Abstract
Human papillomavirus (HPV) infection is associated with almost all cases of cervical cancer, and cervical cancer is a common malignancy in women living in developing countries. A cross-sectional study was conducted to determine the prevalence of HPV infection, human immunodeficiency virus (HIV) infection, and cervical cytologic abnormalities in women presenting to a sexually transmitted infections clinic in Kampala, Uganda. In June and July, 2002, 135 women underwent complete physical exams including Papanicolaou (Pap) smears. HIV status was evaluated by serology. Cervical and vaginal swabs were obtained by clinicians and tested for HPV genotypes by PCR/reverse blot strip assay. Of the 106 women with cervical swabs adequate for HPV testing, the HPV prevalence was 46.2% (49/106). HIV prevalence was 34.9% (37/106). High risk genotypes 52, 58, and 16 were the genotypes detected most commonly. Eighteen percent (9/49) of women infected with HPV were found to have genotypes 16 and/or 18. Seventy-three percent (27/37) of HIV-positive women versus 16% (10/63) of HIV-negative women had abnormal Pap smears (P <0.0001). Among HIV-positive women, abnormal Pap smears were associated with the presence of high risk HPV genotypes (P <0.001). The majority of women infected with HPV attending this sexually transmitted infections clinic in Uganda were infected with high risk HPV genotypes other than 16 and 18. Future studies should focus on whether current HPV vaccine formulations, that are limited to high risk genotypes 16 and 18, would be effective at decreasing the burden of cervical cancer in this population.
Keywords: human papillomavirus (HPV), human immunodeficiency virus (HIV), HPV genotypes, cervical cytologic abnormalities, cervical cancer
INTRODUCTION
Cervical cancer is the most common malignancy in women living in developing countries [Cronje, 2004]. During the past 20 years, it has been established that oncogenic human papillomaviruses (HPV) are associated with almost all cases of cervical cancer and have prompted studies to understand cervical carcinogenesis as well as better means to diagnose and prevent HPV. In African countries, cervical cancer rates are on the rise and parallel the acquired immunodeficiency syndrome (AIDS) epidemic [Wabinga et al., 1993; Mbulaiteye et al., 2005] Studies from Tanzania have correlated human immunodeficiency virus (HIV) infection with HPV genotypes 16 and 18, which have been most frequently associated with malignant transformation [ter Meulen et al., 1992]. HPV prevalence rates range from 30 to 60% in African women with the highest rates seen in women co-infected with HIV [Wright et al., 2000; Baay et al., 2004].
In Uganda, there are currently no formal screening programs for cervical cancer and no technologies readily available to establish HPV infection aside from Papanicolaou (Pap) smears, which are not routinely performed. A vaccine that prevents cervical infection with HPV could have a significant impact in decreasing the rates of cervical cytologic abnormalities and cancer in this country and other developing countries. Recent studies have demonstrated the efficacy of these vaccines in preventing not only the incidence and persistence of cervical HPV infections but also the associated cytologic abnormalities [Koutsky et al., 2002; Harper et al., 2004].
Since immunity to HPV virus-like particle vaccines is type-specific, characterizing the distribution of HPV genotypes in different regions of the world, such as East Africa where there is a high prevalence of both HPV infection and HIV infection, is of critical importance. The goal of this study was to determine the genotypes of HPV infection in Ugandan women presenting to an urban sexually transmitted infections clinic. Secondary endpoints were to assess the co-prevalence of HIV, cervical cytologic abnormalities, and other sexually transmitted infections in these women.
MATERIALS AND METHODS
Setting
The study was conducted at the National Sexually Transmitted Diseases Referral Centre of Mulago Hospital, the major teaching hospital of Makerere University, located in Kampala, Uganda. Ethical approval to conduct this study was obtained from the institutional review boards of Case Western Reserve University/Case Medical Center, the Uganda HIV/AIDS Research Committee, and the Uganda National Council of Science and Technology in Kampala, Uganda. Kampala is the capital city of Uganda covering an area of 169 km2 with a population of more than one million people. Approximately 15 patients per day visit the sexually transmitted infections clinic at the National Sexually Transmitted Diseases Referral Centre. The facility is equipped with a molecular diagnostic laboratory that includes polymerase chain reaction (PCR) capability, among other modern technologies.
