Abstract
Mucins are the most abundant high molecular weight glycoproteins in mucus. Their nature and glycosylation content dictates the biochemical and biophysical properties of viscoelastic secretions, pointing out an important role in diverse biological functions, such as differentiation, cell adhesions, immune responses, and cell signaling. Mucins are expressed in tubular organs by specialized epithelial cells in the body. Their aberrant expression is well documented in a variety of inflammatory or malignant diseases. From a prognosis point of view, their expression and alterations in glycosylation are associated with the development and progression of malignant diseases. Therefore, mucins can be used as valuable markers to distinguish between normal and disease conditions. Indeed, this alteration in glycosylation patterns generates several epitopes in the oligosaccharide side chains that can be used as diagnostic and/or prognostic markers. Furthermore, these characteristic tumor-associated epitopes are extensively used as appropriate immunotargets of malignant epithelial cells. Therefore, in an effort to detect and treat cancer at the earliest stage possible, mucins are analyzed as potential markers of disease for diagnosis, progression, and for therapeutic purposes. In this review, we focused on the current status of the distribution of mucins in normal and pathologic conditions and their clinical use both in cancer diagnosis and therapeutics treatments.
Keywords: Mucins, cancer therapy, cancer diagnosis
1. Introduction
Mucus is the slimy and viscoelastic secretion that covers the epithelial surface of tubular organs such as tracheobronchial, gastrointestinal, reproductive tracts, and other specialized organs. In the body, mucus is secreted by specialized epithelial cells known as goblet cells and are abundant in the epithelium of the gastrointestinal, respiratory and reproductive tracts, and the secretory epithelial surfaces of the liver, pancreas, gall bladder, kidney, salivary, and lacrimal glands [1]. Mucus secretions adhere to the epithelial surface and serve as a protective diffusion barrier against harmful substances and act as a lubricant between the lumen and the cell surface [2,3]. The composition of mucus varies with its location and pathophysiological conditions [4,5], but normally mucus is composed of water, inorganic salts, immunoglobulins, secreted proteins, and mucins. Mucins are the most abundant macromolecules in mucus and are responsible for its biochemical and biophysical properties due to their nature and extent of glycosylation [6,7]. The mucins are a closely related family of O-glycoproteins that play an important role in the renewal and differentiation of the epithelium, cell adhesions, immune response, and cell signaling [3,8–11].
In general, mucins are large (well over 106 Daltons) glycoproteins [12,13] composed of ∼75% carbohydrate and 25% amino acids linked via O-glycosidic bonds between N-acetylgalactosamine and serine/threonine/proline (Ser-Thr-Pro) residues. The hallmark of the mucin family is the large and polymorphic central domain, which is composed of a variable number of tandem repeats (VNTR) rich in Ser-Thr-Pro residues (Table 1) that can be modified with a large number of O-linked oligosaccharides and a few N-glycan chains [3,6,7,14–16]. Till now, about twenty mucin (MUC) genes have been identified and these are designated as MUC1-2, MUC3A, MUC3B, MUC4, MUC5B, MUC5AC, MUC6-9, MUC11-13, MUC15-17, and MUC19-21 [3,17–31]. In this review, we discuss the current status of mucins for cancer diagnosis and therapy. Special emphasis is given on the most commonly occurring lethal cancers.
Table 1. Human mucins and their chromosome localization, domain structures.
| MUCIN GENE | Chromosome location | Domains | AA in tandem repeat region |
|---|---|---|---|
| Soluble/gel-forming | |||
| MUC2 | 11p15.5 | STP, VWC, VWD, CysD, and CK | 50–100 TR of 23 AA |
| MUC5AC | 11p15.5 | STP, VWD, VWC, CysD, and CK | (TR1) 124/(TR2) 17/(TR3)34 and (TR4)66 TR of 8 AA |
| MUC5B | 11p15.5 | STP, VWD, CysD, and CK | 11 TR of the irregular repeat of 29 AA |
| MUC6 | 11p15.5 | STP, VWD, and CK | 169 AA |
| MUC19 | 12q12 | STP, VWD, VWC, and CK | 3–6 TR of 19 AA |
| Soluble | |||
| MUC7 | 4q13.3 | STP | 6 TR of 23 AA |
| MUC8 | 12q24.3 | STP incomplete sequence | 13/41 AA |
| MUC9 | STP | 15 AA | |
| MUC20 | 3q29 | STP | 3 TR of 19 AA |
| Membrane bound | |||
| MUC1 | 1q21-24 | STP AND SEA, TM | 25–125 TR of 20 AA |
| MUC3A | 7q22 | STP, SEA, EGF, and TM | 20 TR of 17 AA |
| MUC3B | 7q22 | STP, SEA, EGF, and TM | 17 AA |
| MUC4 | 3q29 | STP, NTDO, AMOP, VWD, EGF, and TM | 145–395 TR of 16 AA |
| MUC11 | 7q22 | STP, SEA, EGF, and TM | 28 AA |
| MUC12 | 7q22 | STP, SEA, EGF, and TM | 28 AA |
| MUC13 | 3q13.3 | STP, SEA, EGF, and TM | 8 TR of 27 AA |
| MUC15 | 11p14.3 | STP and TM | No TR region |
| MUC16 | 19p13.2 | STP, SEA, and TM | 60 TR of 156 AA |
| MUC17 | 7q22 | STP, SEA, EGF, and TM | 61 TR of 59 AA |
| MUC21 | 6 | STP and TM | 28 TR of 15 AA |
STP, domain rich in serine, threonine and proline, which are heavily glycosylated; VWD von Willebrand factor type D domain; VWC, von Willebrand factor type C domain; CK, Cys-knot domain; SEA, Sea urchin sperm protein, Enterokinase, and Agrin module (MUC16 has six SEA domains—five of them in the tandem repeat region); NIDO, Nidogen-like domain; EGF, epidermal growth factor-like domain (MUC3A. 3B. 4, and 12 have two EGF-like domains and MUC13 has three); AMOP, Adhesion-associated domain in MUC4 and other proteins; TM, transmembrane domain; TR, Tandem repeat; AA, Amino acid.
2. Classification of mucins
Based on physiological fate and nature, mucins are categorized into three subgroups: “secreted/gel-forming”, “membrane-bound”, and “soluble” mucins [3] (Table 1, Fig. 1). The first group is composed of strictly secreted, gel-forming mucins including MUC2, MUC5AC, MUC5B, MUC6, and MUC19, which form oligomeric structures. The second group is composed of mucins either tethered at the cell surface or secreted in the mucus. The mucins of this group, MUC1, MUC3A, MUC3B, MUC4, MUC11, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, and MUC21, harbor a transmembrane domain, a short cytoplasmic tail (CT), and an extensive extracellular domain. The third subgroup, composed of MUC7, MUC8, and MUC9, are exclusively secreted non-gel forming mucins.
Fig. 1.
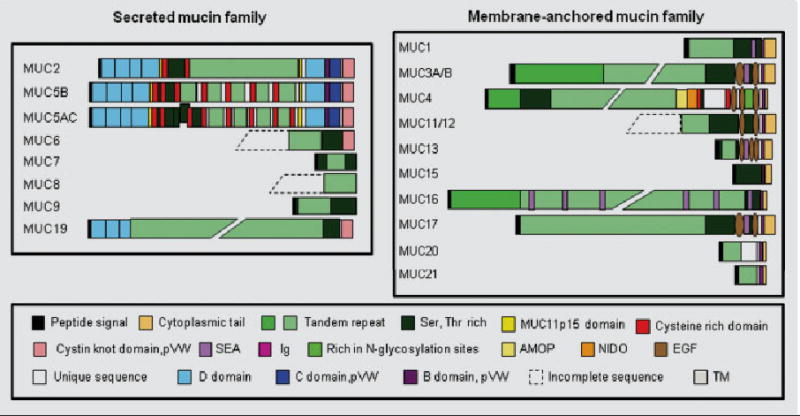
A schematic representation of the deduced amino acid for various MUC genes. SEA, Sea-urchin sperm Protein; Ig, Immunoglobulin; pVW, pro-Von Willebrand; AMOP, Adhesion Associated Domain; NIDO, Nidogen-like Domain; EGF, Epidermal Growth Factor; TM, Transmembrane.
2.1. Secretory/gel-forming mucins
Out of the gel-forming mucin family, MUC2, MUC5AC, MUC5B, and MUC6 genes are mapped to chromosome 11p15.5, in a cluster of 400 kb in size, rich in CpG islands [32]. These are localized between HRAS and IGF2 and arise from a common ancestor gene similar to the human von Willebrand factor gene (vWF) [33,34]. On the other hand, MUC19 is localized to chromosome 12q12. This family of mucins is formed by oligomerization of the tri-dimensional network of the mucus backbone, which provides the mucus its visco-elastic properties. The gel-forming mucins share several structural features including a large central repetitive region flanked by a non-repeating region. The central region is comprised of tandem repeats varying in length from 24 nt for MUC5AC [35] to 507 nt for MUC6 [27]. The tandem repeat domains encompassed several sub-domains rich in cysteine residues. The number of these sub-domains varies depending of the mucin, which allows mucin dimerization. The amino and carboxy-flanking regions share similarities with the B, C, D, and CK domains found in the pro-von Willebrand factor (pro-vWF) [19–21,23,35–37].
MUC2 was first identified and described by Gum et al in 1989 [38]. Its cDNA was isolated from a human small intestine cDNA library [23,38]. The central domain of MUC2 is composed of two highly repetitive sequences. The first, in the central position, is characterized by the perfect repetition of one motif of 23 amino acids and the second, located upstream, is composed of an irregular sequence repeated in tandem with a unit of 347 amino acids. These two sequences are rich in amino acid residues of Ser-Thr-Pro. MUC2 is mainly expressed in intestinal [39] and colorectal goblet cells [38,40,41]. The pathogenesis of colorectal neoplasia is associated with MUC2 expression [42]. Downregulation of MUC2 expression is detected in colorectal cancer [42], gastric cancer [43,44], and ovarian tumors [45]. It is also expressed by adenomas and mucinous carcinomas [42].
The MUC5AC is primarily expressed in tracheobronchial goblet cells, in gastric epithelial cells [46,47], in conjunctiva and lacrimal gland tissues, but not in cornea [48]. The abnormal expression of MUC5AC has been observed in colorectal adenomas [49,50] and pancreatic cancer [51,52]. Furthermore, the expression level of MUC5AC is associated with the degree of cellular dysplasia. It is also expressed at higher level in patients with asthma, bronchitis, cystic fibrosis [53,54], and Sjögren syndrome. Recently published work states that MUC5AC is more frequently expressed in advanced cholangiocarcinoma [55]. In contrast, a decrease in MUC5AC expression is associated with decreased survival in colorectal carcinoma patients [56,57].
MUC5B is the second largest human gel-forming mucin, composed of 5,000 amino acids [19,20]. It is detected in human saliva, salivary glands, tracheobronchial and esophageal epithelia, pancreatobiliary and endocervical epithelial cells [46,47,58]. Both MUC5B and MUC5AC mucin proteins have been identified in human airway secretions from healthy and diseased individuals [59–63]. MUC5AC and 5B expression is decreased in cystic fibrosis airway secretions [64].
MUC6 is expressed in gastric mucosa and mucopeptic cells located at the neck region of the body [65–69]. It is also expressed in gastric and duodenal mucous glands, pancreatobiliary and endocervical epithelial cells [69], and in the stomach of 8-week-old embryos [70]. The aberrant expression of MUC6 is reported in gastric carcinomas. In addition, MUC6 expression is detected at significant levels in well-differentiated cholangiocarcinomas [55], breast cancer [66], and in colonic sessile serrated adenoma [71].
The human MUC19 gene is the largest gel-forming mucin with a size of 180 kb and with a deduced peptidic sequence of more than 7,000 amino acids. It is localized at chromosome 12q12 [72]. In the human middle ear, it is the major gel-forming mucin [73]. It is also expressed in mucous cells of sublingual and submandibular glands, submucosal gland of the tracheal tissue [72] corneal and conjunctival epithelial cells, and lacrimal gland tissues [48]. However MUC19 is not detected in salivary glands [74] and its expression is decreased in conjunctiva of Sjögren syndrome patients.
