Abstract
The sequencing of the Mycobacterium tuberculosis genome revealed the existence of several genes encoding novel proteins with unknown functions, one of which is the proline-threonine repetitive protein (PTRP; Rv0538). Genomic studies of various mycobacterial species and M. tuberculosis clinical isolates demonstrate that ptrp is specific to the M. tuberculosis complex and ubiquitous in clinical isolates. Enzyme-linked immunosorbent assay, Western blot analysis, and electron microscopic evaluation of M. tuberculosis subcellular fractions and intact bacteria confirm that PTRP is a cell wall protein. Antibodies to PTRP are present in serum specimens from human immunodeficiency virus (HIV)–negative, tuberculosis (TB)–positive and HIV-positive, TB-positive patients but not purified protein derivative (PPD)–negative or PPD-positive healthy control subjects, demonstrating its diagnostic potential. Epitope mapping of PTRP delineated 4 peptides that can identify >80% of sputum smear–positive and >50% of smear-negative, HIV-negative, TB-positive patients and >80% of HIV-positive, TB-positive patients. These results demonstrate that immunodominant epitopes of carefully selected M. tuberculosis–specific proteins can be used to devise a simple peptide-based serodiagnostic test for TB.
Tuberculosis (TB) afflicts ∼9 × 106 new persons and is responsible for ∼2 × 106 deaths annually. The diagnosis of TB in countries where TB is endemic relies primarily on sputum smears for acid-fast bacilli, a test that is tedious, requires multiple specimens and patient visits, and identifies ∼30%–60% of the persons who have the disease [1]. The shortcomings of microscopy are exacerbated in human immunodeficiency virus (HIV)–positive, TB-positive patients, among whom there is an increased incidence of smear-negative TB [2]. Rapid point-of-care diagnostic tests to replace acid-fast bacilli microscopy and identify a significant proportion of smear-negative patients are urgently required.
No current commercial serodiagnostic test provides an accurate TB diagnosis [3, 4], but the adaptability of such tests to rapid formats that can be deployed in low-income settings continues to encourage efforts in this direction. A majority of the recent antigen discovery–related proteomic studies of Mycobacterium tuberculosis have focused on the culture filtrate or cytosolic proteins of M. tuberculosis [5–9], and new candidate antigens have been identified [10, 11]. Although we also demonstrated that the cell wall of M. tuberculosis contains highly immunogenic proteins [12], these studies have been hampered by difficulties in isolation and purification.
Our previous studies identified a proline-threonine repetitive protein (PTRP; Rv0538) of M. tuberculosis as a target of humoral responses in aerosol-infected rabbits [13]. PTRP is classified in the cell wall and cell processes functional category [14]. No proteomic studies of M. tuberculosis whole-cell lysate (WCL) [7, 15, 16], culture filtrate [5–9], cytosols [6, 17], membrane [17–20] or cell wall [6, 17] have identified PTRP, although ptrp transcripts were reported in broth-grown M. tuberculosis [21]. In this study, Basic Local Alignment Search Tool (BLAST) analysis of the mycobacterial sequences suggests the lack of a homologue in Mycobacterium avium and Mycobacterium leprae.
The immunogenicity of PTRP in rabbits, its probable cell wall localization, and its absence in 2 major pathogenic mycobacterial species prompted studies aimed at its characterization and diagnostic potential. Results demonstrate that ptrp is indeed specific to the M. tuberculosis complex, being absent in all 10 nontuberculous mycobacterial species examined. Moreover, PTRP is a cell wall protein exposed on the surface of intact M. tuberculosis and is highly immunogenic in persons with TB. Delineation of the immunodominant epitopes of PTRP demonstrates that these epitopes can form the foundation of a peptide-based diagnostic test for TB.
Materials and Methods
Bacterial strains
Stock cultures of the following mycobacterial species were obtained from the American Type Culture Collection: M. tuberculosis H37Rv, M. bovis, M. bovis bacille Calmette-Guerin, M. africanum, M. microti, M. avium, M. kansassi, M. scrofulaceum, M. intracellular, M. fortuitum, M. smegmatis mc2, M. vaccae, M. phlei, M. chelonae, and M. xenopii. The cultures were grown in Difco Middlebrook 7H9 broth (Becton Dickinson) supplemented with 0.2% glycerol, 0.05% Tween 80, and 1× albumin dextrose saline (0.5% bovine serum albumin, fraction V [Sigma]; 0.2% dextrose; and 0.85% sodium chloride) at 37°C with shaking.
Southern hybridization
Genomic DNA of the mycobacterial species listed above was isolated from mid-log-phase bacterial cultures (Genomic-tip System; Qiagen). Genomic DNA of M. tuberculosis H37Rv, M. tuberculosis H37Ra, M. tuberculosis Erdman, and 7 M. tuberculosis clinical isolates was obtained by means of the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) TB Research Materials contract (Colorado State University), and 10 additional M. tuberculosis clinical isolates were obtained from Dr. Barry Kreiswirth (Public Health Research Institute). For each isolate, 4 μg of DNA was digested with XhoI and separated on a 0.8% agarose gel to prepare Southern blots. The digoxigenin (DIG)–labeled ptrp probe (1645 bp) was prepared by amplification of the gene, using appropriate primers (forward: 5′-AGCCAGCCGAAGGAGAGCCCATATGGA-3′; reverse: 5′-AGTGAAGCCGCGACCGAAGCTTGAACC-3′) from M. tuberculosis H37Rv genomic DNA and cloning into pET23b+ vector (PTRP-pET23b+; Novagen [EMD Biosciences]). The plasmid PTRP-pET23b+ DNA was digested with NdeI and HindIII to release ptrp, which was labeled with DIG (DIG probe synthesis kit; Roche Diagnostic). The hybridization and chemiluminescence-based detection was performed according to the manufacturer's protocols (Roche). The complete ptrp gene (1697 bp) was also amplified from the genomic DNAs, using appropriate primers (forward: 5′- TGCCGGGACATTGCTGGTTG-3′; reverse: 5′-TGATCAGAACCCGCCGAATAAG-3′), and the Southern blots were prepared with these polymerase chain reaction products and probed with the DIG-labeled ptrp gene probe described above.