Study Population and Collection of Specimens
Between June 4 and July 12, 2002 consecutive women presenting to the sexually transmitted infections clinic for evaluation were invited to participate in this cross-sectional study. Women were eligible if they were nongravid and between the ages of 18 and 55 years old. After obtaining written informed consent and HIV pre-test counseling, a demographic and clinical questionnaire was administered by personnel on-site at the clinic. A complete physical examination was performed by one of three physicians (JF, SM, JK). On pelvic exam, particular attention was given to the presence, location, and number of genital warts (condyloma accuminata). In addition, visual inspection of the cervix was performed after application of acetic acid to identify acetowhite lesions. Colposcopy was not performed, and no cervical biopsies were taken. Routine cervical and vaginal swabs were obtained for cytologic screening, HPV detection, multiplex PCR, KOH, and wet preps. Additional specimens for multiplex PCR were collected from suspicious ulcerative lesions. Multiplex PCR tested for the presence of Neisseria gonorrhea, Chlamydia trachomatis, Trichomonas vaginalis, herpes simplex virus, Treponema pallidum, and Hemophilus ducreyi. Blood samples were obtained to test for HIV and syphilis. Prevalent syphilis was established with positive rapid plasma reagin (RPR) titers (Human GmbH, Germany) confirmed with microhemagglutinin-treponema pallidum (MHATP) (Human GmbH, Germany).
Cytologic Screening
The Pap smears were prepared conventionally and shipped to the Department of Pathology at University Hospitals of Cleveland/Case Western Reserve University where they were read by a cytopathologytechnician and a cytopathologist (FAK). These Pap smears were classified according to The Bethesda System as negative, atypical squamous cells of uncertain significance, low-grade squamous intraepithelial lesion, or high-grade squamous intraepithelial lesion.
HPV DNA Detection and Typing
After collection, the cervical and vaginal swabs were placed in a −20°C freezer until they could be transported to Indiana University for further analysis. At Indiana University, DNA was extracted using the High Viral Pure Kit (Roche Molecular Diagnostics, Basel, Switzerland). Samples were initially tested for adequacy by co-amplifying the GH20 and PC04 human β-globin targets. The Roche PCR/reverse blot strip assay was used to detect specific HPV types in the cervical and vaginal swab specimens. This assay uses nondegenerate biotinylated primer pairs to amplify HPV genotypes 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 73, 82, 83, and 84 as described elsewhere [Brown et al., 2005]. Reactions were amplified in a Perkin Elmer TC9600 Thermal Cycler (Perkin Elmer, Massachusetts, USA) [Brown et al., 1999]. A separate PCR containing no added DNA was included in each assay as a negative control.
HIV Serologic Detection
Initial testing for HIV-1/2 antibodies was performed using enzyme immunoassay (EIA) (Recombigen HIV-1/2 EIA, Cambridge Diagnostics, Ireland). HIV-1 positive EIA results on the first assay were confirmed with a rapid HIV-1 enzyme-linked immunosorbent assay (ELISA) (Capillus™, Trinity Biotech, Ireland). Women found to have HIV infection were invited for post-test counseling and, thereafter, referred to HIV care and treatment centers, as recommended by the World Health Organization [World Health Organization, 2003].
Statistical Analysis
Statistical analysis was performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC). Demographic risk factors were evaluated for an association with cervical HPV infection by univariate analysis. Differences between categorical variables were evaluated using χ2 analysis, Fisher’s exact test, and Kendall’s Tau-b statistic. Differences between continuous variables were evaluated using Student’s t-test, Wilcoxon Rank Sum, and Kruskal–Wallis statistics. A two-sided P-value <0.05 was considered significant for all comparisons.
Cervical HPV assays were used to represent infection since this source is considered the standard for evaluation. The presence of cervical HPV DNA was coded as a three-level hierarchical variable: negative, low risk HPV, and high risk HPV. The HPV genotypes that were included in the low risk category were 6, 11, 40, 42, 53, 54, 57, and 66. The HPV genotypes considered high risk included 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 55, 56, 58, 59, 68, 73, 82, 83, and 84. Participants with HPV infections caused by multiple HPV genotypes were categorized by the HPV genotype of highest oncogenicity. Associations between the Pap smears and the level of HPV oncogenicity were evaluated using Kendall’s Tau-b statistic to account for the ordinality of risk level. Correlation between cervical and vaginal HPV results were calculated using a kappa statistic.
Pap smears with readings of negative were classified as normal, while those with readings of atypical squamous cells of uncertain significance, low-grade squamous intraepithelial lesion, or high-grade squamous intraepithelial lesion were classified as abnormal. Pap smear results were evaluated for association with HIV status and HPV oncogenicity. The interaction between HIV status and high-risk HPV infection (present/absent) on these associations was tested using logistic regression with Pap smear results collapsed to a dichotomous outcome (normal/abnormal) and including an interaction term between HIV and HPV in the model.
Subjects were considered to have HIV infection only if both the initial ELISA and rapid HIV test were positive. The presence of multiple HPV infections was evaluated overall, and in relation to both HIV status and cervical cytologic abnormalities.