2.2. Soluble mucins
Originally, the MUC7 cDNA clone was isolated from the human submandibular glands (Reddy et al., 1992), and later on, the full-length cDNA was isolated and characterized from the same cDNA library [17]. It is a gene of 10 kb long, localized to chromosome 4q13.3 and composed of three exons only. It is a low-molecular mass mucin (150–200 kDa) and is secreted by the submandibular and sublingual salivary glands. It encodes a 39 kDa protein of 377 amino acid residues and reveals five distinct domains [75]. It functions as an antimicrobial agent in the oral cavity by interacting with microorganisms. It also agglutinate the AIDS virus HIV-1 [76,77] and inhibits the HIV infection [78]. This is mainly due to the two cysteine residues located in the N-terminal region of MUC7 [79]. Moreover, it acts as an anti-candidal activity [80] via its histatin-like domain. The MUC7 is expressed in epithelial cells of the oral cavity, minor salivary gland, respiratory tract [81], normal human conjunctivae [82], pancreas, submucosal glands of the bronchus, and cholangiocarcinomas [83–85]. The MUC7 gene is also expressed in bladder cancer during the malignant transformation of bladder urothelium in preinvasive carcinoma in situ but no MUC7 gene expression is detected in superficial, non-invasive bladder tumors [86].
The MUC8 mucin gene has been mapped to chromosome 12 at position q24.3. The MUC8 gene mRNA and protein is expressed in nasal epithelial (NHNE) cells [87], in middle ear epithelium [88], and in middle ear effusions [89]. The expression of MUC8 is up-regulated in nasal polyp epithelium and it may play an important role in the pathogenesis of chronic sinusitis with polyps [90].
The expression of MUC9 was observed from late follicular development through early cleavage-stage in embryonic development, middle ear epithelium [88], and middle ear effusions [89].
2.3. Transmembrane mucins
Membrane bound mucins are tethered to cells with the help of a unique transmembrane domain. The mucin cytoplasmic tails facilitate their association with cytoskeletal elements and cytosolic adaptor proteins, and therefore, these have a significant role in signal transduction [91]. The family of membrane-associated mucins has several common features such as a specific proteolytic cleavage site, two or three epidermal growth factor (EGF) domains, juxtamembrane domain, tandem repeat, and a cytoplasmic tail. In MUC3A, MUC12, MUC13, and MUC17, the EGF domains are separated by the sea urchin sperm protein (SEA) domain, but not in MUC4.
In 1990, MUC1 was sequenced at the DNA level by four groups [25,92–94]. Subsequently, it was mapped to chromosome 1q21-24, spanning about 4–7 kb of genomic DNA, depending upon the size of the central domain [95–98]. MUC1 consists of seven exons, out of which, exon 1 encodes the leader peptide, exon 2 the central domain, and exon 6 and 7 encode the transmembrane sequence and cytoplasmic tail. The central domain is made up of a sequence repeated in tandem with a perfect unit repetition of 20 amino acid residues [99]. MUC1 was first identified in human milk [100] and is expressed by almost all glandular epithelial surfaces of respiratory, female reproductive, gastrointestinal tracts, middle ear, salivary gland, mammary gland [100–104], and normal pancreatic intralobular ducts [105,106]. Its expression is dramatically increased in breast, ovarian, lung, pancreatic, prostate [85,107,108], and colorectal cancers [109,110]. In secretory epithelial cells, MUC1 is normally expressed on the apical borders, but in tumor cells, its expression is spread throughout the cell surface [100,111]. One major role of the MUC1 protein is to act as an anti-adhesive protein to maintain the luminal integrity of the lining epithelium, thus aiding in the metastasis of tumor cells [112,113].
MUC3A and MUC3B are expressed in gastrointestinal epithelia [114]. The MUC3 gene has been mapped to chromosome 7 in the region q22 [22,115]. It is a large gene, and the sequence is partially known as the organization of its central domain is unclear. The alternative splicing results in four variants which may be membrane bound or secretory [28,116].
The first MUC4 mucin sequence was cloned from a tracheobronchial cDNA library [29] and, subsequently from colon mucosa [117] and pancreatic tumor cell lines [118]. It has been mapped to the chromosome 3q29 [119] close to the small membrane bound mucin MUC20 [120]. MUC4 is made up of 26 exons, in which, the largest one being exon 2 codes for a large central tandem repeat domain rich in Ser/Thr/Pro [18,121]. The first exon covers the 5′ untranslated region and codes for the leader peptide, whereas the remaining 24 exons code for the extracellular, transmembrane, and carboxyl tail of the MUC4. This glycoprotein is expressed in various normal tissues such as the respiratory tract, lung, salivary glands, stomach, colon, eye, vagina, ectocervix, uterus, and prostate [15,46,122–127]. It is not detected in the normal pancreas, gall bladder, liver, and biliary epithelial cells [47,51,70]. Additionally, an abnormal expression of MUC4 has been observed in various inflammatory diseases and cancers such as dysplasia and adenocarcinoma of the esophagus [128], Crohn's disease [129,130], gall bladder carcinomas [131], salivary gland mucoepidermoid carcinoma [132], lung adenocarcinoma [133–135], pancreatic ductal adenocarcinoma [51,136–138], epithelial ovarian carcinomas [139], prostate cancer [140], breast cancer [141], and other inflammatory diseases of airways such as cystic fibrosis (CF) and chronic obstructive pulmonary disease [5,142].
The MUC11 mucin gene was mapped to the human chromosome 7q22 region [143], similar to MUC12 and MUC3. Up to date, only a partial sequence of MUC11 has been isolated, corresponding to a part of the tandem repeat domain. This sequence presents 71% homology with the variable repeat region of MUC12. At the RNA level, the expression of MUC11 was highest in the colon, whereas, a weak expression was observed in the stomach, pancreas, prostate, and uterus [114,143]. The MUC11 is expressed more widely in gastrointestinal, respiratory, reproductive, urinary tracts and also detected in the liver and thymus when compared with MUC12 and MUC3. Its expression is commonly down-regulated in colorectal cancer [143].
The MUC12 mucin gene has been mapped to the human 7q22 region [143]. It spans at least 28 kb long and likely contains 13 exons. It has structural similarities with MUC3, with two EGF-like domains, one transmembrane sequence, and a cytoplasmic tail. MUC12 is most abundantly expressed in the normal colon and stomach, and to a lesser extent in the pancreas [114,144], prostate, and uterus.
The MUC13 gene was mapped to chromosome band 3q13.3 [145]. It encodes a cell surface membrane-anchored mucin expressed in the normal gastrointestinal, respiratory tracts, trachea, middle ear, and kidney [88,114,145,146]. It is aberrantly expressed in colorectal, esophageal, gastric, pancreatic, and lung cancers [144,146]. In colorectal cancers, the highest expression of MUC13 was observed in poorly differentiated tumors. The staining intensity was high in adenocarcinomas (81%) when compared with mucinous tumors (50%). Similarly, the high-intensity of cytoplasmic staining was observed in poorly differentiated and late-stage tumors [146].
The human MUC15 is localized to chromosome 11p14.3 and contains five exons and four introns. It is made of a signal peptide and the majority of the extracellular fraction is encoded by exon 3, which is followed by a 150-bp exon encoding the transmembrane domain (exon 4). The cytoplasmic domain is encoded by exons 4 and 5, which also contains a 274-bp-long 3′ untranslated region and the stop-codon. The MUC15 expression was abundant in placenta, salivary gland, thyroid gland, trachea, esophagus, kidney, testis, and also in leukemia K-562 cell line, but moderate expression was seen in the pancreas, adult and fetal lung, fetal kidney, lymph nodes, adult and fetal thymus [147,148]. Abnormal expression of MUC15 has been observed in (mRNA and protein) colorectal tumors when compared with the normal tissues [149] and its expression enhances the invasive potential of colorectal cells.
The MUC16 encodes a transmembrane-bound molecule also known as CA125. It was cloned from the ovarian cancer cell line OVCAR-3 and was mapped to chromosome 19p13.2 [122]. It encodes splice variants that give rise to polypeptides that differ in the number of SEA domains repeated 7, 12, or 60 times [150]. The first MUC16 cDNA clones were published by two independent groups [151,152]. The MUC16 translated into a large peptide made up of 22,152 amino acids with a molecular weight of 2.5 MDa. It has a large extracellular domain extending up to 500 nm from the cell surface into the glycocalyx, a transmembrane domain, and a small cytoplasmic tail. The extracellular domain is composed of a heavily O-glycosylated N-terminus, a tandem repeat region, and a region with several SEA domains near the membrane-spanning sequence. MUC16 is mainly expressed in the ocular surface, respiratory tract, and female reproductive tract epithelia [153], and middle ear [88]. Its overexpression is correlated with the progression of ovarian cancer [154].
The MUC17 gene was mapped to the mucin cluster at the chromosomal locus 7q22 along with MUC3A/B, MUC11, and MUC12 mucins [155,156]. The first partial length cDNA sequence of MUC17 was identified by Van Klinken and co-workers [157]. MUC17 was extended to its 5′-part using genomic sequences by 5′RACE-PCR [158]. It is made of 13 exons, out of which, the exon 3 is the largest one (12.2 kb) and codes for the Ser/Thr/Pro region. This domain is followed by two EGF modules flanking a SEA domain, a transmembrane (22-amino acid) domain, and an intracytoplasmic tail (78-amino acid). The MUC17 is expressed in the gastrointestinal tract showing the highest expression in the duodenum and transverse colon [156,159]. The MUC17 is also expressed in the stomach, fetal kidney, and conjuctival epithelium [156,160]. The MUC17 is overexpressed in pancreatic cancer cells when compared with the normal pancreas and pancreatitis tissues [138].
MUC20 has been localized to chromosome 3q29 and contains five exons coding for a 76–78 kDa protein. Abundant expression of MUC20 mRNA was detected in the kidney but moderately in placenta, lung, prostate, liver, colon, esophagus, rectum [120], and middle ear [88]. The expression of MUC20 is up-regulated in immunoglobulin A nephropathy (IgAN) [120,161].
The epiglycanin/MUC21 gene was mapped on chromosome 6 in close proximity to the MHC class I and within the susceptibility domain for diffuse panbronchiolitis (DPB) [162]. It spans 4.8 kbp in size and consists of three exons coding a 21 amino acid signal peptide, the tandem repeat domain, the transmembrane domain, and the cytoplasmic tail. It encodes a typical transmembrane mucin with a cytoplasmic tail, a transmembrane domain, a stem domain, and 28 tandem repeats composed of 15 amino acids. Human epiglycanin/MUC21 mRNA expression was examined in various normal tissues by PCR screening using human epiglycanin/MUC21 specific primers. It was demonstrated that expression was remarkably high in lung, thymus, colon, normal bronchi, bronchioles, and bronchial glands, large intestine, and testis [163].
3. Mucins as diagnostic markers for various cancers
Worldwide, mucins are extensively studied for their clinical use as carcinoma markers and for cancer immunotherapy [2,164]. Tumors associated epitopes are generated in the oligosaccharide side chains of mucins due to changes in their glycosylation patterns, including newly, expressed blood-group antigens. Therefore, in an effort to detect cancer at the earliest stage, mucins are analyzed as potential markers of disease progression and diagnosis. Significant findings are discussed below and are subdivided by the tissue where the primary tumor originated.
3.1. Breast cancer
MUC1 is overexpressed frequently in breast cancers and is a target for diagnostic assays and immunotherapy [165]. Fifteen years ago, a helper-T cell independent response directed to the PDTR region of unglycosylated MUC1 TR peptides was reported in the sera of breast, colon, and pancreatic cancer patients [166]. In these studies, IgM antibodies specific to MUC1 were detected using a synthetic 105-residue peptide that mimicked the native structure of unglycosylated MUC1. Humoral responses specific to MUC1 were detected in 8.3% of breast cancer, 16.7% of pancreatic cancer, and 10.0% of colon cancer patients without any correlation to tumor. Thus, as a diagnostic tool, this approach was of little value as fewer cases of the cancer patients studied had detectable circulating MUC1 levels.
In breast cancer patients, a shortened five-year disease-free interval, increasing tumor node metastases stages, positive lymph node status, and increasing histological grade are significantly associated with the expression of the mucin associated Tn antigen [167]. In agreement with these studies, a better overall five-year survival was associated with Sialyl-Tn negativity [168]. Further, the increased expression of Lewis x and sialyl-Lewis x correlated with lymph node metastasis and poorer survival [169,170]. A study that was conducted to compare the superiority of serum biomarker (CEA, CA15.3, and CA27.29) to diagnose patients with primary breast cancer showed that CA15.3 was superior to CA27.29 [171], while other studies reported no difference among these markers [172].
One recent study demonstrated the potential use of mucins as prognostic indicators in breast cancer patients [173]. The circulating tumor cells (CTC) in the blood of breast cancer patients undergoing palliative therapy were evaluated for the expression of MUC1 among other markers. CTC analyzed by immunomagnetic tumor cell selection and multiplex RT-PCR, were detected in 52% of the patients and 86% of this group was MUC1 positive. The presence of CTC correlated with shorter overall survival when compared with patients that did not have detectable CTC. Keeping in mind that CTC were selected by MUC1 specific expression, it can be inferred that MUC1 is a significant prognostic factor as well. The aberrant cytoplasmic and membranous localization of MUC1 in breast cancer tissues is associated with a poor outcome when compared with normal physiological apical localization, [141]. Recently, the MUC1 antibody named PankoMab, which binds specifically to carbohydrate-induced conformational tumor epitope on MUC1, revealed a strong correlation of MUC1 with hormone receptor expression in ductal carcinoma of the breast [174]. In the same study, PankoMab performance was compared with the VU-4-H5 antibody, which recognizes the tandem repeat region of MUC1, and an increased staining intensity was demonstrated in correlation with increased grading and lymph node involvement. Therefore, the combination of PankoMab and VU-4-H5 staining is suggested as a useful prognostic indicator of ductal breast cancer.