Expression and purification of recombinant PTRP
The ptrp was amplified with appropriate primers (forward: 5′-GGATCCATGGACGTCGCTTTGGGGGTT-3′; reverse: 5′-CTCGAGTCAGAACCCGCCGAATCCGTC-3′) containing BamHI and XhoI sites (underlined sequences). The 1659-bp gel-purified polymerase chain reaction product was cloned into pCR4Blunt-TOPO vector (Invitrogen), the resulting plasmid was digested with BamHI and XhoI, and the released fragment was cloned into the glutathione S–transferase (GST) fusion vector pGEX-6P-1 (pGEX-6P-1–PTRP; GE Healthcare). Escherichia coli BL21 (DE3) pLysS (Invitrogen) harboring pGEX-6P-1-PTRP was grown in 2YT broth (Difco) at 37°C for 12 h, followed by induction of GST-PTRP expression with isopropyl thiogalactoside (concentration, 0.5 mmol/L) overnight at 25°C. The harvested cells were resuspended in phosphate-buffered saline (PBS) containing DNAse (0.6 μg/mL), lysed by sonication (model 4710; Cole-Parmer), and centrifuged, and the supernatant was incubated with 1 mL of PBS-equilibrated Glutathione Sepharose 4 Fast Flow (GE Healthcare) at 4°C for 2 h. The protein-bearing resin was washed once each with PBS and PreScission Protease cleavage buffer (Tris-chloride [concentration, 50 mmol/L], sodium chloride [10 mmol/L], ethylenediaminetetraacetic acid [1 mmol/L], and dithiothreitol [1 mmol/L]; pH 7.0), incubated with PreScission protease (100 U; GE Healthcare) overnight at 4°C for cleavage of the GST tag, and loaded on a column from which the recombinant PTRP (rPTRP) was eluted with cold cleavage buffer. The rPTRP was further purified by anion exchange chromatography, using Q Sepharose resin (Amersham Biosciences). Pooled fractions containing rPTRP were dialyzed against 10 mmol/L ammonium bicarbonate, and the rPTRP was sequenced by quadrupole time-of-flight mass spectrometry (New York University Protein Analysis Facility).
Localization of PTRP
Rabbit anti-PTRP antibodies were elicited by immunization with rPTRP in incomplete Freund's adjuvant (Sigma) [22], and immunoglobulin (Ig) G from serum specimens obtained before and after immunization was purified (protein A–Sepharose 4B columns; Amersham Biosciences). Western blots of M. tuberculosis H37Rv subcellular protein fractions (NIH/NIAID TB Research Materials), total cell wall (TCW) proteins, sodium dodecyl sulfate (SDS)–extracted cell wall (SDS-CW) proteins, WCLs, and culture filtrate were probed with rabbit anti-PTRP IgG (1:1000) or anti–malate synthase (MS) IgG (1:5000) and respective preimmune IgG, followed by alkaline phosphatase (AP)–conjugated anti-rabbit IgG (1:2000) and 5-bromo-4-chloro-3-indolyl phosphate–nitro blue tetrazolium chloride substrate (KPL). The band density was calculated by ImageJ software (available at: http://rsb.info.nih.gov/ij/index.html).
M. tuberculosis subcellular fractions (concentration, 2.5–10 μg/mL in PBS) were coated in triplicate in enzyme-linked immunosorbent assay (ELISA) plates, washed with PBS, blocked with 1% bovine serum albumin (BSA; Sigma), washed with PBS containing 0.05% Tween 20 (PBST), and exposed to anti-PTRP IgG or preimmune IgG (1:1000) for 1.5 h at 37°C. After the plates were washed with PBST, the bound antibodies were detected with anti-rabbit AP (1:2000) and the amplification system (Invitrogen). Fifty microliters of serially diluted single-cell suspension of γ-irradiated M. tuberculosis H37Rv or CDC1551 was coated (triplicate wells) in ELISA plates, and the surface-exposed PTRP was detected as already described.
Log-phase M. tuberculosis bacilli were fixed with 3% paraformaldehyde in sodium cacodylate buffer (concentration, 0.1 mol/L) containing 0.1% glutaraldehyde and 4% sucrose, washed, and dehydrated before being embedded in Lowicryl K4M (Polysciences) and polymerized under UV light (360 nm) at −35°C. Ultrathin sections (70 nm) were incubated with anti-PTRP IgG (1:10) or preimmune IgG (1:10) at 4°C overnight, exposed to gold-conjugated protein A (Cell Microscopy Center), stained with uranyl acetate and lead citrate, and examined under a Philips CM12 electron microscope at the New York University Image Core Facility
Serum specimens
The immunogenicity studies were performed with serum specimens obtained with informed consent from 42 purified protein derivative (PPD)–negative and PPD-positive healthy subjects; 80 HIV-negative, TB-positive patients; 45 HIV-positive, TB-positive patients; and 46 asymptomatic HIV-positive, TB-negative patients (table 1).
Table 1. Clinical Characteristics of Study Subjects.
| Infection status, geographic origin | Source | Subjects, no. | Sputum smear status | Culture positive | Treatment status at time of serum sampling |
|---|---|---|---|---|---|
| HIV negative, TB positive (n = 80) | |||||
| India | LRSITRD | 60 | Positive | ND | Untreated |
| India | PGIMER | 20 | Negative | 20 | Untreated |
| HIV positive, TB positive | |||||
| India | PGIMER | 45 | Positive | ND | Untreated |
| HIV positive, TB negativea | |||||
| United States | VAMC | 46 | ND | ND | ART |
| PPD positiveb (n = 27) | |||||
| United States | VAMC | 6 | ND | ND | NA |
| India | VAMC | 10 | ND | ND | NA |
| China | VAMC | 7 | ND | ND | NA |
| Cameroon | VAMC | 2 | ND | ND | NA |
| Japan | VAMC | 1 | ND | ND | NA |
| Colombia | VAMC | 1 | ND | ND | NA |
| PPD negative (n = 15) | |||||
| United States | VAMC | 6 | ND | ND | NA |
| India | VAMC | 6 | ND | ND | NA |
| China | VAMC | 3 | ND | ND | NA |
NOTE. ART, antiretroviral therapy; HIV, human immunodeficiency virus; LRSITRD, Lala Ram Sarup Institute of Tuberculosis and Respiratory Diseases, New Delhi, India; NA, not applicable; ND, not done; PGIMER, Postgraduate Institute of Medical Education and Research, Chandigarh, India; PPD, purified protein derivative TB, tuberculosis; VAMC, Veterans Affairs Medical Center, New York, NY.
The PPD status of these individuals is not known.
Bacille Calmette-Guérin vaccination is routinely provided during childhood in the countries from which PPD-positive subjects originated, except for the United States. These subjects may have been PPD positive because of vaccination or latent Mycobacterium tuberculosis infection.
Detection of anti-PTRP antibodies in serum specimens from patients with TB
Western blots of purified rPTRP (40 ng per lane) were blocked with 3% BSA, washed with PBST, and probed with serum specimens from HIV-negative, TB-positive patients or PPD-positive healthy control subjects (1:50). Similar blots were probed with serum specimens from HIV-positive, TB-positive patients; HIV-positive, TB-negative patients; HIV-negative, PPD-negative subjects; and HIV-negative, PPD-positive subjects (1:100). A mixture of protein A–AP (1:2000; Sigma) and anti–human IgA–AP (1:1000; Sigma) was used to detect bound anti-PTRP antibodies.