RESULTS
Study Population
One-hundred thirty-five of 238 consecutive women presenting to the sexually transmitted infections clinic at the National Sexually Transmitted Diseases Referral Centre over a 6-week period in 2002 consented to participate in the study. Twenty-nine of these women were excluded from analysis when their cervical samples were found to be inadequate for HPV testing, leaving a total of 106 women in the analysis.
The age of these 106 participants ranged from 18 years to 51 years old with a mean of 26.3. The average number of lifetime sex partners for these women was 3.6 with the average age of coital debut being 17.5 years old. Sixty-one percent (65/106) of the women reported a past history of sexually transmitted infections. Two women admitted to engaging in commercial sex. While 86.7% (91/105) reported having had sex with a male who used a condom, only 14.6% (15/103) always used a condom. Women infected with HPV were somewhat younger (25 vs. 27.5 years old on average, respectively, P = 0.06), had fewer lifetime sex partners (medians of 2 vs. 3 respectively, P = 0.03), and fewer children (medians of 1 vs. 2 respectively, P = 0.03). No other demographic risk factors were associated with HPV infection (Table I).
TABLE I.
Baseline Characteristics of 106 Ugandan Women by HPV Status
| HPV +* |
HPV −* |
||
|---|---|---|---|
| Characteristic | (n =49) | (n =57) | P value |
| Age | 25.0 (5.7) | 27.5 (7.4) | 0.06 |
| Marital status | |||
| Married monogamous | 18 (37) | 27 (47) | 0.6 |
| Married polygamous | 3 (6) | 7 (12) | |
| Co-habitating | 2 (4) | 1 (2) | |
| Separated/divorced | 7 (14) | 5 (9) | |
| Widowed | 3 (6) | 2 (4) | |
| Never married | 16 (33) | 15 (26) | |
| Number of children | 1.3 (1.6) | 2.0 (1.8) | 0.03 |
| Past history of sexually transmitted infections | 28 (57) | 37 (65) | 0.4 |
| History of cervical cytologic abnormalities or cervical cancer | 0 | 0 | |
| Age of first sex | 17.2 (2.7) | 17.7 (2.6) | 0.3 |
| Engaged in commercial sex | 1 (2) | 1 (2) | 0.9 |
| Have used a condom with sex | 44 (90) | 47 (84) | 0.4 |
| Always use a condom with sex | 9 (19) | 6 (11) | 0.3 |
| Number of sex partners in life | 3.2 (4.9) | 3.8 (2.8) | 0.03 |
| New partners in past 2 months | 1 (2) | 3 (5) | 0.6 |
| Current smoker | 1 (2) | 1 (2) | 0.9 |
| Current alcohol drinker (drink at least 1 day per week) | 24 (50) | 26 (46) | 0.7 |
| Reason for visit | |||
| Genital discharge | 34 (69) | 34 (60) | 0.3 |
| Lower abdominal tenderness | 21 (43) | 26 (46) | 0.8 |
| Dysuria | 22 (45) | 21 (37) | 0.4 |
| Vaginal bleeding | 1 (2) | 5 (9) | 0.2 |
| Partner has sexually transmitted infection | 9 (18) | 11 (19) | 0.9 |
| Genital ulcer, no blister | 13 (27) | 10 (18) | 0.3 |
| Genital ulcer, with blisters | 11 (22) | 14 (25) | 0.8 |
| Asymptomatic check-up | 1 (2) | 0 | 0.9 |
| Genital itching | 35 (71) | 40 (70) | 0.9 |
| Other | 5 (10) | 2 (4) | 0.2 |
| Visit type | |||
| Initial visit | 39 (80) | 33 (62) | 0.08 |
| Established patient | 10 (20 | 20 (38) | |
Reported as count data (%) or mean (S.D.).
Clinical Findings
The most common reason for visiting the sexually transmitted infections clinic was genital itching (71%, 75/106). Other reasons included genital discharge (64%, 68/106), lower abdominal tenderness (44%, 47/106), dysuria (41%, 43/106), vaginal bleeding (6% 6/106), genital ulcers without blisters (22%, 23/106), genital ulcers with blisters (24%, 25/106), a partner with a sexually transmitted infection (19%, 20/106), or other (7%, 7/106). Most women (85%, 90/106) presented with multiple symptoms. Only one woman was asymptomatic.
The clinical diagnoses given to these women by the on-site physicians included candidiasis (34.2%, 36/105), trichomoniasis (3.9%, 4/102), bacterial vaginosis (25.5%, 26/102), mucopurulent cervicitis (8.7%, 9/104), pelvic inflammatory disease (21.7%, 23/106), warts (15.1%, 16/106), genital ulcer disease (20.8%, 22/106), vaginal discharge (50.9%, 54/106) and other (34.0%, 34/100). Seven women were noted to have oral candidiasis, all of whom were found subsequently to have HIV infection. Sixteen women were found to have genital warts, but warts observed on physical examination were not associated with the presence of HPV infection by PCR determination (P = 0.4).