Another mucin, MUC3, was evaluated for breast cancer diagnosis. MUC3 membranous expression in breast cancer tissues is associated with poor prognostic outcome, higher tumor grade, and poorer Nottingham Prognostic Index (NPI) [141]. MUC3 expression was detected in 91% of human breast cancer tissues, and its expression associated with increased local recurrence and lymph node stage [141]. In other studies, the MUC5B was detected in primary breast tumors (81%), non-malignant breast diseases (92.8%), samples of normal breast epithelia adjacent to cancer (42.1%), and in none of the normal control breast epithelium [175].
3.2. Colon cancer
The tumor-associated glycoprotein (TAG-72) is aberrantly expressed in a variety of epithelial malignant tissues. The increased levels of serum TAG-72 antigen was observed in 48% of 56 gastric carcinoma patients and 67% of 45 colorectal carcinoma patients[176]. The B72.3 monoclonal antibody (MAb) detects an epitope carried by high-molecular-weight mucins (tumor-associated glycoprotein, TAG-72) recently identified as sialyl-Tn, and it has a restricted pattern of reactivity with normal tissues but it reacts with a high proportion of epithelial cancers. This antibody reacted strongly with 100% of the specimens of the mucosa adjacent to human colorectal adenocarcinomas (transitional mucosa) and with 81% of the specimens of adjacent colon cancer. Therefore, the expression of TAG-72 antigen occurs in the colon during the process of epithelial cell transformation [177].
The largest study evaluating the prognostic value of MUC1 and MUC3 expression in colorectal carcinoma samples was published in 2007 [178]. High throughput tissue microarray technology was used to evaluate 462 tumor samples. The study found that 32% of the tumors were positive to MUC1, and 74% were positive to MUC3. Although there was no correlation of both mucins in the clinicopathological variables, such as tumor stage and vascular invasion by univariate analysis, but multivariate analysis indicated that MUC1 expression was an independent marker of prognosis. Patients with higher MUC1 expression had a significant reduction in disease specific survival. Interestingly, the expression of MUC1 in colorectal carcinoma is a valuable indicator of poor prognosis in Caucasian patients when compared with those of African–American descent [179].
MUC2, MUC6, MUC12, and MUC13 expression was also investigated in colon cancer. Increased expression of MUC13 in colorectal cancers is associated with poorly differentiated tumors [146], whereas, MUC2 expression tends to be related to the indolent behavior of human neoplasms and a favorable outcome of the patients [180]. In the case of MUC6, it was found to have 100% specificity in distinguishing colonic sessile serrated adenoma (N = 26; positive staining) from hyperplastic polyp (N = 48; negative staining) [71]. In the case of MUC12 expression, this was down-regulated or absent in 6 out of 15 (40%) colorectal tumors samples, when compared with matched normal colonic tissues and the expression was down-regulated or not detected in any of six colorectal cancer cell lines examined in published work [155]. On the contrary, in colorectal cancers, the highest expression of MUC13 was observed in poorly differentiated tumors. The staining intensity was high in adenocarcinomas (81%) when compared with mucinous tumors (50%). Similarly, the high-intensity of cytoplasmic staining was observed in poorly differentiated and late-stage tumors [146].
3.3. Gastric cancer
In an attempt to evaluate whether membrane mucins or secretory mucins had a better prognosis value in gastric carcinoma, the expression of MUC1, MUC2, MUC3, MUC5AC, and MUC6 was evaluated in 46 tumor samples [181]. Normal gastric samples were positive to MUC1, MUC5AC, and MUC6. A shift in mucin expression was found on intestinal metaplasia, where an increase in MUC2 and MUC3 and a decrease in MUC1, MUC5AC, and MUC6 were obtained. Nevertheless, a drastic shift was not observed with gastric carcinoma samples as a measure of cancer progression. The main findings of this study was that a high expression of MUC1 correlated with a better prognosis, including smaller tumor size and an absence of metastasis, whereas MUC3 expression correlated with opposite trend. In addition, no prognostic correlation was found for the expression of secretory mucins and the development of gastric cancer.
In a recent published study, 133 mucinous gastric carcinomas (MGC) were compared with nonmucinous gastric carcinomas (NMGC) [182]. Key findings included that MGC tumor had deeper invasion, frequently metastasized to lymph nodes, and had a more advanced pathologic state than NMGC. In particular, MUC2 was expressed in 95.5% of MGC and in 33.4% of NMGC, suggesting a correlation between a mucinous histology and MUC2 expression in gastric carcinoma. However, the mucinous expression itself could not be identified as an independent prognostic factor of the progression of the disease. It appears that the aggressive characteristics of the MGC are a consequence of the advanced stage of the disease and the extracellular mucin facilitates the spreading of metastasis to the surrounding tissues.
In gastric cancer, MUC5AC expression was observed more frequently (100%) in lower grade carcinomas when compared to late stage carcinomas (58.6%) [68,183], and its expression is associated with better prognosis, whereas, its reduced expression is associated with shorter survival [184]. On the other hand, overexpression of MUC1 has been associated with poor clinicopathological parameters and poor survival [184]. The expression of sialyl-Lewis a [185] and Lewis x [186] epitopes indicates poor survival.
3.4. Lung cancer
Similar to other types of cancers, the MUC1 overexpression in squamous cell cancer of the lung is associated with poor survival [187]. Another mucin that has been envisioned as a potential candidate for tumor diagnosis is MUC4. Lung carcinoma tissues have been evaluated for the expression of MUC4 protein as well as mRNA levels [188]. Twenty-nine tumor samples were included in this study and it was found that 67% were positive for MUC4 protein expression and 72% were positive to MUC4 mRNA expression. Unfortunately, this expression could not be correlated to the clinical stage of cancer. Nevertheless, opposing to these results, a recent study demonstrated that the expression of MUC4 is in fact correlated with poor patient prognosis [189]. It was showed that patients, whose tumors had high MUC4 levels, had a disease-free curve significantly lower than in patients who had lower MUC4 levels [189]. Although only 13.5% of the patients included in the study had a high expression of MUC4, these results indicate that MUC4 expression is an indicator of poor prognosis in lung adenocarcinoma. Consistent with these studies, MUC4 overexpression in lung adenocarcinoma proved to be useful in distinguishing lung adenocarcinoma from malignant mesothelioma, with 91.4% sensitivity and 100% specificity [133].
3.5. Ovarian cancer
Ovarian cancer is another hidden disease that presents no detectable signs and hence eludes early detection. Recently, our group has published a comprehensive review of mucins in diagnosis, prognosis, and therapy of ovarian cancer [190]. From the diagnostic point of view, the concentration of MUC16 (CA125) increases in 80–90% of advanced stage ovarian cancers [191] and is the more widely used marker for its diagnosis [139,192]. Nevertheless, the detection of MUC16 as a diagnosis marker is limited due to its elevation in endometriosis, pelvic inflammatory disease, and pregnancy. Recently, Chauhan et al. showed that MUC4 (92%), MUC1 (83%), and MUC16 (79%) were significantly overexpressed in ovarian tumors and the expression of MUC4 was significantly higher when compared to MUC1 and MUC16 in early-stage ovarian tumors with 100% incidence. They suggested that, MUC4 could be a possible candidate biomarker for the early diagnosis of ovarian carcinoma and also indicated that MUC4 can be utilized in combination with MUC1 and MUC16 to achieve greater sensitivity for the detection of late-stage tumors [139]. Other studies that show contradictory results found no association between MUC4 expression and prognosis [193]. Other suggestions for an improved detection of stage 1 ovarian cancer include the combination of MUC16 (CA125), MUC1 (CA15-3), and tumor-associated glycoprotein 72 (CA72-4) [194].
3.6. Pancreatic cancer
To date, there is no specific tumor marker to diagnose pancreatic cancer, but mucins have been researched as potential candidates for this aggressive disease. Metzgar et al. (1982) raised monoclonal antibodies, such as DUPAN-2, specific to human pancreatic antigens (MUC1) secreted in the sera of pancreatic cancer patients [195]. Subsequently, this antibody was extensively studied in the United States [196,197] and in Japan [198] using a competitive assay and indicated a high positivity rate for DUPAN-2 in pancreatic and biliary tract carcinomas but low positivity in colorectal and gastric cancers. Later on, several researchers reported that mucin associated antigens like CA19.9, CA50, and CA195 [199–203] are elevated in advanced colorectal and pancreatic cancer and thus can be used as diagnostic markers.
The examination of MUC1 levels in serum was shown to be a good survival predictor for metastatic pancreatic cancer patients [204]. Similarly, determination of CA19-9 or sialylated Lewis antigen, along with ultrasound, computed tomography (CT) scan or endoscopic retrograde cholangiopancreatography (ERCP), can increase the positive predictive value from 62–71% to 100% [205]. In another study, the sensitivity of the CA19-9 serum assay ranged from 69 to 93% and the specificity varied between 46 and 98% [206]. The patients with invasive carcinoma of pancreatic and intrahepatic bile duct tumors showed poor outcome when the expression pattern was MUC1 positive and MUC2 negative, whereas, the non-invasive tumors with a favorable outcome exhibited an expression pattern of MUC1 negative and MUC2 positive [44,207]. Therefore, the expression pattern of MUC1 and MUC2 can be used as potential markers to differentiate between the invasive and non-invasive carcinomas of the pancreas and intrahepatic bile duct.
MUC4 is one of the candidate biomarkers in the diagnosis of pancreatic cancer [136,140]. It is not expressed in the normal pancreas or chronic pancreatitis, but it is highly expressed in human pancreatic tumors [51,136,208]. The MUC4 is expressed as early as in precancerous pancreatic intraepithelial neoplastic (PanIN) lesions, and its expression increases with the disease progression [137]. Studies have also shown that the detection of MUC4 is one of the useful markers for the early diagnosis of pancreatic cancer in fine-needle aspirates (FNA), exhibiting 91% sensitivity and 100% specificity [209]. It has also been reported that, MUC4 overexpression and a decreased expression of clusterin-β in pancreatic adenocarcinoma are useful in distinguishing reactive ductal cells from the cells of pancreatic adenocarcinoma in FNA samples.
Detection of circulating MUC1 by PAM4-based immunoassay could be useful in the diagnosis of pancreatic cancer [210]. In the same way, the quantitative assessments of mRNA, such as MUC1 and MUC5AC in pancreatic juice are potential candidate genes for preoperative diagnosis of pancreatic cancer [176]. A recent review described the presence of MUC4-specific antibody in the serum and MUC4 transcript in peripheral blood mononuclear cells (PBMCs) of cancer patients, positioning MUC4 as a promising diagnostic biomarker in high-risk individuals as well as in those with established cancer [211].
3.7. Prostate cancer
In clinical studies developed for targeted radioimmunotherapy in metastatic prostate cancer patients, the BrE3 antibody, which recognizes epitopes in the VNTR region of MUC1, showed a strong correlation of prostate cancer progression [212]. These results demonstrate the potential of hypoglycosylated MUC1 as a marker for aggressive prostate cancer.
3.8. Other cancers
The efficacy of mucins in diagnosis has been investigated in other less common cancers as well, and some of these examples are mentioned below, including bladder cancer, extrahepatic bile duct carcinoma, and cholangiocarcinomas. MUC2 expression is detected by the monoclonal antibody 4F1 in ∼40% of the cases of carcinomas of bladder, whereas normal urothelium showed no MUC2 expression [213]. In other study, the 111Indium-labeled anti-MUC1 mucin monoclonal antibody was detected both in primary and recurrent invasive bladder tumors with distant metastases. The use of this MUC1 radio labeled antibody will likely improve the clinical staging of cancers and assist in selecting radical therapy [53]. In extrahepatic bile duct carcinoma, MUC1/Df3 was the most useful indicator for prognosis among the various glycoforms of the MUC1 mucin [214]. Finally, the MUC1 expression in cholangiocarcinomas has been associated with dedifferentiation, infiltrative growth pattern, and patient survival, while an abnormal MUC1 expression is correlated with the progression of cholangiocarcinoma [55].
4. Evaluation of mucins in immunologic therapeutics
The use of mucins as targets for cancer therapy has been evaluated for the last 3 decades [2,140,215–225]. Altogether, most of the approaches involve instructing the immune system to recognize these glycoproteins presented in malignant tumor cells and eradicate them. A summary of the key findings in the immunotherapy of breast, colon, lung, pancreatic, prostate, and renal adenocarcinomas is presented below (Table 2).