Mapping immunodominant regions of PTRP
Fifty-four overlapping peptides (20 aa in length with a 10-aa overlap; PT1–PT54) covering the entire PTRP sequence, each linked with a biotin residue at the N-terminal, were synthesized in PEPscreen format (Sigma Genosys). The reactivity of each peptide was tested with serum specimens from 13 PPD-negative healthy subjects, 23 PPD-positive healthy subjects, and 60 smear-positive, HIV-negative, TB-positive patients. For this, 50 μL of each peptide (concentration, 2.5 μg/mL) diluted in blocking buffer (7.5% fetal bovine serum [HyClone] and 2.5% BSA in PBS) was added to wells of streptavidin-coated ELISA plates (Roche) for 1 h at 37°C. The capture of peptides onto streptavidin-coated wells via the biotin residue ensures equal binding of the peptides. Subsequently, 50 μL of diluted serum (1:20 in 0.1× blocking buffer) from healthy subjects and patients was added for 1 h at 37°C. After 4 washes with PBST, a mixture of protein A–AP (1:2000; Sigma) and anti–human IgA–AP (1: 1000; Sigma) was added to each well for 1 h at 37°C. After 6 washes with PBST, color was developed with p-nitrophenyl phosphate (pNPP) (pNPP [concentration, 1 mg/mL] in diethanolamine buffer [1 mol/L] containing magnesium chloride [0.5 mmol/L]; pH 9.8) and read at 405 nm. The cutoff used to determine positive responses in patients with TB was the mean optical density (OD) plus 3 standard deviations (SDs) for the serum specimens from PPD-positive and PPD-negative individuals. Peptides that were recognized by antibodies in serum specimens from >40% of the 60 HIV-negative, TB-positive patients were retested for reactivity on 2 additional occasions. Selected immunodominant peptides were tested for reactivity with serum specimens from smear-negative, HIV-negative, TB-positive patients; HIV-positive, TB-positive patients; and HIV-positive, TB-negative patients. Serum specimens showing positive reactivity at least 2 of 3 times were considered positive.
Bioinformatics and statistical analysis
BLAST searches were performed on the National Center for Biotechnology Information Web site (available at: http://www.ncbi.nlm.nih.gov/). The reactivity of serum specimens from PPD-negative and PPD-positive healthy control subjects was compared, as was the reactivity of serum specimens from PPD-positive and PPD-negative healthy control subjects and patients with TB. P values were calculated with the nonparametric Mann-Whitney test using GraphPad Prism software (version 5; GraphPad Software), and differences were considered statistically significant at P < .05.
Results
ptrp is specific for M. tuberculosis(complex)
Protein BLAST analysis of PTRP showed the presence of homologous (∼100% identity) protein in M. tuberculosis CDC1551, M. tuberculosis F11, M. tuberculosis C, M. tuberculosis H37Ra, M. bovis AF2122/97, and M. bovis bacille Calmette-Guérin strain Pasteur 1173P2 but not in the sequenced pathogenic nontuberculous mycobacterial species (M. avium paratuberculosis K-10, M. avium 104, and M. ulcerans Agy99); 1 hypothetical protein with ∼55%–60% identity was identified in each of these nontuberculous mycobacterial species. Nucleotide BLAST analysis of ptrp identified an ∼100% identical gene in M. tuberculosis complex species and clinical isolates but not in the listed nontuberculous mycobacterial species. In M. leprae TN, the corresponding gene is predicted to be a pseudogene. To determine the distribution of ptrp in additional nontuberculous mycobacterial species, Southern hybridization with genomic DNA was performed. The DIG-labeled M. tuberculosis ptrp probe hybridized with a single ∼9.5-kb band in the fractionated genomic DNA from M. tuberculosis complex species but not from any of the nontuberculous mycobacterial species tested (figure 1A). Restriction of ptrp to M. tuberculosis complex and 17 M. tuberculosis clinical isolates tested was further confirmed when the ∼1.7-kb ptrp was amplified only from them and not from any nontuberculous mycobacterial species tested (figure 1B and 1C).
Figure 1.
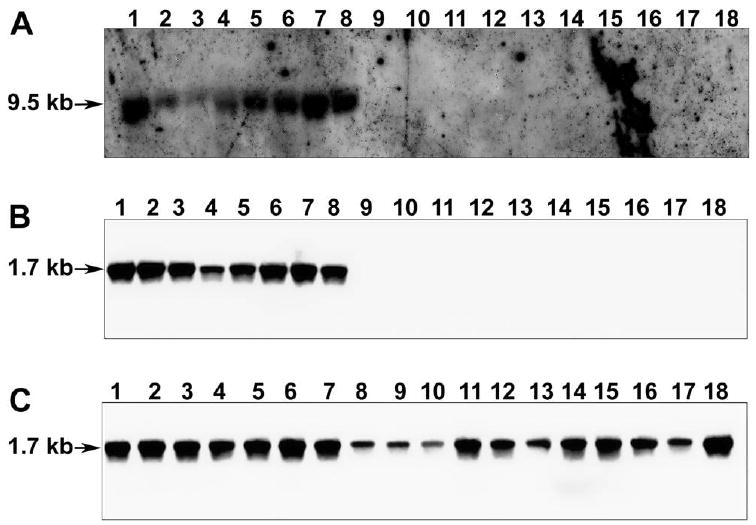
Distribution of ptrp in various mycobacterial species. A, Southern blot prepared from XhoI-digested genomic DNA of mycobacterial species was probed with digoxigenin (DIG)–labeled ptrp (1645 bp). Lane 1, Mycobacterium tuberculosis H37Rv; 2, M. tuberculosis Erdman; 3, M. tuberculosis CDC1551; 4, M. tuberculosis H37Ra; 5, M. bovis; 6, M. bovis bacille Calmette-Guérin; 7, M. microti; 8, M. africanum; 9, M. avium; 10, M. xenopi; 11, M. kansassi; 12, M. scrofulaceum; 13, M. vaccae; 14, M. intracellulare; 15, M. phlei; 16, M. fortuitum; 17, M. smegmatis mc2 155; and 18, M. chelonae. B, and C, Southern blots prepared from ptrp amplified from genomic DNA of mycobacterial species. M. tuberculosis clinical isolates were probed with DIG-labeled ptrp (1697 bp) amplified from M. tuberculosis H37Rv genomic DNA. B, Lanes have the same contents as those in A. C, Lane 1, M. tuberculosis H37Rv; 2, M. tuberculosis CSU11; 3, M. tuberculosis CSU17; 4, M. tuberculosis CSU19; 5, M. tuberculosis CSU22; 6, M. tuberculosis CSU25; 7, M. tuberculosis CSU26; 8, M. tuberculosis CSU 27; 9, M. tuberculosis 10738 (W200); 10, M. tuberculosis 10591 (W187); 11, M. tuberculosis 10813 (W148); 12, M. tuberculosis AI10 (TN10692); 13, M. tuberculosis AI46 (TN11533); 14, M. tuberculosis 11159 (BE); 15, M. tuberculosis 11164 (H17); 16, M. tuberculosis 11165 (MB2); 17, M. tuberculosis 11168 (001); and 18, M. tuberculosis 11177 (001). Arrows indicate position of hybridizing fragments.