Laboratory Findings
HIV prevalence was 34.9% (37/106). There was a low prevalence of syphilis (1.0%, 1/102) based on serology. Multiplex PCR of cervical swabs also demonstrated that there was a low prevalence of chlamydia (3.9%, 4/102), gonorrhea (3.9%, 4/102), and trichomoniasis (4.9%, 5/102). No significant associations were found between HPV infection and other sexually transmitted infections overall or if stratified by HIV status.
HPV Prevalence
The HPV prevalence from cervical specimens in these 106 women was 46.2% (49/106). HPV infection was detected in 39.1% (27/69) of HIV-negative women and 59.5% (22/37) of HIV-positive women (P = 0.065). High-risk genotypes were detected in all but three of the women infected with HPV. While high-risk genotypes were detected in 88.9% (24/27) of the HIV-negative women infected with HPV, high-risk genotypes were detected in all (22/22) of the HIV-positive women co-infected with HPV.
Ninety-seven of the 106 women with cervical swabs that were adequate for HPV analysis also had vaginal swabs that were adequate for evaluation. Out of this group, 95.9% (47/49) with a positive cervical swab had a vaginal swab positive for HPV. Similarly, 87.5% (42/48) with a negative cervical HPV test had a negative vaginal HPV test. The kappa statistic for agreement among these observations was 0.83 (95% CI: 0.72, 0.94).
HPV Genotype Distribution
Of the 27 HPV genotypes included on the Roche HPV assay, there were 23 identified in the 49 women infected with HPV. High risk genotypes 52 (14.2%, 15/106), 16 (7.5%, 8/106), 58 (7.5%, 8/106), and low risk genotype 6 (6.6%, 7/106) were the most commonly detected genotypes (Fig. 1). Genotype 16 and/or 18 was identified in 18.4% (9/49) of the women infected with HPV.
Fig. 1.
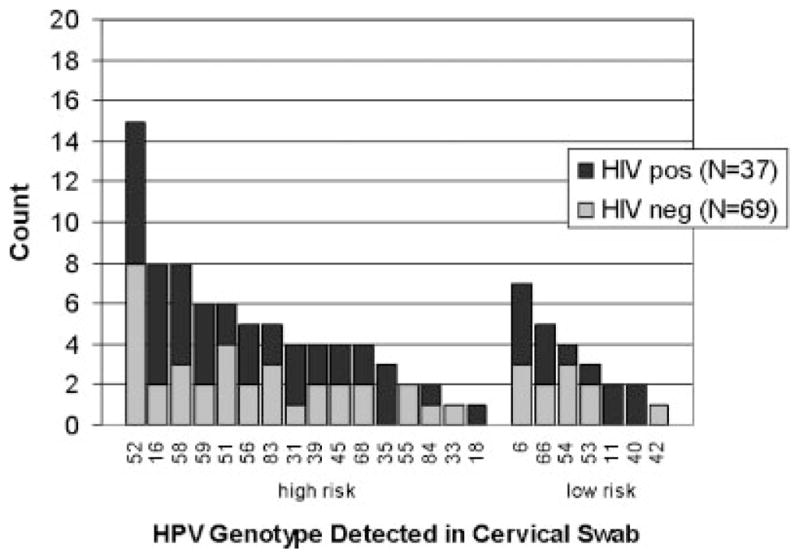
Frequency of HPV types in cervical swabs from 106 women by HIV status.
Nineteen (17.9%, 19/106) women were infected with a single HPV genotype, while 30 (28.3%, 30/106) women were infected with multiple HPV genotypes. Out of those infected with multiple genotypes 14 women were infected with two, 11 were infected with three, 3 were infected with four, and 2 were infected with five genotypes (Table II). Infection with multiple HPV genotypes was associated with HIV status (P <0.01) as was abnormal Pap smear (P <0.01). However, after adjusting for HIV status, women infected with multiple HPV genotypes were no more likely to have an abnormal Pap smear than women infected with a single HPV genotype (P =0.2).
TABLE II.
HPV Infections Based on Number of Genotypes Stratified by HIV Status and Pap Smear Results in 106 Ugandan Women
| HIV – negative (N = 69) |
HIV-positive (N =37) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pap Smear Result |
Pap Smear Result |
||||||||||
| No. of HPV genotypes | No. of women | Not known | Neg. | ASC-US | LSIL | HSIL | Not known | Neg. | ASC-US | LSIL | HSIL |
| 0 | 57 | 5 | 31 | 3 | 1 | 2 | 0 | 8 | 4 | 0 | 3 |
| 1 | 19 | 0 | 14 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 2 | 14 | 0 | 4 | 0 | 1 | 2 | 0 | 1 | 2 | 2 | 2 |
| 3 | 11 | 1 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 3 |
| 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| 5 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total* | 106 | 6 | 53 | 4 | 2 | 4 | 0 | 10 | 6 | 6 | 15 |
Reported as number of women.