Table 2. Mucins used as therapeutic targets in various cancers.
| Primary cancer | Mucins in therapy | Therapy approaches | Major therapy outcomes |
|---|---|---|---|
| Breast | MUC1 | TR peptides
|
|
| Colon | MUC1, Thomsen-Friedrich (T-Tn), Sialyl-Tn antigens |
|
|
| Lung | MUC1 |
|
|
| Ovarian | MUC1 |
|
|
| Pancreatic | MUC1 |
|
|
| MUC4 |
|
|
|
| Prostate | MUC1 |
|
|
| Renal | MUC1 |
|
|
TR, Tandem Repeat; KLH, Key Limpet Hemocyanin; DC, Dendritic Cell; CTL, Cytotoxic T Lymphocytes; NK, Natural Killer Cells; CEA, Carcinoembryonic Antigen; PADRE, Pan-DR-epitope
4.1. Breast cancer
Breast cancer is the most investigated cancer for the development of mucin therapies. One of the earliest studies highlighting the immunogenicity of mucins in breast and pancreatic cancer dated from 2 decades ago [223]. Cytotoxic T lymphocytes (CTLs) were generated from lysed tumor cells collected from tumor draining lymph nodes of breast cancer patients in a MHC-unrestricted manner. The CTLs were cytotoxic to breast and pancreatic cancer cell lines and not to normal counterparts, implying that immunologic epitopes are not revealed in normal cells.
A clinical trial including 10 breast cancer patients that were immunized with MUC1 33-mer and 106-mer MUC1 tandem repeat peptide found high levels of circulating MUC1 antibodies after the immunizations [226]. MUC1 antibodies, present in the plasma of immunized patients, were cultured with peripheral blood mononuclear cells (PBMCs) isolated from healthy donors. It was demonstrated that tumor cell lysis specific to MUC1 was induced by the stimulated PBMCs. Natural killer cells were the essential effector cells for the MUC1 antibody-mediated cytotoxicity, indicating the involvement of both innate and adaptive immune systems.
In an effort to increase the immunogenicity of the MUC1 vaccine, the mucin has been conjugated with several proteins. A promising study with metastatic breast cancer patients that were immunized with a 16-mer MUC1 peptide (BP16) conjugated to the carrier protein keyhole limpet hemocyanin (KLH), shows that MHC I restricted CTLs precursors were generated in vitro with synthetic MUC1 peptide [227]. In another approach, in a phase I clinical trial involving 25 patients with metastatic adenocarcinoma of breast, colon, rectum, and stomach, strong humoral responses were generated when oxidized mannan-MUC1 fusion protein was injected in the patients [228]. Around 70% of the patients receiving eight injections yielded strong MUC1-antibodies responses, while CTL responses were present in only two out of 10 patients. In a recent published clinical study, the vaccine formulation also consisted of oxidized mannan-MUC1, but this time the vaccinations were tested on early-stage breast cancer patients [229]. A group of 31 patients with stage II breast cancer were injected with the vaccine formulation. This was the first evidence of a successful immunotherapy outcome of any MUC1 vaccine, as no recurrence of disease occurred over 5-year-period after the immunizations. The control group, which received only placebo, had a 27% recurrence rate. Of the 10 patients, whose samples were available for T cell proliferation assays, the T cells isolated from four patients (40%) were shown to be specific for MUC1 VNTR. Conversely, anti-VNTR serum antibodies were induced in 69% of the patients. Important findings of this study emphasize the importance of conducting immunotherapy trials on cancer patients before their immune system is compromised with the tumor burden of an advanced stage.
Other approaches to use MUC1 on immune therapeutics against breast cancer include a live recombinant vaccinia virus that expressed MUC1 and IL-2 genes [230]. This small study included nine patients with advanced breast cancer and, as opposed to the studies discussed previously, none of the patients produced high antibody titers to MUC1 after vaccination and only two had cellular responses as indicated by the production of MUC1-specific CTLs.
In addition, vaccinations with MUC1 peptide-pulsed dendritic cells (DCs) have been carried out in different trials. Metastatic breast and ovarian cancer patients that were vaccinated with autologous MUC1 peptide-pulsed DC produced CD8+ cytotoxic T cells that were detected in the peripheral blood after three vaccinations over 28 days [231].
4.2. Colon cancer
Clinical studies done on patients with resected colon carcinoma investigated vaccines containing the Thomsen-Friedenreich antigen (T) and sialyl–Tn antigen (sTn), which are overexpressed in the mucins of colorectal carcinomas [232,233]. In the first approach, 20 patients were subdivided in three groups that were immunized with partially desialylated ovine submaxillary gland mucin (modified OSM) alone, OSM administered with DETOX, or modified OSM injected with Bacillus Calmette-Guérin (BCG), respectively [232]. Although the modified OSM alone did not induced any immune response in the patients, nine out of 15 patients immunized with modified OSM and the adjuvants (DETOX or BCG) produced increased IgG titers of OSM specific antibodies, primarily against sTn. In another clinical trial, T and sTn antigens were evaluated against colorectal cancer, in an approach involving the conjugation of these antigens to the immunogenic carrier protein KLH and injection with several adjuvants in order to improve the immune response [233]. The patients injected with DETOX and QS-21 generated both IgM and IgG antibodies against the synthetic peptides. However, when analyzing the induced antibodies against natural antigens, the responses were weak to moderate for IgM and undetectable for IgG. This study demonstrates the importance of designing vaccines that will induce the immune system to recognize antigens as they are presented on a tumor environment.
Viral vaccines that expresses carcinoembryonic antigen (CEA) and MUC1 had been developed by Therion Biologics Corporation [234,235]. The PANVAC-VF consists of vaccinia or fowlpox viral vectors expressing CEA and MUC1 along with the three T cell costimulatory molecules B7.1, ICAM-1, and LAF-3 (TRICOM™). In this first National Cancer Institute (NCI)-sponsored study, 25 patients with advanced metastatic carcinoma from different tissue origins, mainly from colon, were injected with PANVAC-VF. Over half of the analyzed patients had CD8 and CD4 immune responses specific to MUC1 and/or CEA. In addition to these promising responses, a follow up of an ovarian cancer patient confirmed a duration of the clinical response for 18 months, whereas a breast cancer patient had a measurable decrease liver metastasis.
4.3. Lung cancer
As recently reviewed, cancer associated mucins are potent biomolecules to develop novel therapies against lung cancer [236,237]. One of the antigen-specific vaccination strategies investigated was the MUC1 liposomal vaccine preparation BLP25 (L-BLP25). The vaccine consists of BLP25 lipopeptide, which targets the exposed core of the MUC1 peptide, the adjuvant monophosphoryl lipid A, and three lipids including cholesterol, dimyristoyl phosphatidylglycerol, and dipalmitoyl phosphatidylcholine. In a phase I clinical trial, five of the 12 patients with advanced non-small cell lung cancer (NSCLC) that completed the vaccination regimen had cellular responses specific to MUC1 [238]. Although no antitumor responses were obtained, the higher dose injected extended the median survival time significantly. Likewise, in a phase II clinical trial with 65 NSCLC patients, 54% of the patients that received the MUC1 liposomal vaccination increased their survival for almost a year when compared with the control group [239]. In a 53 months follow-up study conducted by the same research group, the overall survival of patients receiving the L-BLP25 vaccine was further increased to 30.6 months versus the 13.3 months of non-immunized patients [240].
4.4. Ovarian cancer
High MUC1-antibody levels in ovarian cancer patients are associated with greater survival, but it does not represent a significant prognostic indicator [241]. In agreement with the reports on humoral responses to MUC1 discussed for breast cancer, IgM antibodies were detected in ovarian cancer patients with the 105-residue MUC1 peptide. In these studies, the levels of MUC1 antibodies were higher in healthy women, making unfeasible the correlation of this immune response with ovarian cancer. One of the possible explanations to these findings relies on the possible variety of immune responses against MUC1, as glycosylation patterns among different types of adenocarcinomas are not homogenous. A CTL cell line, which was derived from ovarian tumor-infiltrating or tumor-associated lymphocytes, that recognizes MUC1 positive tumors in a non-MHC restricted manner [222]. In the same study, MUC1 transfected cells were also lysed, and in this latter case it was MHC restricted. The main outcome of this study was that the CTL cell line recognizes epitopes on the core peptide of MUC1 and thus, suggested that mucin therapy can be feasible for the treatment of ovarian cancer.
A recent review published by our group, summarizes the mucin immunotherapeutic strategies against ovarian cancer [190]. One of the recent studies discussed here included a clinical trial where 11 patients with stage III-IV ovarian, fallopian tube, and peritoneal cancers were immunized with heptavalent antigens individually conjugated to KLH and administered with the adjuvant QS-21 [242]. All the mucin antigens included in the vaccine (Tn, STn, TF, Tn-MUC1) induced the higher antibody titers when compared with the nonmucin antigens and, specifically, Tn-MUC1 was able to induce both IgM and IgG responses. Refer to the review for more examples of therapies involving the mucins MUC1, MUC4, and MUC16 in ovarian cancer.
4.5. Pancreatic cancer
In a recent review describing novel therapies against pancreatic cancer, MUC1 and MUC4 are mentioned as potential targets for treatment of this malignancy [243]. One of the earlier immunogenic studies done in pancreatic cancer patients generated a cytotoxic cell line derived from lymph nodes of a patient with pancreatic adenocarcinoma, which induced MHC-unrestricted recognition of MUC1 [216]. The CTLs lysed pancreatic, breast, colon, ovarian, and other cancer cell lines that expressed mucin peptide epitopes, whereas the tumor cells lacking mucin peptide were not killed.
In a phase I clinical trial done over a decade ago, 24 pancreatic cancer patients, nine breast cancer patients, and 30 colorectal cancer patients were immunized with a MUC1 105-mer peptide that contain five immunologic epitopes [220]. The main goal of this trial was to determine the toxicities of the vaccine, using Bacillus-Calmette-Guérin (BCG) as an adjuvant, and, secondarily, to determine if the vaccine induced a hypersensitive (DHT) response specific to MUC1. Altogether, the tolerability of the vaccine was proved and 67% of the biopsies analyzed indicated a strong T cell infiltration at the injection site only when the peptide with the immunogenic epitopes was present in the formulation. Enhanced responses to MUC1 were reported in seven out of 22 patients.
In a more recent phase I study, 10 patients with advanced pancreatic cancer were immunized with the recombinant vaccinia and fowl pox viral vectors expressing CEA and MUC1, in combination with the three costimulatory molecules B7.1, ICAM-1, and LFA-3 (TRICOM™) [234]. In this study, the granulocyte-macrophage colony-stimulating factor (GM-CSF) was used as an adjuvant, and the whole vaccine formulation is known as PANVAC-VF. The prime immunizations consisted of vaccinia virus and subsequent boost injections consisted of fowl-pox virus in order to increase T cell immunity. Antigen specific T cell responses were detectable in five out of eight patients within 2 months of the immunizations. The encouraging results of the study included a significant increase in the median survival of the patients with T cell immune responses (15.1 months) when compared with the ones that did not have the T cell activation (3.9 months).
A potential immunotherapy strategy against pancreatic cancer was developed last year, where a dendritic cell-based vaccine was designed to stimulate both T cell arms of the immune system: CD4+ T helper cells and cytotoxic CD8+ T cells [244]. Dendritic cells were transduced with the universal DR-restricted T helper epitope (PADRE) combined with human leukocyte antigen HLA-A1 and HLA-A2 restricted epitopes from MUC4 using adenovirus vector. The in vitro studies showed that immunogenicity of the formulation was increased and cytotoxicity was HLA-A2 restricted and MUC4 specific. The advantage of PADRE resides on its ability to increase immunogenic responses more than three-fold when compared with natural T cell epitopes.
4.6. Prostate cancer
As recently reviewed, MUC1 shows promising results for the therapy of prostate cancer [245]. Some of these approaches for targeting MUC1 are discussed below. A vaccinia virus expressing MUC-1 and IL-2 genes was evaluated in a phase I clinical trial of previously treated patients with advanced prostate cancer, whose tumors were shown to be positive for MUC1 [246]. The progression of the disease was measured by the levels of prostate-specific antigen (PSA). Of the 16 patients enrolled in the study, only one had an objective tumor response and two showed long-term stabilization of PSA. Unfortunately, at the end of the clinical study, 14 patients had progressive disease and mild adverse effects were reported. These secondary effects are possibly caused by the IL-2 rather than the MUC-1 expression, and to implement the vaccine for future studies, more modifications need to be done in order to minimize these undesirable effects while increasing its immunogenicity.