Expression and purification of rPTRP
The purified rPTRP appeared as an ∼52-kD band on SDS-polyacrylamide gel (figure 2A), which is similar to the theoretical molecular weight of 55 kD. Protein sequencing of this band confirmed its identity (table 2).
Figure 2.
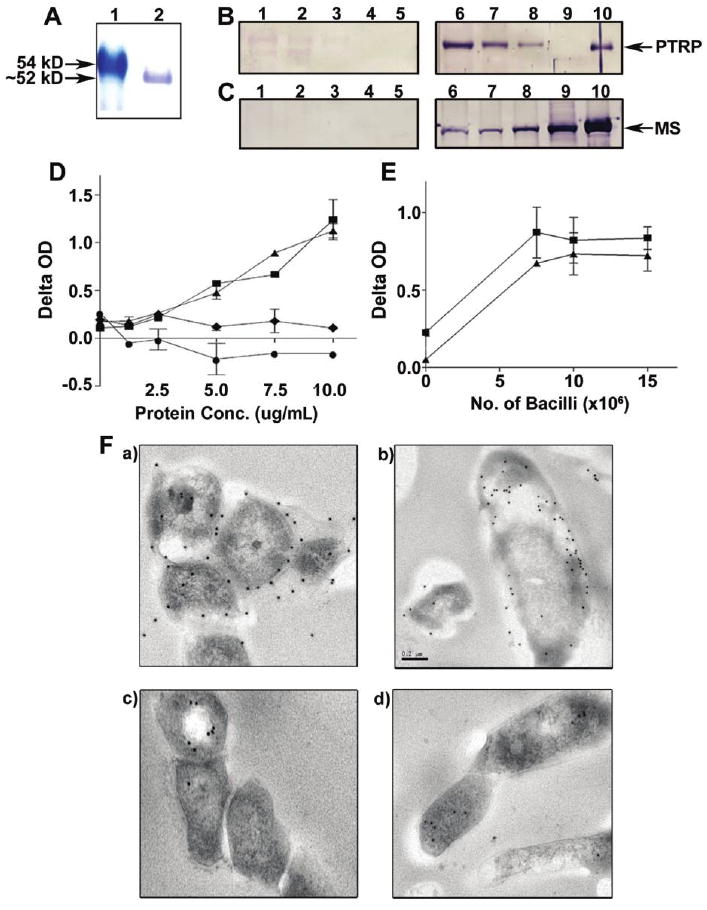
Expression of recombinant (r) proline-threonine repetitive protein (PTRP) and localization of PTRP in Mycobacterium tuberculosis. A, Purified rPTRP was fractionated on sodium dodecyl sulfate (SDS)–polyacrylamide gel and stained with Coomassie blue. Lane 1, molecular weight markers; 2, rPTRP. B, C, Western blots of M. tuberculosis subcellular protein fractions (10 μg per lane) were probed with preimmune immunoglobulin (Ig) G (lanes 1–5), anti-PTRP IgG (B; lanes 6–10), or anti–malate synthase (MS) IgG (C; lanes 6–10). Lanes 1 and 6 contain SDS-extracted cell wall proteins; lanes 2 and 7, total cell wall; lanes 3 and 8, whole-cell lysate; lanes 4 and 9, culture filtrate; and lanes 5 and 10, rPTRP (B), or recombinant MS (C). D, Detection of PTRP by enzyme-linked immunosorbent assay (ELISA) in M. tuberculosis total cell wall (squares), SDS-extracted cell wall (triangles), whole-cell lysate (diamonds), and culture filtrate protein (circles) preparations. Plotted values represent means ± standard deviations (SDs) for the delta optical density (OD; calculated as the OD with anti-PTRP IgG minus the OD with preimmune IgG) at various concentrations (Conc.) of the subcellular preparations. E, Presence of PTRP on the surface of M. tuberculosis H37Rv (squares) and M. tuberculosis CDC1551 (triangles) bacilli, as demonstrated by bacterial ELISA. Values represent means ± SDs for the difference in OD (OD for anti-PTRP IgG minus OD for preimmune IgG) obtained with different numbers of bacilli. F Immunoelectron microscopy of ultrathin sections of M. tuberculosis probed with anti-PTRP IgG (a, b) and preimmune IgG (c, d).
Table 2. Sequencing of Recombinant Proline-Threonine Repetitive Protein (PTRP) by Quadrupole Time-of-Flight Mass Spectrometry.
| PTRP peptide fragments mapped | Peptides identified, no. | PTRP amino acid position |
|---|---|---|
| LGDQALAPGDVFLVGR | 27 | 155–170 |
| SAEHTTVLADQLR | 7 | 171–183 |
| VQTPDDPTFALAR | 21 | 190–202 |
| GAAMAAGAATMAHPALVADATTSLPR | 9 | 203–228 |
| AEAGQSGSEGEQLAYSQASDYELLPVDEYEEHDEYGAAADR | 1 | 229–269 |
PTRP is a cell wall protein of M. tuberculosis
The anti-PTRP IgG identified an ∼52-kD protein in the TCW and the SDS-CW preparations of M. tuberculosis; weak reactivity with this protein was detectable in the WCL preparation, and no similar protein was detected in the culture filtrate (figure 2B). To confirm the integrity of the preparations and estimate the relative abundance of PTRP in them, MS detection and density analysis of each protein band on Western blots was performed [22] (figure 2C). With the quantity of MS in each fraction considered 100%, the WCLs contained less PTRP (∼20%), and the TCW and the SDS-CW contained more (∼140% and ∼320%, respectively). In contrast to MS, no PTRP was detected in the culture filtrate. Anti-PTRP antibodies demonstrated a dose-dependent reactivity with different concentrations of TCW and SDS-CW preparations (figure 2D). Anti-PTRP antibodies also bound to intact M. tuberculosis H37Rv and M. tuberculosis CDC1551 bacterial cells in a dose-dependent manner (figure 2E). Finally, immunoelectron microscopy confirmed the presence of PTRP on the cell wall (figure 2F). Together, these results conclusively demonstrate that PTRP is a cell wall protein of M. tuberculosis.