Neg, negative; ASC-US, atypical cells of uncertain significance; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
Cytologic Findings
There were six women whose Pap smears were unsatisfactory for evaluation. Out of the 100 women with evaluable Pap smears, 37 were abnormal. There were 63 normal, 10 with atypical squamous cells of uncertain significance, 8 with low-grade squamous intraepithelial lesions, and 19 with high-grade squamous intraepithelial lesions. Seventy-three percent (27/37) of HIV-positive women versus 16% (10/63) of HIV-negative women had abnormal Pap smears (P <0.0001) (Fig. 2).
Fig. 2.
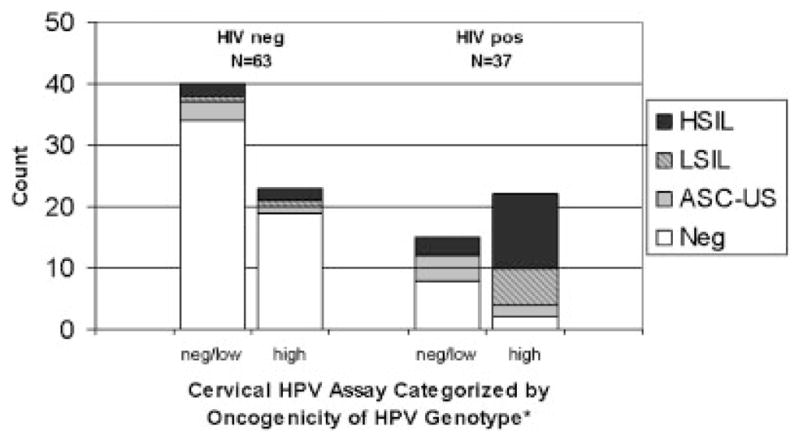
Frequency of cervical cytologic abnormalities stratified by HIV status and oncogenic risk of HPV genotypes. * Subjects with HPV infections caused by multiple HPV genotypes were categorized by the HPV genotype of highest oncogenicity. Neg, negative; ASC-US, atypical cells of uncertain significance; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
Fifty-nine percent (22/37) of HIV-positive women versus 37% (23/63) of HIV-negative women had at least one detectable HPV genotype of high oncogenic risk (P =0.015). The association between high risk HPV genotypes and abnormal Pap smears depended on HIV status in these women (P =0.049). Among HIV-positive women, the presence of high risk HPV was significantly associated with abnormal findings on Pap smear (P <0.001), while among HIV-negative women, no statistically significant association between high risk HPV and abnormal Pap smear was observed (P =0.9). In addition, 20 women co-infected with HIV and HPV had abnormal Pap smears, and, of those, 70% (14/20) had HPV genotypes which did not include 16 or 18.
Thirty-nine percent (15/37) of the HIV-positive women versus 6% (4/63) of the HIV-negative women had high-grade squamous intraepithelial lesions detected on Pap smear (P <0.0001) (Table II). Table III shows the HPV genotypes from cervical HPV assays of women with high-grade squamous intraepithelial lesions on Pap smear. The most commonly isolated genotypes were 16, 52, and 58. While genotype 16 was identified in five women, genotypes 52 and 58 each were identified in four women.
TABLE III.
HPV Genotypes from the 14 Women* With Pap Smears Showing High-Grade Squamous Intraepithelial Lesions (HSIL) Who Had Virus Identified on the HPV Assays
| Number of genotypes | HIV-negative | HIV-positive |
|---|---|---|
| 1 | 59 | |
| 58 | ||
| 52 | ||
| 16 | ||
| 2 | 33, 6 | |
| 56, 6 | ||
| 52, 45 | ||
| 16, 51 | ||
| 3 | 58, 83, 35 | |
| 18, 58, 51 | ||
| 16, 68, 54 | ||
| 4 | 16, 52, 66, 53 | |
| 16, 52, 56, 31 | ||
| 5 | 58, 56, 45, 66, 11 |
There were five women with HSIL who had no HPV isolated on cervical HPV assay.
The distribution of HPV infection varied between cytologic groups. Thirty-eight percent (24/63) of women with normal Pap smears had a positive cervical HPV assay. While 30% (3/10) of women with atypical squamous cells of uncertain significance on Pap smear had a positive cervical HPV assay, 77.8% (21/27) of women with low-grade squamous intraepithelial lesions or high-grade squamous intraepithelial lesions were HPV-positive on this assay.