As discussed for patients with lung cancer, the liposomal vaccine BLP25 also shows promise for prostate cancer patients as demonstrated in a small clinical trial of 16 patients [247]. Even though the study was of small size, the liposomal vaccine prolonged the protein specific antigen (PSA) doubling time in half of the patients, which may delay the clinical relapse of the disease.
4.7. Renal cancer
In a study including patients with advanced cancer immunized with oxidized mannan conjugated to MUC1 VNTR, demonstrated that the vaccine did not induce antibody responses but only cell responses [248]. This trial included patients with renal, colon, breast, and ovarian among other types of cancer. IFNγ-secreting T cells specific to the VNTR MUC1 region were identified in the 10 patients included in the study, whereas nine out of them had DTH responses. Even though the anti-tumor response was not proven, the immune responses were still detectable after a year of the last immunization.
In another promising phase I trial with metastatic renal cancer patients, 30% of the MUC1- immunized patients had a noticeable regression of metastasis [249]. The vaccine consisted of autologous DCs pulsed with MUC1 peptides and the T helper epitope PAN-DR-binding peptide (PADRE) was included as an adjuvant therapy. Cytotoxic T cell responses were detected roughly in half of the patients after three to four vaccine injections. After five immunizations, the patients were treated with low doses of IL-2 as part of the combination therapy. It is important to mention that the immunologic responses discussed previously were detected before the treatment with IL-2. With these promising results, the same group published a short review suggesting the potential of MUC1-pulsed DCs to induce immunological and clinical responses in patients with advanced renal cell carcinoma [249].
5. Mucins as conjugated therapeutics
Because they are differentially expressed in malignant tissues, mucins have been investigated for targeted therapies. Several approaches include conjugating mucin antibodies to radioactive elements and/or cytotoxic drugs. Once the distinct epitopes of mucins are targeted by the conjugated antibodies, the malignant cells are selectively destroyed by radiation and/or the cytotoxic drug.
5.1. Breast cancer
Work that was published this year describes a novel approach against epithelial cancer cells [250]. The potential therapy consisted of using phototoxic single stranded DNA aptamers that display a peptide corresponding to five MUC1 tandem repeats modified with up to 15 GalNAc sugars (Tn antigen). The specific entry of the phototoxic aptamers to MUC1 positive cancer cells (breast, colon, lung, ovarian, and pancreatic) occurred by receptor-mediated endocytosis. The toxicity was increased over 500-fold after light activation when compared with the drug alone. Even if this work was demonstrated on in vitro experiments, it shows the potential of using mucins to deliver cytotoxic drugs, such as photodynamic therapy drugs into cancer cells to eradicate them.
Another technique that is currently being developed is a MUC1 pre-targeting molecule for imaging and pre-targeting radioimmunotherapy of metastatic breast and prostate cancers [251]. The three major components of the pre-targeting molecule are a tumor antigen binding module or single chain antibody fragments (scFv), a radioactive hapten capturing molecule, and a bifunctional polyethylene glycol (PEG) for the covalent attachment of both. Preclinical studies are being performed and different valency of the antibody fragments is being compared on in vivo experiments to determine the best formulation.
5.2. Ovarian cancer
In a randomized phase III study, patients with ovarian cancer were treated with intraperitoneal radioimmunotherapy using 90Y-HMFG1 monoclonal antibody targeting MUC1 [252]. The patients that entered the study with complete remission after chemotherapy had a median survival of 78% over 10 years of follow up studies. In a similar study published more recently, the same radiolabeled murine antibody was analyzed in a phase III clinical trial to determine the immunogenicity of the drug in ovarian cancer patients and the outcome of the disease [224]. It was found that MUC1 IgG antibodies were induced in the patients, but no difference in disease outcome was observed in the patients that did not receive the injections. However, when patients receiving the drug were compared, the ones that had greater levels of MUC1 antibodies had an improved disease outcome than those that did not respond to the vaccine.
5.3. Prostate cancer
A combination therapy of 111In/90Y-monoclonal antibody, m170, which targets expressed MUC1, and paclitaxel given to patients with metastatic prostate cancer increased the therapeutic index of targeted radiation therapy [212]. The metastatic cancer was successfully targeted in 25 out of 26 patients, indicating the specificity of MUC1 recognition in prostate tumor cells. This study confirmed an increased efficacy of targeted radiation when compared to previous studies done with radioimmunotherapy alone.
6. Other approaches for the use of mucins as valuable therapeutic tools
Clearly, numerous studies discussed in this review have shown that mucins can be successfully recognized when injected as a vaccine and the focus has been mainly to target cytotoxic T cells. However, some studies emphasize the importance of developing vaccines that prime not only the cytotoxic T cell responses but also the activation of mucin-specific helper T cells, in concordance with the hypothesis that a cancer vaccine should prime the activation of both arms of the immune system [253]. One of the studies that emphasizes the activation of humoral responses or helper T cell responses has just been published [254]. The strategy evaluated in murine models indicates that the induction of antibodies specific to sialylated Thomsen-noveau (STn) antigens, which can be carried on various mucins, created significant delay in tumor growth. Similarly, in other study performed in a MUC1-tolerant colon cancer murine model, the vaccine included MHC class I-restricted MUC1 peptides as well as MHC class II-restricted pan helper peptide [255]. In addition, the vaccine formulation included unmethylated CpG oligodeoxynucleotide, and GM-CSF as adjuvants. This promising study was successful in breaking MUC1 self-tolerance in MUC1.Tg mice, both prophylactically and therapeutically.
Other in vitro approaches involved the development of self-inactivating lentiviral vectors encoding the human MUC1-specific MHC-unrestricted single chain T cell receptor and a fusion suicide gene to eradicate transduced cells in a safe manner [256]. This represents a novel and safe approach to target MUC1 in malignant tissues to activate innate and adaptive immune responses.
In addition, studies involving other mucins have shown promise for further clinical analysis. One such examples is an anti-idiotype MUC16 antibody fused with IL-6 that induced specific humoral responses against MUC16 (CA125) in ovarian cancer [257].
7. Future perspectives
More than 200 clinical studies have been done over the last three decades to evaluate mucins as prognostic indicators or therapeutic constituents. The current need to find alternative treatments against cancer and the promise of mucins is denoted by the current clinical trials involving these glycoproteins in observational as well as in interventional therapeutics studies against cancer. Nevertheless, it is important to keep in mind that outcomes obtained from clinical trials done in patients with advanced cancer might underestimate the therapeutic value of new vaccine formulations. Altogether, there is no doubt that these macromolecules represent a potential tool for the diagnosis and therapy of a wide range of cancers and their incorporation into clinical medicine represents an advance in the continuous search for the cure of cancer. Advances in our knowledge on splice variants, domain structrures, development of new MUC specific antibodies, and characterization of glycosylations on these proteins will form the basis for new clinical trials.
Acknowledgments
The authors on this work, in part, are supported by the grants from the National Institutes of Health (CA78590, EDRN UO1 CA111294, CA133774 and CA131944).
References
- 1.Forstner JF. Intestinal mucins in health and disease. Digestion. 1978;17:234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 3.Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–D1206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- 4.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 5.Lamblin G, Degroote S, Perini JM, Delmotte P, Scharfman A, Davril M, Lo-Guidice JM, Houdret N, Dumur V, Klein A, Rousse P. Human airway mucin glycosylation: a combinatory of carbohydrate determinants which vary in cystic fibrosis. Glycoconj J. 2001;18:661–684. doi: 10.1023/a:1020867221861. [DOI] [PubMed] [Google Scholar]
- 6.Carraway KL, Hull SR. O-glycosylation pathway for mucin-type glycoproteins. Bioessays. 1989;10:117–121. doi: 10.1002/bies.950100406. [DOI] [PubMed] [Google Scholar]
- 7.Hanisch FG. O-glycosylation of the mucin type. Biol Chem. 2001;382:143–149. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56:2237–2244. [PubMed] [Google Scholar]
- 9.Fukuda M. Roles of mucin-type O-glycans in cell adhesion. Biochim Biophys Acta. 2002;1573:394–405. doi: 10.1016/s0304-4165(02)00409-9. [DOI] [PubMed] [Google Scholar]
- 10.Satoh S, Hinoda Y, Hayashi T, Burdick MD, Imai K, Hollingsworth MA. Enhancement of metastatic properties of pancreatic cancer cells by MUC1 gene encoding an anti-adhesion molecule. Int J Cancer. 2000;88:507–518. doi: 10.1002/1097-0215(20001115)88:4<507::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gindzienski A, Zwierz K. The effect of SDS and 2-mercaptoethanol on the supramolecular structure of human gastric mucus gel. Biochem Med Metab Biol. 1987;38:347–354. doi: 10.1016/0885-4505(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 13.Paszkiewicz-Gadek A, Gindzienski A, Porowska H. The use of preparative polyacrylamide gel electrophoresis and electroelution for purification of mucus glycoproteins. Anal Biochem. 1995;226:263–267. doi: 10.1006/abio.1995.1224. [DOI] [PubMed] [Google Scholar]
- 14.Corfield AP, Myerscough N, Gough M, Brockhausen I, Schauer R, Paraskeva C. Glycosylation patterns of mucins in colonic disease. Biochem Soc Trans. 1995;23:840–845. doi: 10.1042/bst0230840. [DOI] [PubMed] [Google Scholar]
- 15.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 16.Strous GJ, Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- 17.Bobek LA, Tsai H, Biesbrock AR, Levine MJ. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7) J Biol Chem. 1993;268:20563–20569. [PubMed] [Google Scholar]
- 18.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desseyn JL, Guyonnet-Duperat V, Porchet N, Aubert JP, Laine A. Human mucin gene MUC5B, the 10.7-kb large central exon encodes various alternate subdomains resulting in a super-repeat. Structural evidence for a 11p15.5 gene family. J Biol Chem. 1997;272:3168–3178. doi: 10.1074/jbc.272.6.3168. [DOI] [PubMed] [Google Scholar]
- 20.Desseyn JL, Aubert JP, Van SI, Porchet N, Laine A. Genomic organization of the 3′ region of the human mucin gene MUC5B. J Biol Chem. 1997;272:16873–16883. doi: 10.1074/jbc.272.27.16873. [DOI] [PubMed] [Google Scholar]
- 21.Desseyn JL, Buisine MP, Porchet N, Aubert JP, Laine A. Genomic organization of the human mucin gene MUC5B. cDNA and genomic sequences upstream of the large central exon. J Biol Chem. 1998;273:30157–30164. doi: 10.1074/jbc.273.46.30157. [DOI] [PubMed] [Google Scholar]
- 22.Gum JR, Hicks JW, Swallow DM, Lagace RL, Byrd JC, Lamport DT, Siddiki B, Kim YS. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990;171:407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- 23.Gum JR, Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 24.Itoh Y, Kamata-Sakurai M, da-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M, Irimura T. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 25.Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J Biol Chem. 1990;265:15294–15299. [PubMed] [Google Scholar]