Immunogenicity of PTRP in patients with TB
Serum antibodies from 4 of 6 HIV-negative, TB-positive and 5 of 6 HIV-positive, TB-positive patients showed strong reactivity with the ∼52-kDa rPTRP (figure 3). In contrast, serum specimens from none of the 6 HIV-negative, PPD-positive subjects, 6 HIV-negative, PPD-negative subjects, or 6 HIV-positive, TB-negative patients showed strong reactivity (figure 3), demonstrating that PTRP is immunogenic in both HIV-negative, TB-positive and HIV-positive, TB-positive patients.
Figure 3.
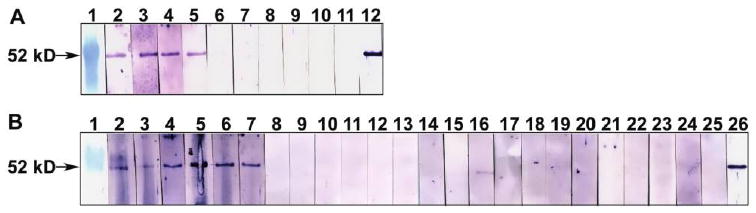
Reactivity of recombinant (r) proline-threonine repetitive protein (PTRP) with serum specimens from patients with tuberculosis (TB) and healthy control individuals. A, Western blots of rPTRP were probed with serum specimens from 6 human immunodeficiency virus (HIV)–negative, TB-positive patients (lanes 2–7) or 4 purified protein derivative (PPD)–positive healthy control subjects (lanes 8–11) or anti-PTRP immunoglobulin (Ig) G (lane 12). Lane 1 contains molecular weight markers. B, Blots of rPTRP were probed with serum specimens from 6 HIV-positive, TB-positive patients (lanes 2–7), 6 HIV-positive, TB-negative patients (lanes 8–13), 6 PPD-negative healthy subjects (lanes 14–19), or 6 PPD-positive healthy subjects (lanes 20–25). Anti-PTRP IgG was used to probe lane 26. Lane 1 contains molecular weight markers.
Identification of immunogenic epitopes of PTRP
The specificity of PTRP to M. tuberculosis, its ubiquitous presence in clinical isolates, and its immunogenicity in patients with TB encouraged evaluation of its diagnostic potential. When serum specimens from 13 PPD-negative and 23 PPD-positive healthy control subjects were compared for reactivity to 53 overlapping peptides (1 peptide was insoluble), there was no significant difference in reactivity for 49 peptides (P = .561−.986). For the 4 remaining peptides, the ODs for the individual serum specimens were higher in the PPD-positive group for 3 and in the PPD-negative group for 1 (data not shown). By use of the mean OD plus 3 SDs for all 36 control subjects as the cutoff, no control serum tested positive with 22 of 53 peptides, and the remaining peptides were recognized by antibodies in only 1 or 2 serum specimens (figure 4A). In contrast, 37 of 53 peptides were recognized by antibodies in serum specimens from 20%–68% of the smear-positive, HIV-negative, TB-positive patients (figure 4A); 16 of 53 were recognized by antibodies from >40% of the patients with TB. Antibodies to ≥1 of these 16 peptides were present in 57 (95%) of 60 HIV-negative, TB-positive patients, demonstrating universal recognition of the protein in patients with TB. Of these 16 peptides, 12 were reproducibly recognized by antibodies in serum specimens from ≥40% of the patients with TB (figure 4A; data not shown). Together, these 12 peptides were recognized by serum specimens from 87% of HIV-negative, TB-positive patients. The following 4 peptides were recognized by antibodies in specimens from >50% of patients with TB: PT9 (LQDSGVHDVAVISEAQAATA; recognized by specimens from 58%), PT13 (LSVVGDPDAPPTMVAVAPVA; 53%), PT40 (PIPVPIIIPPFPGWQPGMPT; 52%), and PT41 (FPGWQPGMPTIPTAPPTTPV; 55%) (figure 4B); 82% of the HIV-negative, TB-positive patients had antibodies to ≥1 of these 4 peptides (data not shown).
Figure 4.
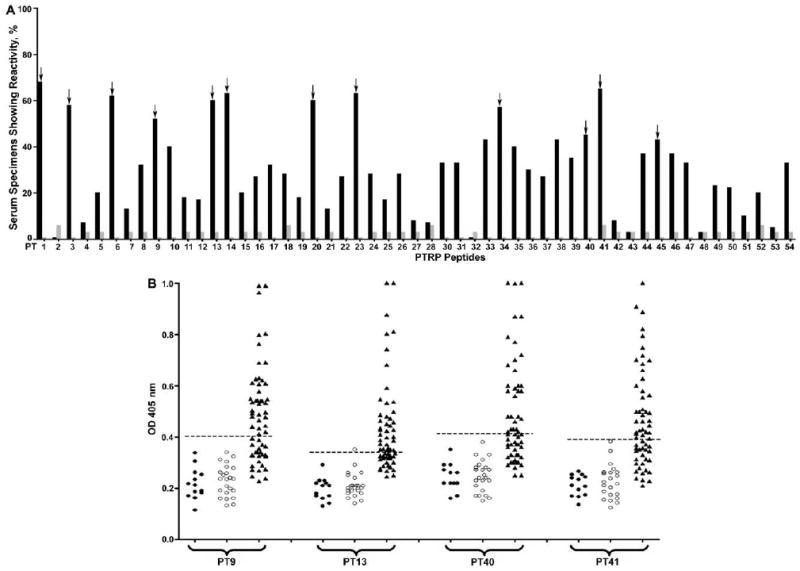
Reactivity of overlapping peptides of proline-threonine repetitive protein (PTRP) with serum specimens from patients with tuberculosis (TB) and healthy control subjects. A, PTRP peptides were probed with serum specimens from 60 smear-positive, human immunodeficiency virus (HIV)– negative, TB-positive patients and 36 purified protein derivative (PPD)–positive or PPD-negative healthy control subjects. The cutoff for identifying positive responses was the mean optical density (OD) at 405 nm plus 3 standard deviations for serum specimens from PPD-positive or PPD-negative subjects. Plotted values represent percentages of serum specimens from HIV-negative, TB-positive patients (black bars) and PPD-positive or PPD-negative healthy control subjects (gray bars) showing positive reactivity with each PTRP peptide. Arrows indicate PTRP peptides that demonstrated reactivity with serum specimens from ≥40% of HIV-negative, TB-positive patients when tested 3 times. B, Reactivity of immunodominant PTRP peptides PT9, PT13, PT40, and PT41 with serum specimens from 60 smear-positive, HIV-negative, TB-positive patients and 13 PPD-negative and 23 PPD-positive healthy control subjects. The cutoff was the same as that described in A (dashed line). Values represent ODs for reactivity of each peptide with individual serum specimens from PPD-negative (closed circles) and PPD-positive (open circles) healthy subjects and HIV-negative, TB-positive patients (closed triangles) in 1 representative experiment. ODs of >1.0 are depicted as 1.0. There was no significant difference between ODs obtained with the serum specimens from PPD-negative and PPD-positive healthy subjects for any of these peptides (P = .24–.99). Compared with ODs for PPD-negative or PPD-positive healthy subjects, those for HIV-negative, TB-positive patients were significantly higher (P < .001 for all 4 peptides).