DISCUSSION
This small study provides valuable information regarding the distribution of HPV genotypes in women presenting to a sexually transmitted infections clinic in Uganda. Genotype 52 was detected most commonly from cervical HPV assays, followed by genotypes 58 and 16. These results are consistent with the distribution documented by De Vuyst et al. at a family practice clinic in Nairobi, Kenya where HPV 52 also was isolated most commonly [De Vuyst et al., 2003]. In addition, they are similar to the findings of Lin et al. who found that HPV genotypes 16, 52, 58 were most prevelant in a population of adult women visiting a general gynecologic practitioner in South Taiwan [Lin et al., 2006]. These genotype distributions are different than the patterns documented in the United States, where HPV 16, 83, 51, 55, and 56 have been found to predominate [Brown et al., 2002].
This data may have important public health implications, because the results suggest that a HPV vaccine containing genotypes 16 and 18 could play only a limited role in Uganda. Although the HPV vaccine has shown promising results [Koutsky et al., 2002; Harper et al., 2004], the current preparation may prevent only oncogenic infections with genotypes 16 and 18. In this study, 18.4% of women infected with HPV had one or both of these HPV genotypes, suggesting that the majority of HPV infections were caused by other genotypes. In addition, a large proportion of Ugandan women had multiple infections as detailed in Table II, which adds complexity to the prevention of this disease. It may be worthwhile in the future, therefore, to formulate a HPV vaccine tailored more specifically to the genotype distribution in East Africa.
Because HPV assays never have been performed on cervical cancer specimens isolated from Uganda women, it is not known if the HPV genotypes isolated from cervical samples in this study are the ones that actually cause progressive cervical cytologic abnormalities leading to carcinoma. Table III documents the HPV genotypes from cervical HPV assays of women with high-grade squamous intraepithelial lesions on Pap smear. The findings shown in this table may give, perhaps, the best available approximation of the genotypes associated with advanced cervical lesions in these Ugandan women. The same genotypes as were found in all participants infected with HPV (genotypes 16, 52, and 58) were isolated also, most commonly, from the women with high-grade squamous intraepithelial lesions.
The results of this study suggest that HIV-positive women may have the most to gain from interventions to decrease the burden of HPV infection in Uganda. There was a trend towards a higher prevalence of HPV infection in subjects co-infected with HIV (P =0.065). Although this association was not statistically significant, an association between the prevalence of these two viral infections has been found in other studies in Uganda. A cross-sectional study of HPV infection in rural Uganda found that 44.3% of HIV-positive women compared to 10.2% of HIV-negative women were infected with HPV by a hybrid capture assay of self-collected vaginal swabs (RR =5.36, 95% CI, 3.81–7.54) [Serwadda et al., 1999]. In addition, other studies from Africa have suggested that HIV-positive women are co-infected more often with HPV [Marais et al., 2000; Hawes et al., 2003]. The reasons for this association have not been delineated but likely are related to the immunosuppressive properties of HIV as well as social and behavioral risk factors. There is also the possibility that HPV infection in turn increases the transmission of HIV [Rein et al., 2000].
While studies from both the developed world [Serraino et al., 1999; Frisch et al., 2000] and the developing world [Hawes et al., 2003] have shown an association between cervical cancer and HIV infection, this study suggests that the same relationship may exist in Uganda as well. Figure 2, as well as Table II, show that HIV-positive participants have a higher prevalence of cervical cytologic abnormalities than HIV-negative women. In fact, the contrast of high-grade squamous intraepithelial lesions between HIV-positive and HIV-negative women is striking (39% vs. 6%, P <0.0001).
In this study, high risk HPV genotypes were associated with abnormal Pap smear findings in HIV-positive women. This relationship can be seen best in Figure 2, which demonstrates that HIV-positive women who were infected with high-risk HPV genotypes account for the majority of low-grade squamous intraepithelial lesions and high-grade squamous intraepithelial lesions. To corroborate these results, HIV-positive participants were found to be infected more often with high risk HPV genotypes than HIV-negative participants. One reasonable explanation for these observations is that people with HIV cannot control HPV expression due to their immunosuppression[Williams et al., 1994; Ho et al., 1995; Heard et al., 2000]. While HPV infections in many HIV-negative women are self-limited, HPV infection in HIV-positive women are persistent causing increased exposure to HPV oncoproteins and the eventual carcinogenic cell transformation leading to cancer [Heard et al., 2000].
Because there is currently no structured program for cervical cancer screening in Uganda, a method of cervical cancer screening that may be practical is the self-collection of vaginal swabs for HPV evaluation. Based on a recent meta-analysis, it is believed that the sensitivity of vaginal specimens for HPV-DNA is more than 70% [Oglivie et al., 2005]. Other studies have suggested that this method may work well for HIV-positive women in Africa. For example, a study in the Gambia in 377 rural women showed that self-collected vaginal swabs had a sensitivity of 63.9% compared to cervical cytobrush [Lack et al., 2005]. In this study, the cervical and vaginal HPV swabs had a relatively good correlation with a kappa statistic of 0.83. These results support the concept that self-collected vaginal swabs may be appropriate in populations that do not have access to formal screening programs.