- 26.Shankar V, Gilmore MS, Elkins RC, Sachdev GP. A novel human airway mucin cDNA encodes a protein with unique tandem-repeat organization. Biochem J. 1994;300:295–298. doi: 10.1042/bj3000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW, Gum JR, Jr, Byrd JC, Siddiki B, Kim YS. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993;268:5879–5885. [PubMed] [Google Scholar]
- 28.Williams SJ, Munster DJ, Quin RJ, Gotley DC, McGuckin MA. The MUC3 gene encodes a transmembrane mucin and is alternatively spliced. Biochem Biophys Res Commun. 1999;261:83–89. doi: 10.1006/bbrc.1999.1001. [DOI] [PubMed] [Google Scholar]
- 29.Porchet N, Dufosse J, Audie JP, Duperat VG, Perini JM, Nguyen VC, Degand P, Aubert JP. Structural features of the core proteins of human airway mucins ascertained by cDNA cloning. Am Rev Respir Dis. 1991;144:S15–S18. doi: 10.1164/ajrccm/144.3_pt_2.S15. [DOI] [PubMed] [Google Scholar]
- 30.Dufosse J, Porchet N, Audie JP, Guyonnet DV, Laine A, Van-Seuningen I, Marrakchi S, Degand P, Aubert JP. Degenerate 87-base-pair tandem repeats create hydrophilic/hydrophobic alternating domains in human mucin peptides mapped to 11p15. Biochem J. 1993;293:329–337. doi: 10.1042/bj2930329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lapensee L, Paquette Y, Bleau G. Allelic polymorphism and chromosomal localization of the human oviductin gene (MUC9) Fertil Steril. 1997;68:702–708. doi: 10.1016/s0015-0282(97)00317-8. [DOI] [PubMed] [Google Scholar]
- 32.Pigny P, Guyonnet-Duperat V, Hill AS, Pratt WS, Galiegue-Zouitina S, d'Hooge MC, Laine A, Van-Seuningen I, Degand P, Gum JR, Kim YS, Swallow DM, Aubert JP, Porchet N. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996;38:340–352. doi: 10.1006/geno.1996.0637. [DOI] [PubMed] [Google Scholar]
- 33.Desseyn JL, Buisine MP, Porchet N, Aubert JP, Degand P, Laine A. Evolutionary history of the 11p15 human mucin gene family. J Mol Evol. 1998;46:102–106. doi: 10.1007/pl00006276. [DOI] [PubMed] [Google Scholar]
- 34.Desseyn JL, Aubert JP, Porchet N, Laine A. Evolution of the large secreted gel-forming mucins. Mol Biol Evol. 2000;17:1175–1184. doi: 10.1093/oxfordjournals.molbev.a026400. [DOI] [PubMed] [Google Scholar]
- 35.Guyonnet Duperat V, Audie JP, Debailleul V, Laine A, Buisine MP, Galiegue-Zouitina S, Pigny P, Degand P, Aubert JP, Porchet N. Characterization of the human mucin gene MUC5AC: a consensus cysteine-rich domain for 11p15 mucin genes? Biochem J. 1995;305:211–219. doi: 10.1042/bj3050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gum JR, Jr, Hicks JW, Toribara NW, Rothe EM, Lagace RE, Kim YS. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem. 1992;267:21375–21383. [PubMed] [Google Scholar]
- 37.Toribara NW, Ho SB, Gum E, Gum JR, Jr, Lau P, Kim YS. The carboxyl-terminal sequence of the human secretory mucin, MUC6. Analysis Of the primary amino acid sequence. J Biol Chem. 1997;272:16398–16403. doi: 10.1074/jbc.272.26.16398. [DOI] [PubMed] [Google Scholar]
- 38.Gum JR, Byrd JC, Hicks JW, Toribara NW, Lamport DT, Kim YS. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989;264:6480–6487. [PubMed] [Google Scholar]
- 39.Toribara NW, Gum JR, Jr, Culhane PJ, Lagace RE, Hicks JW, Petersen GM, Kim YS. MUC-2 human small intestinal mucin gene structure. Repeated arrays and polymorphism. J Clin Invest. 1991;88:1005–1013. doi: 10.1172/JCI115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escande F, Porchet N, Bernigaud A, Petitprez D, Aubert JP, Buisine MP. The mouse secreted gel-forming mucin gene cluster. Biochim Biophys Acta. 2004;1676:240–250. doi: 10.1016/j.bbaexp.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Tytgat KM, Buller HA, Opdam FJ, Kim YS, Einerhand AW, Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994;107:1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 42.Blank M, Klussmann E, Kruger-Krasagakes S, Schmitt-Graff A, Stolte M, Bornhoeft G, Stein H, Xing PX, McKenzie IF, Verstijnen CP. Expression of MUC2-mucin in colorectal adenomas and carcinomas of different histological types. Int J Cancer. 1994;59:301–306. doi: 10.1002/ijc.2910590302. [DOI] [PubMed] [Google Scholar]
- 43.Reis CA, David L, Carvalho F, Mandel U, de BC, Mirgorodskaya E, Clausen H, Sobrinho-Simoes M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000;48:377–388. doi: 10.1177/002215540004800307. [DOI] [PubMed] [Google Scholar]
- 44.Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, Tanaka S, Irimura T, Kim YS, Sato E. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998;4:2605–2614. [PubMed] [Google Scholar]
- 45.Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, McGuckin MA. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. J Pathol. 1997;183:311–317. doi: 10.1002/(SICI)1096-9896(199711)183:3<311::AID-PATH917>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41:1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- 47.Vandenhaute B, Buisine MP, Debailleul V, Clement B, Moniaux N, Dieu MC, Degand P, Porchet N, Aubert JP. Mucin gene expression in biliary epithelial cells. J Hepatol. 1997;27:1057–1066. doi: 10.1016/s0168-8278(97)80150-x. [DOI] [PubMed] [Google Scholar]
- 48.Yu DF, Chen Y, Han JM, Zhang H, Chen XP, Zou WJ, Liang LY, Xu CC, Liu ZG. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjogren syndrome patients. Exp Eye Res. 2008;86:403–411. doi: 10.1016/j.exer.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Bartman AE, Sanderson SJ, Ewing SL, Niehans GA, Wiehr CL, Evans MK, Ho SB. Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int J Cancer. 1999;80:210–218. doi: 10.1002/(sici)1097-0215(19990118)80:2<210::aid-ijc9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 50.Buisine MP, Janin A, Maunoury V, Audie JP, Delescaut MP, Copin MC, Colombel JF, Degand P, Aubert JP, Porchet N. Aberrant expression of a human mucin gene (MUC5AC) in rectosigmoid villous adenoma. Gastroenterology. 1996;110:84–91. doi: 10.1053/gast.1996.v110.pm8536891. [DOI] [PubMed] [Google Scholar]
- 51.Balague C, Gambus G, Carrato C, Porchet N, Aubert JP, Kim YS, Real FX. Altered expression of MUC2, MUC4, and MUC5 mucin genes in pancreas tissues and cancer cell lines. Gastroenterology. 1994;106:1054–1061. doi: 10.1016/0016-5085(94)90767-6. [DOI] [PubMed] [Google Scholar]
- 52.Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, Kim YS. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- 53.Hughes OD, Perkins AC, Frier M, Wastie ML, Denton G, Price MR, Denley H, Bishop MC. Imaging for staging bladder cancer: a clinical study of intravenous 111indium-labelled anti-MUC1 mucin monoclonal antibody C595. BJU Int. 2001;87:39–46. doi: 10.1046/j.1464-410x.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 54.Temann UA, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, Rankin JA. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol. 1997;16:471–478. doi: 10.1165/ajrcmb.16.4.9115759. [DOI] [PubMed] [Google Scholar]
- 55.Park SY, Roh SJ, Kim YN, Kim SZ, Park HS, Jang KY, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact. Oncol Rep. 2009;22:649–657. doi: 10.3892/or_00000485. [DOI] [PubMed] [Google Scholar]
- 56.Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470–477. doi: 10.1046/j.1440-1827.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 57.Kocer B, McKolanis J, Soran A. Humoral immune response to MUC5AC in patients with colorectal polyps and colorectal carcinoma. BMC Gastroenterol. 2006;6:4. doi: 10.1186/1471-230X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thornton DJ, Khan N, Mehrotra R, Howard M, Veerman E, Packer NH, Sheehan JK. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology. 1999;9:293–302. doi: 10.1093/glycob/9.3.293. [DOI] [PubMed] [Google Scholar]
- 59.Sheehan JK, Howard M, Richardson PS, Longwill T, Thornton DJ. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem J. 1999;338:507–513. [PMC free article] [PubMed] [Google Scholar]
- 60.Sheehan JK, Brazeau C, Kutay S, Pigeon H, Kirkham S, Howard M, Thornton DJ. Physical characterization of the MUC5AC mucin: a highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem J. 2000;347(Pt 1):37–44. [PMC free article] [PubMed] [Google Scholar]
- 61.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 62.Thornton DJ, Carlstedt I, Howard M, Devine PL, Price MR, Sheehan JK. Respiratory mucins: identification of core proteins and glycoforms. Biochem J. 1996;316:967–975. doi: 10.1042/bj3160967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thornton DJ, Howard M, Khan N, Sheehan JK. Identification of two glycoforms of the MUC5B mucin in human respiratory mucus. Evidence for a cysteine-rich sequence repeated within the molecule. J Biol Chem. 1997;272:9561–9566. doi: 10.1074/jbc.272.14.9561. [DOI] [PubMed] [Google Scholar]
- 64.Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK. MUC5AC and MUC5B Mucins are decreased in cystic fibrosis airway secretions. Am J Respir Cell Mol Biol. 2004;31:86–91. doi: 10.1165/rcmb.2003-0345OC. [DOI] [PubMed] [Google Scholar]
- 65.Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997;113:455–464. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]
- 66.de BC, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 67.Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, Niehans GA. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995;55:2681–2690. [PubMed] [Google Scholar]
- 68.Reis CA, David L, Correa P, Carneiro F, de BC, Garcia E, Mandel U, Clausen H, Sobrinho-Simoes M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–1007. [PubMed] [Google Scholar]
- 69.Bartman AE, Buisine MP, Aubert JP, Niehans GA, Toribara NW, Kim YS, Kelly EJ, Crabtree JE, Ho SB. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J Pathol. 1998;186:398–405. doi: 10.1002/(SICI)1096-9896(199812)186:4<398::AID-PATH192>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 70.Buisine MP, Devisme L, Degand P, Dieu MC, Gosselin B, Copin MC, Aubert JP, Porchet N. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J Histochem Cytochem. 2000;48:1667–1676. doi: 10.1177/002215540004801210. [DOI] [PubMed] [Google Scholar]
- 71.Owens SR, Chiosea SI, Kuan SF. Selective expression of gastric mucin MUC6 in colonic sessile serrated adenoma but not in hyperplastic polyp aids in morphological diagnosis of serrated polyps. Mod Pathol. 2008;21:660–669. doi: 10.1038/modpathol.2008.55. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, Ann DK, Wu R. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–165. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- 73.Kerschner JE, Khampang P, Erbe CB, Kolker A, Cioffi JA. Mucin gene 19 (MUC19) expression and response to inflammatory cytokines in middle ear epithelium. Glycoconj J. doi: 10.1007/s10719-009-9245-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rousseau K, Kirkham S, Johnson L, Fitzpatrick B, Howard M, Adams EJ, Rogers DF, Knight D, Clegg P, Thornton DJ. Proteomic analysis of polymeric salivary mucins: no evidence for MUC19 in human saliva. Biochem J. 2008;413:545–552. doi: 10.1042/BJ20080260. [DOI] [PubMed] [Google Scholar]
- 75.Gururaja TL, Ramasubbu N, Venugopalan P, Reddy MS, Ramalingam K, Levine MJ. Structural features of the human salivary mucin, MUC7. Glycoconj J. 1998;15:457–467. doi: 10.1023/a:1006978818555. [DOI] [PubMed] [Google Scholar]
- 76.Bergey EJ, Cho MI, Hammarskjold ML, Rekosh D, Levine MJ, Blumberg BM, Epstein LG. Aggregation of human immunodeficiency virus type 1 by human salivary secretions. Crit Rev Oral Biol Med. 1993;4:467–474. doi: 10.1177/10454411930040033001. [DOI] [PubMed] [Google Scholar]
- 77.Bergey EJ, Cho MI, Blumberg BM, Hammarskjold ML, Rekosh D, Epstein LG, Levine MJ. Interaction of HIV-1 and human salivary mucins. J Acquir Immune Defic Syndr. 1994;7:995–1002. [PubMed] [Google Scholar]
- 78.Nagashunmugam T, Malamud D, Davis C, Abrams WR, Friedman HM. Human submandibular saliva inhibits human immunodeficiency virus type 1 infection by displacing envelope glycoprotein gp120 from the virus. J Infect Dis. 1998;178:1635–1641. doi: 10.1086/314511. [DOI] [PubMed] [Google Scholar]
- 79.Liu B, Rayment S, Oppenheim FG, Troxler RF. Isolation of human salivary mucin MG2 by a novel method and characterization of its interactions with oral bacteria. Arch Biochem Biophys. 1999;364:286–293. doi: 10.1006/abbi.1999.1141. [DOI] [PubMed] [Google Scholar]
- 80.Liu B, Rayment SA, Gyurko C, Oppenheim FG, Offner GD, Troxler RF. The recombinant N-terminal region of human salivary mucin MG2 (MUC7) contains a binding domain for oral Streptococci and exhibits candidacidal activity. Biochem J. 2000;345:557–564. [PMC free article] [PubMed] [Google Scholar]