Reactivity of immunodominant PTRP peptides with serum specimens from other classes of patients with TB
Antibodies to the 4 immunodominant peptides were demonstrated in serum specimens from 25%–45% of 20 smear-negative, HIV-negative, TB-positive patients tested; together, they were recognized by serum specimens from 55% (11 of 20) of these patients (figure 5A). The 4 peptides were also recognized by serum specimens from 60%–76% of the HIV-positive, TB-positive patients; together, they identified 87% (39 of 45) of the HIV-positive, TB-positive patients (figure 5B). However, in contrast to specimens from the PPD-positive and PPD-negative healthy subjects, serum specimens from 4%–13% of the HIV-positive, TB-negative subjects reacted with the 4 peptides (figure 5B). PT9 and PT41 were less cross-reactive, being recognized by serum specimens from 7% (3 of 46) of the HIV-positive, TB-negative subjects; >70% of the HIV-positive, TB-positive patients had antibodies to PT9 and/or PT41 (figure 5B).
Figure 5.
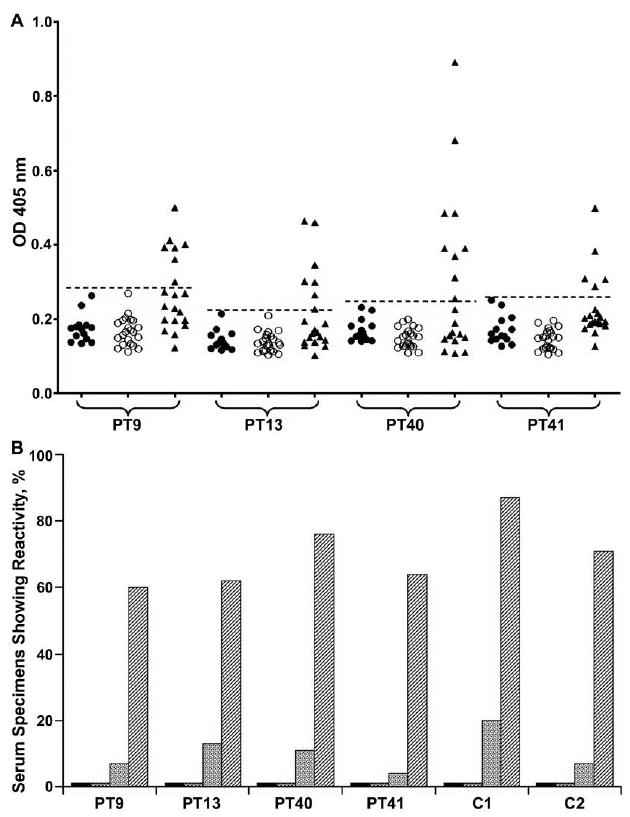
Reactivity of immunodominant proline-threonine repetitive protein (PTRP) peptides with serum specimens from different groups of patients with tuberculosis (TB) and control subjects. A, Reactivity with serum specimens from smear-negative, human immunodeficiency virus (HIV)–negative, TB-positive patients. The cutoff for identifying positive responses was the mean optical density (OD) at 405 nm plus 3 standard deviations for serum specimens from PPD-positive or PPD-negative subjects (dashed line). Values represent ODs for reactivity of each peptide with individual serum specimens from PPD-negative (closed circles) or PPD-positive (open circles) healthy subjects or smear-negative, HIV-negative, TB-positive patients (closed triangles) in 1 representative experiment. B, Reactivity with the 4 immunodominant peptides of serum specimens from PPD-positive (black bars) and PPD-negative (checked bars) healthy control subjects, asymptomatic HIV-positive, TB-negative patients (dotted bars), and HIV-positive, TB-positive patients (striped bars). The cutoff was the same as that described in A. C1 indicates the additive reactivity of all 4 peptides, and C2 indicates the additive reactivity of 2 peptides (PT9 and PT41) showing minimal cross-reactivity with serum specimens from HIV-positive, TB-negative patients.
Discussion
The sequencing of the M. tuberculosis genome revealed the existence of several genes encoding novel proteins with unknown function [14], one of which is PTRP. Our studies demonstrate that PTRP is a highly immunogenic bona fide M. tuberculosis-specific cell wall protein. The cell wall location and the absence of a homologue among common (M. leprae and M. avium) and occasional human pathogens (M. kansassi, M. ulcerans, and M. chelonae) implicates PTRP as playing an important role in host-pathogen interaction during clinical TB. Furthermore, the ubiquitous presence of ptrp in clinical isolates and the association of anti-PTRP antibodies with active TB merits investigation into its potential as a vaccine and diagnostic candidate for TB.
PTRP was predicted to be a cell wall protein [14], and, consistent with this prediction, it was identified in the TCW and SDS-CW preparations of M. tuberculosis and on the surface of intact bacteria. Neither earlier proteomic studies nor our current investigation identified PTRP in the M. tuberculosis culture filtrate [5–9], and the relative paucity of PTRP in the WCLs explains the lack of detection in proteomic studies of M. tuberculosis cytosol [6, 17]. Considering that the SDS-CW and TCW preparations contain ample PTRP, it is surprising that it was not identified in earlier studies in the M. tuberculosis cell wall [6, 17] or, despite the predicted presence of 4 transmembrane regions, in the membrane fraction [17–20]. In our experience, although PTRP-GST could be expressed and the GST tag cleaved to obtain small quantities of PTRP, all attempts to scale up production were unsuccessful owing to rapid precipitation of the recombinant protein (data not shown). Possibly, like other putative cell wall proteins of M. tuberculosis, PTRP requires association with other proteins or chaperones for stabilization [23].
In recent years, peptide-based diagnostic tests have been devised for other bacterial, viral, and parasitic diseases [24–26]. Besides easy production at relatively low cost, peptides offer the advantage of increased specificity by eliminating nonspecific regions of the protein [24, 26]. Considering the difficulties encountered in obtaining purified PTRP, and in view of the instability, misfolding, degradability, batch-to-batch variation, and expense involved in producing high-quality recombinant proteins, we sought to identify the immunodominant epitopes of PTRP. As expected, the protein has both immunogenic and immunosilent regions. Antibodies to ≥1 PTRP peptide were present in 95% of smear-positive, HIV-negative, TB-positive patients; the high immunogenicity of PTRP may be attributed to its presentation to the immune system in the context of the mycobacterial cell wall, a strong adjuvant. Although the presence of antibodies in a vast majority of patients with TB suggests that the major histocompatibility complex haplotype does not affect recognition of the PTRP peptides, all patients with TB in this study were from the same geographical setting, and the recognition of these peptides in patients from other parts of the world is the subject of ongoing study.