There are several limitations to this study, most important of which is the small sample size. Participation in the study was relatively low, because the study protocol required a pelvic examination in a region of the world where invasive procedures of this nature are not done routinely. Consequently, the small total number of abnormal smears makes it difficult to generalize about the associated HPV genotypes in these women. Similarly, it is important to recognize that no cervical biopsies were done in this study. Although Pap smears are effective screening tests for cervical cytologic abnormalities, their reliability does not compare to biopsy [Wright et al., 2002]. Before a more definitive statement can be made regarding the HPV genotypes associated with cervical cancer in Uganda, a study will need to be conducted that evaluates the HPV genotypes of cervical biopsies isolated from Uganda women.
Other limitations include the fact that the study was conducted in a sexually transmitted infections clinic where participants are likely at higher than normal risk of HPV infection. Although the prevalence of HPV infection was 46%, and consistent with the HPV prevalences found in studies done at other sexually transmitted infections clinics in East Africa [De Vuyst et al., 2003] and around the world [Brown et al., 2002], the overall prevalence of HPV infection in Uganda is likely to be lower. For example, the study of the hybrid capture assay in self-collected vaginal swabs for detection of HPV in rural Uganda found an HPV prevalence of 16.7% [Serwadda et al., 1999]. In addition, this study was cross-sectional by design, so no insight could be gained in regards to persistence of HPV infection in these women. A reasonable follow-up, therefore, would be a longitudinal study to evaluate outcomes in women with and without HIV infection presenting to the sexually transmitted infections clinic with HPV infections.
Overall, the findings suggest that HIV-positive women in Uganda are at higher risk of cervical cytologic abnormalities than HIV-negative women. HPV 16, 52, and 58 were the most common genotypes encountered overall and within the group of participants who had high-grade squamous intraepithelial lesions on Pap smear at this sexually transmitted infections clinic. Although further studies with a larger number of participants are needed to more completely characterize the HPV genotype distribution in cervical cancer in this country, our study raises important issues that should be investigated in the future to develop robust screening programs for cervical cancer and to optimize HPV vaccination planning in resource-constrained countries.
Acknowledgments
We are indebted to Bobbie Van Der Pol for her assistance with the processing of the HPV assays. We would like also to thank Pamela Siekkinen for her help with the processing and evaluation of the Pap smears. Sponsorship: this research was supported by the Center for AIDS Research and the Fogarty AIDS International Training and Research Program.
References
- Baay MFD, Kjetland EF, Ndhlovu PD, Deschoolmeester V, Mduluza T, Gomo E, Friis H, Midzi N, Gwanzura L, Mason PR, Vermorken JB, Gundersen SG. Human papillomavirus in a rural community in Zimbabwe: the impact of HIV co-infection on HPV genotype distribution. J Med Virol. 2004;73:481–485. doi: 10.1002/jmv.20115. [DOI] [PubMed] [Google Scholar]
- Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in condyloma accuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–3322. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Legge D, Qadadri B. Distribution of human papillomavirus types in cervicovaginal washings from women evaluated in a sexually transmitted diseases clinic. Sex Transm Dis. 2002;29:763–768. doi: 10.1097/00007435-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, Juliar BE, Breen TE, Fortenberry JD. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–192. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronje HS. Screening for cervical cancer in developing countries. Int J Gynaecol Obstet. 2004;84:101–108. doi: 10.1016/j.ijgo.2003.09.009. [DOI] [PubMed] [Google Scholar]
- De Vuyst H, Steyaert S, Van Renterghem L, Claeys P, Muchiri L, Sitati S, Vansteelandt S, Quint W, Kleter B, Van Marck E, Temmerman M. Distribution of human papillomavirus in a family planning population in Nairobi, Kenya. Sex Transm Dis. 2003;30:137–142. doi: 10.1097/00007435-200302000-00009. [DOI] [PubMed] [Google Scholar]
- Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomized controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- Hawes SE, Critchlow CW, Faye Niang MA, Diouf MB, Diop A, Toure P, Kasse AA, Dembele B, Sow PS, Coll-Seck AM, Kuypers JM, Kiviat NB. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J Infect Dis. 2003;188:555–563. doi: 10.1086/376996. [DOI] [PubMed] [Google Scholar]
- Heard I, Tassie JM, Schmitz V, Mandelbrot L, Kazatchkine MD, Orth G. Increased risk of cervical disease among human immunodeficiency virus-infected women with severe immunosuppression and high human papillomavirus load. Obstet Gynecol. 2000;96:403–409. doi: 10.1016/s0029-7844(00)00948-0. [DOI] [PubMed] [Google Scholar]
- Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, Basu J, Tachezy R, Lewis R, Romney S. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–1375. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- Lack N, West B, Jeffries D, Ekpo G, Morison L, Soutter WP, Walraven G, Boryseiwicz L. Comparison of non-invasive sampling methods for detection of HPV in rural african women. Sex Transm Infect. 2005;81:239–241. doi: 10.1136/sti.2004.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Ma YY, Moh JS, Ou YC, Shen SY, ChangChien CC. High prevalence of genital human papillomavirus type 52 and 58 infection in women attending gynecologic practitioners in South Taiwan. Gynecol Oncol. 2006;101:40–45. doi: 10.1016/j.ygyno.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Marais DJ, Vardas E, Ramjee G, Allan B, Kay P, Rose RC, Williamson AL. The impact of human immunodeficiency virus type 1 status on human papillomavirus (HPV) prevalence and HPV antibodies in serum and cervical secretions. J Infect Dis. 2000;182:1239–1242. doi: 10.1086/315815. [DOI] [PubMed] [Google Scholar]
- Mbulaiteye SM, Katabira ET, Wabinga H, Parkin DM, Virgo P, Ochai R, Workneh H, Coutinho A, Engels EA. Spectrum of cancers among HIV-infected persons in Africa: The Uganda AIDS-Cancer Registry Match Study. Int J Cancer. 2006;118:985–990. doi: 10.1002/ijc.21443. [DOI] [PubMed] [Google Scholar]
- Oglivie GS, Patrick DM, Schulzer M, Sellars JW, Petric M, Chambers K, White R, Fitzgerald JM. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: A meta-analysis. Sex Transm Infect. 2005;81:207–212. doi: 10.1136/sti.2004.011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein MF. The interaction between HIV and the classic sexually transmitted diseases. Curr Infect Dis Rep. 2000;2:87–95. doi: 10.1007/s11908-000-0093-x. [DOI] [PubMed] [Google Scholar]
- Serraino D, Carrieri P, Pradier C, Bidoli E, Dorrucci M, Ghetti E, Schiesari A, Zucconi R, Pezzotti P, Dellamonica P, Franceschi S, Rezza G. Risk of invasive cervical cancer among women with, or at risk for, HIV infection. Int J Cancer. 1999;82:334–337. doi: 10.1002/(sici)1097-0215(19990730)82:3<334::aid-ijc5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Serwadda D, Wawer MJ, Shah KV, Sewankambo NK, Daniel R, Li C, Lorinez A, Meehan MP, Wabwire-Mangen F, Gray RH. Use of a hybrid capture assay of self-collected vaginal swabs in rural Uganda for detection of human papillomavirus. J Infect Dis. 1999;180:1316–1319. doi: 10.1086/315026. [DOI] [PubMed] [Google Scholar]
- ter Meulen J, Eberhardt HC, Luande J, Mgaya HN, Change-Claude J, Mtiro H, Mhina M, Kashaija P, Ockert S, Yu X. Human papillomavirus (HPV) infection, HIV infection and cervical cancer in Tanzania, East Africa. Int J Cancer. 1992;51:515–521. doi: 10.1002/ijc.2910510403. [DOI] [PubMed] [Google Scholar]
- Wabinga HR, Parkin DM, Wabwire-Mangen F, Mugerwa J. Cancer in Kampala, Uganda: Changes in incidence in the era of AIDS. Int J Cancer. 1993;54:26–36. doi: 10.1002/ijc.2910540106. [DOI] [PubMed] [Google Scholar]
- Williams AB, Darragh TM, Vranizan K, Ochia C, Moss AR, Palefsky JM. Anal and cervical human papillomavirus infection and risk of anal and cervical epithelial abnormalities in human immunodeficiency virus-infected women. Obstet Gynecol. 1994;83:205–211. [PubMed] [Google Scholar]
- World Health Organization. HIV Testing and Counselling: The Gateway to Treatment, Care and Support. [Accesseds October 28, 2005];2003 Available at http://www.who.int/3by5/publications/briefs/hivtestingcounselling/en/index.html.
- Wright TC, Denny L, Kuhn L, Pollack A, Lorincz A. HPV testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283:81–86. doi: 10.1001/jama.283.1.81. [DOI] [PubMed] [Google Scholar]
- Wright TC, Cox T, Massad S. JAMA; The 2001 ASCCP-Sponsored Consensus Conference. 2001 Consensus Guidelines for the Management of Women with Cervical Cytological Abnormalities; 2002. pp. 2120–2129. [DOI] [PubMed] [Google Scholar]