- 81.Li S, Intini G, Bobek LA. Modulation of MUC7 mucin expression by exogenous factors in airway cells in vitro and in vivo. Am J Respir Cell Mol Biol. 2006;35:95–102. doi: 10.1165/rcmb.2005-0305OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea. 2003;22:41–45. doi: 10.1097/00003226-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Sasaki M, Nakanuma Y, Ho SB, Kim YS. Cholangiocarcinomas arising in cirrhosis and combined hepatocellular-cholangiocellular carcinomas share apomucin profiles. Am J Clin Pathol. 1998;109:302–308. doi: 10.1093/ajcp/109.3.302. [DOI] [PubMed] [Google Scholar]
- 84.Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA, Engelhardt JF. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol. 1998;19:30–37. doi: 10.1165/ajrcmb.19.1.3054. [DOI] [PubMed] [Google Scholar]
- 85.Zhang S, Zhang HS, Reuter VE, Slovin SF, Scher HI, Livingston PO. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Cancer Res. 1998;4:295–302. [PubMed] [Google Scholar]
- 86.Retz M, Lehmann J, Roder C, Plotz B, Harder J, Eggers J, Pauluschke J, Kalthoff H, Stockle M. Differential mucin MUC7 gene expression in invasive bladder carcinoma in contrast to uniform MUC1 and MUC2 gene expression in both normal urothelium and bladder carcinoma. Cancer Res. 1998;58:5662–5666. [PubMed] [Google Scholar]
- 87.Kim CH, Kim HJ, Song KS, Seong JK, Kim KS, Lee JG, Yoon JH. MUC8 as a ciliated cell marker in human nasal epithelium. Acta Otolaryngol. 2005;125:76–81. doi: 10.1080/00016480410015785. [DOI] [PubMed] [Google Scholar]
- 88.Kerschner JE. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007;117:1666–1676. doi: 10.1097/MLG.0b013e31806db531. [DOI] [PubMed] [Google Scholar]
- 89.Takeuchi K, Yagawa M, Ishinaga H, Kishioka C, Harada T, Majima Y. Mucin gene expression in the effusions of otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2003;67:53–58. doi: 10.1016/s0165-5876(02)00361-0. [DOI] [PubMed] [Google Scholar]
- 90.Seong JK, Koo JS, Lee WJ, Kim HN, Park JY, Song KS, Hong JH, Yoon JH. Upregulation of MUC8 and downregulation of MUC5AC by inflammatory mediators in human nasal polyps and cultured nasal epithelium. Acta Otolaryngol. 2002;122:401–407. doi: 10.1080/00016480260000094. [DOI] [PubMed] [Google Scholar]
- 91.Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA. Cell signaling through membrane mucins. Bioessays. 2003;25:66–71. doi: 10.1002/bies.10201. [DOI] [PubMed] [Google Scholar]
- 92.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 93.Ligtenberg MJ, Vos HL, Gennissen AM, Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990;265:5573–5578. [PubMed] [Google Scholar]
- 94.Wreschner DH, Hareuveni M, Tsarfaty I, Smorodinsky N, Horev J, Zaretsky J, Kotkes P, Weiss M, Lathe R, Dion A. Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms. Eur J Biochem. 1990;189:463–473. doi: 10.1111/j.1432-1033.1990.tb15511.x. [DOI] [PubMed] [Google Scholar]
- 95.Lancaster CA, Peat N, Duhig T, Wilson D, Taylor-Papadimitriou J, Gendler SJ. Structure and expression of the human polymorphic epithelial mucin gene: an expressed VNTR unit. Biochem Biophys Res Commun. 1990;173:1019–1029. doi: 10.1016/s0006-291x(05)80888-5. [DOI] [PubMed] [Google Scholar]
- 96.Peat N, Gendler SJ, Lalani N, Duhig T, Taylor-Papadimitriou J. Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res. 1992;52:1954–1960. [PubMed] [Google Scholar]
- 97.Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci USA. 1988;85:2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Swallow DM, Gendler S, Griffiths B, Kearney A, Povey S, Sheer D, Palmer RW, Taylor-Papadimitriou J. The hypervariable gene locus PUM, which codes for the tumour associated epithelial mucins, is located on chromosome 1, within the region 1q21–24. Ann Hum Genet. 1987;51:289–294. doi: 10.1111/j.1469-1809.1987.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 99.Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988;263:12820–12823. [PubMed] [Google Scholar]
- 100.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6:339–353. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 101.Brayman M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol. 2004;2:4. doi: 10.1186/1477-7827-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee MJ, Lee HS, Kim WH, Choi Y, Yang M. Expression of mucins and cytokeratins in primary carcinomas of the digestive system. Mod Pathol. 2003;16:403–410. doi: 10.1097/01.MP.0000067683.84284.66. [DOI] [PubMed] [Google Scholar]
- 103.Ringel J, Lohr M. The MUC gene family: their role in diagnosis and early detection of pancreatic cancer. Mol Cancer. 2003;2:9. doi: 10.1186/1476-4598-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buisine MP, Devisme L, Degand P, Dieu MC, Gosselin B, Copin MC, Aubert JP, Porchet N. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J Histochem Cytochem. 2000;48:1667–1676. doi: 10.1177/002215540004801210. [DOI] [PubMed] [Google Scholar]
- 105.Masaki Y, Oka M, Ogura Y, Ueno T, Nishihara K, Tangoku A, Takahashi M, Yamamoto M, Irimura T. Sialylated MUC1 mucin expression in normal pancreas, benign pancreatic lesions, and pancreatic ductal adenocarcinoma. Hepatogastroenterology. 1999;46:2240–2245. [PubMed] [Google Scholar]
- 106.Terada T, Ohta T, Sasaki M, Nakanuma Y, Kim YS. Expression of MUC apomucins in normal pancreas and pancreatic tumours. J Pathol. 1996;180:160–165. doi: 10.1002/(SICI)1096-9896(199610)180:2<160::AID-PATH625>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 107.Burdick MD, Harris A, Reid CJ, Iwamura T, Hollingsworth MA. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem. 1997;272:24198–24202. doi: 10.1074/jbc.272.39.24198. [DOI] [PubMed] [Google Scholar]
- 108.Girling A, Bartkova J, Burchell J, Gendler S, Gillett C, Taylor-Papadimitriou J. A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int J Cancer. 1989;43:1072–1076. doi: 10.1002/ijc.2910430620. [DOI] [PubMed] [Google Scholar]
- 109.Ajioka Y, Allison LJ, Jass JR. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J Clin Pathol. 1996;49:560–564. doi: 10.1136/jcp.49.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zotter S, Lossnitzer A, Hageman PC, Delemarre JF, Hilkens J, Hilgers J. Immunohistochemical localization of the epithelial marker MAM-6 in invasive malignancies and highly dysplastic adenomas of the large intestine. Lab Invest. 1987;57:193–199. [PubMed] [Google Scholar]
- 111.Kim YS, Gum J, Jr, Brockhausen I. Mucin glycoproteins in neoplasia. Glycoconj J. 1996;13:693–707. doi: 10.1007/BF00702333. [DOI] [PubMed] [Google Scholar]
- 112.Taylor-Papadimitriou J, Burchell JM, Plunkett T, Graham R, Correa I, Miles D, Smith M. MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia. 2002;7:209–221. doi: 10.1023/a:1020360121451. [DOI] [PubMed] [Google Scholar]
- 113.Yin L, Li Y, Ren J, Kuwahara H, Kufe D. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem. 2003;278:35458–35464. doi: 10.1074/jbc.M301987200. [DOI] [PubMed] [Google Scholar]
- 114.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 115.Fox MF, Lahbib F, Pratt W, Attwood J, Gum J, Kim Y, Swallow DM. Regional localization of the intestinal mucin gene MUC3 to chromosome 7q22. Ann Hum Genet. 1992;56:281–287. doi: 10.1111/j.1469-1809.1992.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 116.Crawley SC, Gum JR, Jr, Hicks JW, Pratt WS, Aubert JP, Swallow DM, Kim YS. Genomic organization and structure of the 3′ region of human MUC3: alternative splicing predicts membrane-bound and soluble forms of the mucin. Biochem Biophys Res Commun. 1999;263:728–736. doi: 10.1006/bbrc.1999.1466. [DOI] [PubMed] [Google Scholar]
- 117.Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert JP. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J. 1999;338:325–333. [PMC free article] [PubMed] [Google Scholar]
- 118.Khorrami AM, Choudhury A, Andrianifahanana M, Varshney GC, Bhattacharyya SN, Hollingsworth MA, Kaufman B, Batra SK. Purification and characterization of a human pancreatic adenocarcinoma mucin. J Biochem. 2002;131:21–29. doi: 10.1093/oxfordjournals.jbchem.a003073. [DOI] [PubMed] [Google Scholar]
- 119.Gross MS, Guyonnet-Duperat V, Porchet N, Bernheim A, Aubert JP, Nguyen VC. Mucin 4 (MUC4) gene: regional assignment (3q29) and RFLP analysis. Ann Genet. 1992;35:21–26. [PubMed] [Google Scholar]
- 120.Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, Yamamoto Y, Munger W, Sun HW, Falk RJ, Jennette JC, Alcorta DA, Li H, Yamamoto T, Saito Y, Nakamura M. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem. 2004;279:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 121.Escande F, Lemaitre L, Moniaux N, Batra SK, Aubert JP, Buisine MP. Genomic organization of MUC4 mucin gene. Towards the characterization of splice variants. Eur J Biochem. 2002;269:3637–3644. doi: 10.1046/j.1432-1033.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- 122.Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- 123.Juusola J, Ballantyne J. Multiplex mRNA profiling for the identification of body fluids. Forensic Sci Int. 2005;152:1–12. doi: 10.1016/j.forsciint.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 124.Gipson IK. Mucins of the human endocervix. Front Biosci. 2001;6:D1245–D1255. doi: 10.2741/gipson. [DOI] [PubMed] [Google Scholar]
- 125.Liu B, Offner GD, Nunes DP, Oppenheim FG, Troxler RF. MUC4 is a major component of salivary mucin MG1 secreted by the human submandibular gland. Biochem Biophys Res Commun. 1998;250:757–761. doi: 10.1006/bbrc.1998.9390. [DOI] [PubMed] [Google Scholar]
- 126.Reid CJ, Gould S, Harris A. Developmental expression of mucin genes in the human respiratory tract. Am J Respir Cell Mol Biol. 1997;17:592–598. doi: 10.1165/ajrcmb.17.5.2798. [DOI] [PubMed] [Google Scholar]
- 127.Balague C, Audie JP, Porchet N, Real FX. In situ hybridization shows distinct patterns of mucin gene expression in normal, benign, and malignant pancreas tissues. Gastroenterology. 1995;109:953–964. doi: 10.1016/0016-5085(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 128.Arul GS, Moorghen M, Myerscough N, Alderson DA, Spicer RD, Corfield AP. Mucin gene expression in Barrett's oesophagus: an in situ hybridisation and immunohistochemical study. Gut. 2000;47:753–761. doi: 10.1136/gut.47.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buisine MP, Desreumaux P, Debailleul V, Gambiez L, Geboes K, Ectors N, Delescaut MP, Degand P, Aubert JP, Colombel JF, Porchet N. Abnormalities in mucin gene expression in Crohn's disease. Inflamm Bowel Dis. 1999;5:24–32. doi: 10.1097/00054725-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 130.Buisine MP, Desreumaux P, Leteurtre E, Copin MC, Colombel JF, Porchet N, Aubert JP. Mucin gene expression in intestinal epithelial cells in Crohn's disease. Gut. 2001;49:544–551. doi: 10.1136/gut.49.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buisine MP, Devisme L, Degand P, Dieu MC, Gosselin B, Copin MC, Aubert JP, Porchet N. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J Histochem Cytochem. 2000;48:1667–1676. doi: 10.1177/002215540004801210. [DOI] [PubMed] [Google Scholar]
- 132.Handra-Luca A, Lamas G, Bertrand JC, Fouret P. MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: diagnostic and prognostic implications. Am J Surg Pathol. 2005;29:881–889. doi: 10.1097/01.pas.0000159103.95360.e8. [DOI] [PubMed] [Google Scholar]
- 133.Llinares K, Escande F, Aubert S, Buisine MP, de BC, Batra SK, Gosselin B, Aubert JP, Porchet N, Copin MC. Diagnostic value of MUC4 immunostaining in distinguishing epithelial mesothelioma and lung adenocarcinoma. Mod Pathol. 2004;17:150–157. doi: 10.1038/modpathol.3800027. [DOI] [PubMed] [Google Scholar]
- 134.Copin MC, Buisine MP, Devisme L, Leroy X, Escande F, Gosselin B, Aubert JP, Porchet N. Normal respiratory mucosa, precursor lesions and lung carcinomas: differential expression of human mucin genes. Front Biosci. 2001;6:D1264–D1275. doi: 10.2741/copin. [DOI] [PubMed] [Google Scholar]