Interestingly, 4 immunodominant peptides were sufficient to identify >80% of smear-positive and >50% of smear-negative, HIV-negative, TB-positive patients; however, the titers in the latter patients were low. Although the parent protein or fragments thereof that present conformational epitopes may provide higher sensitivity than the linear peptides delineated, obtaining sufficient quantities of pure recombinant stable proteins is likely to be challenging, considering that >80 million TB tests are performed every year. The absence of anti-PTRP antibodies in PPD-positive subjects demonstrates that individuals with latent M. tuberculosis infection and/or bacille Calmette-Guérin vaccination lack these antibodies. Because ∼2 billion individuals are probably latently infected with M. tuberculosis and bacille Calmette-Guérin vaccination is routine in TB-endemic countries, the ability of these peptides to discriminate latent from active TB is important.
Rapid diagnosis of TB is especially critical for HIV-positive, TB-positive patients, in whom coinfection accelerates the progression of both diseases [2, 27]. It is encouraging that the 4 immunodominant peptides were also recognized by serum specimens from >80% of HIV-positive, TB-positive patients. Moreover, although PT13 and PT40 were somewhat cross-reactive with serum specimens from asymptomatic HIV-positive, TB-negative individuals, PT9 and PT41 were sufficient to identify >70% of HIV-positive, TB-positive patients. The reasons underlying this cross-reactivity are unclear, because all the HIV-positive, TB-negative patients were at very low risk for TB, being from United States and receiving highly active antiretroviral therapy. The presence of anti-HIV antibodies has been reported in HIV-negative, TB-positive patients and HIV-negative, leprosy-positive patients, suggesting possible similarities between some mycobacterial and HIV epitopes [28, 29]. It is also possible that our 20-mer PTRP peptides contain amino acids that are not relevant to the core epitope [30]; further dissection of the 4 immunodominant peptides and identification of additional immunodominant epitopes from other highly antigenic proteins are being performed [13, 31–34]. Moreover, to devise a commercial test, multiple peptides, derived from a single or from multiple immunogenic proteins, will have to be used in combination or as a single entity (multiepitope peptide).
Although the function of PTRP is unknown, it shows structural similarity with heparin-binding hemagglutinin (HBHA; Rv0475) of M. tuberculosis and the laminin-binding protein (ML-LBP21; ML1683) of M. leprae, in that all 3 have multiple tandem repeats of specific amino acid motifs at the C-terminal [13, 35, 36]. Both HBHA and laminin-binding protein are adhesins of mycobacteria that promote adherence and entry into nonphagocytic cells, and HBHA also contributes to dissemination of M. tuberculosis from the lungs to spleen in vivo [35–40]. Whether PTRP is also an M. tuberculosis adhesin that contributes to the host-pathogen interaction is under investigation.
In summary, PTRP is an M. tuberculosis complex–specific cell wall protein of M. tuberculosis that is highly immunogenic in HIV-negative, TB-positive and HIV-positive, TB-positive patients. Four immunodominant peptides of PTRP have been delineated that can identify >80% of the smear-positive and >50% of the smear-negative patients with TB. Although the core epitopes in these 4 peptides need to be defined, and additional epitopes will be needed to devise an accurate diagnostic test for TB, these results demonstrate that dominant epitopes of carefully selected M. tuberculosis–specific proteins can be used to devise a peptide-based diagnostic test for TB. Further studies to evaluate these peptides in larger patient cohorts from different geographical regions are also ongoing.
Acknowledgments
We thank Dr. Sunil Sethi, Postgraduate Institute of Medical Education and Research Chandigarh, India, for providing serum specimens from smear-negative patients with tuberculosis.
Financial support: Department of Veterans Affairs (merit review grant); National Institutes of Health (NIH; grant AI-056257); Fogarty International Center, NIH (grant TW001409); National Institute of Allergy and Infectious Diseases (contract NO1-AI-40091, TB Vaccine Testing and Research Materials).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: Immunodiagnosis of Tuberculosis: New Questions, New Tools conference, 21-23 September 2008, Virginia Beach, VA.
References
- 1.Foulds J, O'Brien R. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int J Tuberc Lung Dis. 1998;2:778–83. [PubMed] [Google Scholar]
- 2.Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196(Suppl 1):S15–27. doi: 10.1086/518656. [DOI] [PubMed] [Google Scholar]
- 3.Steingart KR, Henry M, Laal S, et al. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Thorax. 2007;62:911–8. doi: 10.1136/thx.2006.075754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steingart KR, Henry M, Laal S, et al. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007;4:e202. doi: 10.1371/journal.pmed.0040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnenberg MG, Belisle JT. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing and electrospray mass spectrometry. Infect Immun. 1997;65:4515–24. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenkrands I, Weldingh K, Jacobsen S, et al. Mapping and identification of Mycobacterium tuberculosis proteins by two-dimensional gel electrophoresis, microsequencing and immunodetection. Electrophoresis. 2000;21:935–48. doi: 10.1002/(SICI)1522-2683(20000301)21:5<935::AID-ELPS935>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Jungblut PR, Schaible UE, Mollenkopf HJ, et al. Comparative proteome analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG strains: towards functional genomics of microbial pathogens. Mol Microbiol. 1999;33:1103–17. doi: 10.1046/j.1365-2958.1999.01549.x. [DOI] [PubMed] [Google Scholar]
- 8.Mattow J, Schaible UE, Schmidt F, et al. Comparative proteome analysis of culture supernatant proteins from virulent Mycobacterium tuberculosis H37Rv and attenuated M. bovis BCG Copenhagen. Electrophoresis. 2003;24:3405–20. doi: 10.1002/elps.200305601. [DOI] [PubMed] [Google Scholar]
- 9.Malen H, Berven FS, Fladmark KE, Wiker HG. Comprehensive analysis of exported proteins from Mycobacterium tuberculosis H37Rv. Proteomics. 2007;7:1702–18. doi: 10.1002/pmic.200600853. [DOI] [PubMed] [Google Scholar]
