile ductules) indicates irreversible destruction in most cases. The presence of massive elevations of serum cannilicular enzymes, particularly gamma glutamyl transpeptidase levels, is an ominous sign as well. Vigorous rescue efforts with increasing immunosuppression in this setting should be abandoned in favor of elective RETX.
In all cases, thorough and repeated efforts should be made to exclude the possibility of correctable lesions, such as drug toxicity, biliary leaks, obstruction, or intraabdominal abscess. Angiography in the face of overt graft failure is an unnecessary risk since RETX will be necessary anyway, but it may allow an earlier decision to be made for RETX in less obvious situations.
SUMMARY
RETX resulted in significant improvements in survival in a large series of liver transplant patients. One-year survival following RETX is not as good as following primary transplants, but the rate approaches 50% for all patients, 60% for children, and 45% for adults. RETX occurs in a variety of clinical settings but results from one of three major causes: rejection, PNF, or TECs. The need for RETX can be emergent, acute, or elective. The decision for RETX is usually obvious in the emergent and acute settings. Guidelines for making the decision in the elective situation have been outlined.
Acknowledgments
Supported by research grants from the Veterans Administration and project grant No. AM-29961 from the National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Ann Surg. 1984;200:524. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calne RY, Williams R, Lindop M, et al. Br Med J. 1981;283:115. doi: 10.1136/bmj.283.6284.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Klintmalm GBG, Weil R, III, et al. N Engl J Med. 1981;305:266. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw BW, Jr, Hakala TR, Rosenthal JT, et al. Surg Gynecol Obstet. 1982;155:321. [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenthal JT, Shaw BW, Jr, Hardesty RL, et al. Ann Surg. 1983;198:617. doi: 10.1097/00000658-198311000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Hakala TR, Shaw BW, Jr, et al. Surg Gynecol Obstet. 1984;158:223. [PMC free article] [PubMed] [Google Scholar]
- 8.Iwatsuki S, Rabin BS, Shaw BW, Jr, et al. Transplant Proc. this issue. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Harlgrimson CC, Koep LJ, et al. Surg Gynecol Obstet. 1979;149:737. [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw BW, Jr, Iwatsuki S, Starzl TE. Surg Gynecol Obstet. 1984;159:265. [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw BW, Jr, Iwatsuki S, Bron K, et al. Surg Gynecol Obstet. in press. [PMC free article] [PubMed] [Google Scholar]