- 10.Abebe F, Holm-Hansen C, Wiker HG, Bjune G. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand J Immunol. 2007;66:176–91. doi: 10.1111/j.1365-3083.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- 11.Steingart KR, Dendukuri N, Henry M, et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol. 2009;16:260–76. doi: 10.1128/CVI.00355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laal S, Samanich KM, Sonnenberg GM, Zolla-Pazner S, Phadtare JM, Belisle JT. Human humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high molecular mass antigens. Clin Diag Lab Immunol. 1997;4:49–56. doi: 10.1128/cdli.4.1.49-56.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh KK, Zhang X, Patibandla AS, Chien P, Jr, Laal S. Antigens of Mycobacterium tuberculosis expressed during preclinical tuberculosis: serological immunodominance of proteins with repetitive amino acid sequences. Infect Immun. 2001;69:4185–91. doi: 10.1128/IAI.69.6.4185-4191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 15.Betts JC, Dodson P, Quan S, et al. Comparison of the proteome of Mycobacterium tuberculosis strain H37Rv with clinical isolate CDC 1551. Microbiology. 2000;146:3205–16. doi: 10.1099/00221287-146-12-3205. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt F, Donahoe S, Hagens K, et al. Complementary analysis of the Mycobacterium tuberculosis proteome by two-dimensional electrophoresis and isotope-coded affinity tag technology. Mol Cell Proteomics. 2004;3:24–42. doi: 10.1074/mcp.M300074-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Mawuenyega KG, Forst CV, Dobos KM, et al. Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Mol Biol Cell. 2005;16:396–404. doi: 10.1091/mbc.E04-04-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Y, Chalmers MJ, Gao FP, Cross TA, Marshall AG. Identification of Mycobacterium tuberculosis H37Rv integral membrane proteins by one-dimensional gel electrophoresis and liquid chromatography electrospray ionization tandem mass spectrometry. J Proteome Res. 2005;4:855–61. doi: 10.1021/pr0500049. [DOI] [PubMed] [Google Scholar]
- 19.Gu S, Chen J, Dobos KM, Bradbury EM, Belisle JT, Chen X. Comprehensive proteomic profiling of the membrane constituents of a Mycobacterium tuberculosis strain. Mol Cell Proteomics. 2003;2:1284–96. doi: 10.1074/mcp.M300060-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S, Kosalai K, Arora S, et al. Immunogenic membrane-associated proteins of Mycobacterium tuberculosis revealed by proteomics. Microbiology. 2005;151:2411–9. doi: 10.1099/mic.0.27799-0. [DOI] [PubMed] [Google Scholar]
- 21.Geiman DE, Kaushal D, Ko C, et al. Attenuation of late-stage disease in mice infected by the Mycobacterium tuberculosis mutant lacking the SigF alternate sigma factor and identification of SigF-dependent genes by microarray analysis. Infect Immun. 2004;72:1733–45. doi: 10.1128/IAI.72.3.1733-1745.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinhikar A, Vargas D, Li H, et al. Mycobacterium tuberculosis malate synthase is a laminin binding adhesin. Mol Microbiol. 2006;60:999–1013. doi: 10.1111/j.1365-2958.2006.05151.x. [DOI] [PubMed] [Google Scholar]
- 23.Strong M, Sawaya MR, Wang S, Phillips M, Cascio D, Eisenberg D. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006;103:8060–5. doi: 10.1073/pnas.0602606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomara MJ, Haro I. Synthetic peptides for the immunodiagnosis of human diseases. Curr Med Chem. 2007;14:531–46. doi: 10.2174/092986707780059698. [DOI] [PubMed] [Google Scholar]
- 25.Alcaro MC, Peroni E, Rovero P, Papini AM. Synthetic peptides in the diagnosis of HIV infection. Curr Protein Pept Sci. 2003;4:285–90. doi: 10.2174/1389203033487117. [DOI] [PubMed] [Google Scholar]
- 26.Noya O, Patarroyo ME, Guzman F, Alarcon de Noya B. Immunodiagnosis of parasitic diseases with synthetic peptides. Curr Protein Pept Sci. 2003;4:299–308. doi: 10.2174/1389203033487153. [DOI] [PubMed] [Google Scholar]
- 27.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188:1146–55. doi: 10.1086/378676. [DOI] [PubMed] [Google Scholar]
- 28.Hussain T, Sinha S, Katoch K, et al. Serum samples from patients with mycobacterial infections cross-react with HIV structural proteins Gp41, p55 and p18. Lepr Rev. 2007;78:137–47. [PubMed] [Google Scholar]
- 29.Swaminathan S, Hanna LE, Sundaramurthi JC, et al. Prevalence and pattern of cross-reacting antibodies to HIV in patients with tuberculosis. AIDS Res Hum Retroviruses. 2008;24:941–6. doi: 10.1089/aid.2007.0211. [DOI] [PubMed] [Google Scholar]
- 30.Xu JY, Gorny MK, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using 10 human monoclonal antibodies. J Virol. 1991;65:4832–8. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samanich K, Belisle JT, Laal S. Homogeneity of antibody responses in tuberculosis patients. Infect Immun. 2001;69:4600–9. doi: 10.1128/IAI.69.7.4600-4609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh KK, Dong Y, Sai Patibandla A, McMurray D, Arora VK, Laal S. Immunogenicity of Mycobacterium tuberculosis PPE55 (Rv3347c) protein during incipient and clinical tuberculosis. Infect Immun. 2005;73:5004–14. doi: 10.1128/IAI.73.8.5004-5014.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh KK, Dong Y, Belisle JT, Harder J, Arora VK, Laal S. Antigens of Mycobacterium tuberculosis recognized by antibodies during incipient, subclinical tuberculosis. Clin Diagn Lab Immunol. 2005;12:354–8. doi: 10.1128/CDLI.12.2.354-358.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sartain MJ, Slayden RA, Singh KK, Laal S, Belisle JT. Disease state differentiation and identification of tuberculosis biomarkers via native antigen array profiling. Mol Cell Proteomics. 2006;5:2102–13. doi: 10.1074/mcp.M600089-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Shimoji Y, Ng V, Matsumura K, Fischetti VA, Rambukkana A. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc Natl Acad Sci U S A. 1999;96:9857–62. doi: 10.1073/pnas.96.17.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menozzi FD, Bischoff R, Fort E, Brennan MJ, Locht C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc Natl Acad Sci U S A. 1998;95:12625–30. doi: 10.1073/pnas.95.21.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menozzi FD, Rouse JH, Alavi M, et al. Identification of a heparinbinding hemagglutinin present in mycobacteria. J Exp Med. 1996;184:993–1001. doi: 10.1084/jem.184.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pethe K, Aumercier M, Fort E, Gatot C, Locht C, Menozzi FD. Characterization of the heparin-binding site of the mycobacterial heparinbinding hemagglutinin adhesin. J Biol Chem. 2000;275:14273–80. doi: 10.1074/jbc.275.19.14273. [DOI] [PubMed] [Google Scholar]
- 39.Pethe K, Alonso S, Biet F, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–4. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 40.de Melo Marques MA, Mahapatra S, Nandan D, et al. Bacterial and host-derived cationic proteins bind alpha2-laminins and enhance Mycobacterium leprae attachment to human Schwann cells. Microbes Infect. 2000;2:1407–17. doi: 10.1016/s1286-4579(00)01294-6. [DOI] [PubMed] [Google Scholar]