- 135.Tsutsumida H, Goto M, Kitajima S, Kubota I, Hirotsu Y, Wakimoto J, Batra SK, Imai K, Yonezawa S. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer. 2007;55:195–203. doi: 10.1016/j.lungcan.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 136.Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, Buchler MW, Aubert JP, Batra SK. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 137.Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 138.Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633–1638. doi: 10.1038/sj.bjc.6602163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chauhan SC, Singh AP, Ruiz F, Johansson SL, Jain M, Smith LM, Moniaux N, Batra SK. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod Pathol. 2006;19:1386–1394. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- 140.Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–436. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 141.Rakha EA, Boyce RW, bd El-Rehim D, Kurien T, Green AR, Paish EC, Robertson JF, Ellis IO. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–1304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 142.Leikauf GD, Borchers MT, Prows DR, Simpson LG. Mucin apoprotein expression in COPD. Chest. 2002;121:166S–182S. doi: 10.1378/chest.121.5_suppl.166s. [DOI] [PubMed] [Google Scholar]
- 143.Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083–4089. [PubMed] [Google Scholar]
- 144.Packer LM, Williams SJ, Callaghan S, Gotley DC, McGuckin MA. Expression of the cell surface mucin gene family in adenocarcinomas. Int J Oncol. 2004;25:1119–1126. [PubMed] [Google Scholar]
- 145.Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 146.Walsh MD, Young JP, Leggett BA, Williams SH, Jass JR, McGuckin MA. The MUC13 cell surface mucin is highly expressed by human colorectal carcinomas. Hum Pathol. 2007;38:883–892. doi: 10.1016/j.humpath.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 147.Pallesen LT, Berglund L, Rasmussen LK, Petersen TE, Rasmussen JT. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. Eur J Biochem. 2002;269:2755–2763. doi: 10.1046/j.1432-1033.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- 148.Pallesen LT, Pedersen LR, Petersen TE, Knudsen CR, Rasmussen JT. Characterization of human mucin (MUC15) and identification of ovine and caprine orthologs. J Dairy Sci. 2008;91:4477–4483. doi: 10.3168/jds.2008-1204. [DOI] [PubMed] [Google Scholar]
- 149.Huang J, Che MI, Huang YT, Shyu MK, Huang YM, Wu YM, Lin WC, Huang PH, Liang JT, Lee PH, Huang MC. Overexpression of MUC15 activates extracellular signal-regulated kinase 1/2 and promotes the oncogenic potential of human colon cancer cells. Carcinogenesis. 2009;30:1452–1458. doi: 10.1093/carcin/bgp137. [DOI] [PubMed] [Google Scholar]
- 150.Maeda T, Inoue M, Koshiba S, Yabuki T, Aoki M, Nunokawa E, Seki E, Matsuda T, Motoda Y, Kobayashi A, Hiroyasu F, Shirouzu M, Terada T, Hayami N, Ishizuka Y, Shinya N, Tatsuguchi A, Yoshida M, Hirota H, Matsuo Y, Tani K, Arakawa T, Carninci P, Kawai J, Hayashizaki Y, Kigawa T, Yokoyama S. Solution structure of the SEA domain from the murine homologue of ovarian cancer antigen CA125 (MUC16) J Biol Chem. 2004;279:13174–13182. doi: 10.1074/jbc.M309417200. [DOI] [PubMed] [Google Scholar]
- 151.O'Brien TJ, Beard JB, Underwood LJ, Shigemasa K. The CA 125 gene: a newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol. 2002;23:154–169. doi: 10.1159/000064032. [DOI] [PubMed] [Google Scholar]
- 152.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 153.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, Gipson IK. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 154.Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, Baggerly KA, Atkinson EN, Skates S, Zhang Z, Lokshin A, Menon U, Jacobs I, Lu K. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl 3):274–281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 155.Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083–4089. [PubMed] [Google Scholar]
- 156.Gum JR, Jr, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun. 2002;291:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- 157.Van Klinken BJ, Van Dijken TC, Oussoren E, Buller HA, Dekker J, Einerhand AW. Molecular cloning of human MUC3 cDNA reveals a novel 59 amino acid tandem repeat region. Biochem Biophys Res Commun. 1997;238:143–148. doi: 10.1006/bbrc.1997.7258. [DOI] [PubMed] [Google Scholar]
- 158.Moniaux N, Junker WM, Singh AP, Jones AM, Batra SK. Characterization of human mucin MUC17. Complete coding sequence and organization. J Biol Chem. 2006;281:23676–23685. doi: 10.1074/jbc.M600302200. [DOI] [PubMed] [Google Scholar]
- 159.Malmberg EK, Pelaseyed T, Petersson AC, Seidler UE, De JH, Riordan JR, Hansson GC. The C-terminus of the transmembrane mucin MUC17 binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine. Biochem J. 2008;410:283–289. doi: 10.1042/BJ20071068. [DOI] [PubMed] [Google Scholar]
- 160.Corrales RM, Galarreta DJ, Herreras JM, Calonge M, Chaves FJ. Normal human conjunctival epithelium expresses MUC13, MUC15, MUC16 and MUC17 mucin genes. Arch Soc Esp Oftalmol. 2003;78:375–381. [PubMed] [Google Scholar]
- 161.Li GS, Zhang H, Lu JC, Hou P, Zhou Y, Ma XZ, Wang HY. Variable number of tandem repeats polymorphism of MUC20 is associated with the progression of IgA nephropathy. Zhonghua Yi Xue Za Zhi. 2005;85:1333–1338. [PubMed] [Google Scholar]
- 162.Keicho N, Ohashi J, Tamiya G, Nakata K, Taguchi Y, Azuma A, Ohishi N, Emi M, Park MH, Inoko H, Tokunaga K, Kudoh S. Fine localization of a major disease-susceptibility locus for diffuse panbronchiolitis. Am J Hum Genet. 2000;66:501–507. doi: 10.1086/302786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Itoh Y, Kamata-Sakurai M, da-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M, Irimura T. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 164.Devine PL, McKenzie IF. Mucins: structure, function, and associations with malignancy. Bioessays. 1992;14:619–625. doi: 10.1002/bies.950140909. [DOI] [PubMed] [Google Scholar]
- 165.Apostolopoulos V, McKenzie IF. Cellular mucins: targets for immunotherapy. Crit Rev Immunol. 1994;14:293–309. doi: 10.1615/critrevimmunol.v14.i3-4.40. [DOI] [PubMed] [Google Scholar]
- 166.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–2860. [PubMed] [Google Scholar]
- 167.Inufusa H, Nakatani Y, Adachi T, Wakano T, Nakajima A, Nakamura M, Suzuki M, Ando O, Kurimoto M, Miyake M, Shindo K, Yasutomi M. Correlation of prognosis of breast cancer patients and expression of Ley which acts as a cofactor of tumor procoagulant. Int J Oncol. 1998;13:481–487. doi: 10.3892/ijo.13.3.481. [DOI] [PubMed] [Google Scholar]
- 168.Leivonen M, Nordling S, Lundin J, von BK, Haglund C. p27 expression correlates with short-term, but not with long-term prognosis in breast cancer. Breast Cancer Res Treat. 2001;67:15–22. doi: 10.1023/a:1010623326118. [DOI] [PubMed] [Google Scholar]
- 169.Nakagoe T, Fukushima K, Itoyanagi N, Ikuta Y, Oka T, Nagayasu T, Ayabe H, Hara S, Ishikawa H, Minami H. Expression of ABH/Lewis-related antigens as prognostic factors in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:257–264. doi: 10.1007/s00432-002-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yamaguchi A, Ding K, Maehara M, Goi T, Nakagawara G. Expression of nm23-H1 gene and Sialyl Lewis X antigen in breast cancer. Oncology. 1998;55:357–362. doi: 10.1159/000011878. [DOI] [PubMed] [Google Scholar]
- 171.Rodriguez de PL, Arnaiz F, Estenoz J, Ortuno B, Lanzos E. Study of serum tumor markers CEA CA 15.3 and CA 27.29 as diagnostic parameters in patients with breast carcinoma. Int J Biol Markers. 1995;10:24–29. doi: 10.1177/172460089501000105. [DOI] [PubMed] [Google Scholar]
- 172.Correale M, Abbate I, Gargano G, Catino A, Dragone CD, Musci MD, Serio G, Paradiso A, De LM. Analytical and clinical evaluation of a new tumor marker in breast cancer: CA 27.29. Int J Biol Markers. 1992;7:43–46. doi: 10.1177/172460089200700106. [DOI] [PubMed] [Google Scholar]
- 173.Tewes M, Aktas B, Welt A, Mueller S, Hauch S, Kimmig R, Kasimir-Bauer S. Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treatment. 2009;115:581–590. doi: 10.1007/s10549-008-0143-x. [DOI] [PubMed] [Google Scholar]
- 174.Dian D, Janni W, Kuhn C, Mayr D, Karsten U, Mylonas I, Friese K, Jeschke U. Evaluation of a novel anti-mucin 1 (MUC1) antibody (PankoMab) as a potential diagnostic tool in human ductal breast cancer; comparison with two established antibodies. Onkologie. 2009;32:238–244. doi: 10.1159/000209280. [DOI] [PubMed] [Google Scholar]
- 175.Sonora C, Mazal D, Berois N, Buisine MP, Ubillos L, Varangot M, Barrios E, Carzoglio J, Aubert JP, Osinaga E. Immunohistochemical analysis of MUC5B apomucin expression in breast cancer and non-malignant breast tissues. J Histochem Cytochem. 2006;54:289–299. doi: 10.1369/jhc.5A6763.2005. [DOI] [PubMed] [Google Scholar]
- 176.Ohuchida K, Mizumoto K, Yamada D, Fujii K, Ishikawa N, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. Quantitative analysis of MUC1 and MUC5AC mRNA in pancreatic juice for preoperative diagnosis of pancreatic cancer. Int J Cancer. 2006;118:405–411. doi: 10.1002/ijc.21317. [DOI] [PubMed] [Google Scholar]
- 177.Xu M, Real FX, Welt S, Schussler MH, Oettgen HF, Old LJ. Expression of TAG-72 in normal colon, transitional mucosa, and colon cancer. Int J Cancer. 1989;44:985–989. doi: 10.1002/ijc.2910440607. [DOI] [PubMed] [Google Scholar]
- 178.Duncan TJ, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Oncol. 2007;5:31. doi: 10.1186/1477-7819-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Manne U, Weiss HL, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. 2000;6:4017–4025. [PubMed] [Google Scholar]
- 180.Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics. 2008;8:3329–3341. doi: 10.1002/pmic.200800040. [DOI] [PubMed] [Google Scholar]
- 181.Wang RQ, Fang DC. Alterations of MUC1 and MUC3 expression in gastric carcinoma: relevance to patient clinicopathological features. J Clin Pathol. 2003;56:378–384. doi: 10.1136/jcp.56.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Choi JS, Lee KMA, Lee HE, Lee HS, Kim WH. Mucinous gastric carcinomas. Cancer. 2009;115:3581–3590. doi: 10.1002/cncr.24422. [DOI] [PubMed] [Google Scholar]
- 183.Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simoes M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 184.Kocer B, Soran A, Kiyak G, Erdogan S, Eroglu A, Bozkurt B, Solak C, Cengiz O. Prognostic significance of mucin expression in gastric carcinoma. Dig Dis Sci. 2004;49:954–964. doi: 10.1023/b:ddas.0000034554.96191.66. [DOI] [PubMed] [Google Scholar]
- 185.Nakamori S, Furukawa H, Hiratsuka M, Iwanaga T, Imaoka S, Ishikawa O, Kabuto T, Sasaki Y, Kameyama M, Ishiguro S, Irimura T. Expression of carbohydrate antigen sialyl Le(a): a new functional prognostic factor in gastric cancer. J Clin Oncol. 1997;15:816–825. doi: 10.1200/JCO.1997.15.2.816. [DOI] [PubMed] [Google Scholar]
- 186.Mayer B, Funke I, Johnson JP. High expression of a Lewis(x)-related epitope in gastric carcinomas indicates metastatic potential and poor prognosis. Gastroenterology. 1996;111:1433–1446. doi: 10.1016/s0016-5085(96)70004-5. [DOI] [PubMed] [Google Scholar]
- 187.Guddo F, Giatromanolaki A, Koukourakis MI, Reina C, Vignola AM, Chlouverakis G, Hilkens J, Gatter KC, Harris AL, Bonsignore G. MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlates with poor survival in node positive patients. J Clin Pathol. 1998;51:667–671. doi: 10.1136/jcp.51.9.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hanaoka J, Kontani K, Sawai S, Ichinose M, Tezuka N, Inoue S, Fujino S, Ohkubo I. Analysis of MUC4 mucin expression in lung carcinoma cells and its immunogenicity. Cancer. 2001;92:2148–2157. doi: 10.1002/1097-0142(20011015)92:8<2148::aid-cncr1557>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 189.Tsutsumida H, Goto M, Kitajima S, Kubota I, Hirotsu Y, Wakimoto J, Batra SK, Imai K, Yonezawa S. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer (Amsterdam, Netherlands) 2007;55:195–203. doi: 10.1016/j.lungcan.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 190.Singh AP, Senapati S, Ponnusamy MP, Jain M, Lele SM, Davis JS, Remmenga S, Batra SK. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 2008;9:1076–1085. doi: 10.1016/S1470-2045(08)70277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fritsche HA, Bast RC. CA 125 in ovarian cancer: advances and controversy. Clin Chem. 1998;44:1379–1380. [PubMed] [Google Scholar]
- 192.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98:737–740. doi: 10.1002/ijc.10250. [DOI] [PubMed] [Google Scholar]
- 193.Giuntoli RL, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58:5546–5550. [PubMed] [Google Scholar]
- 194.Skates SJ, Horick N, Yu Y, Xu FJ, Berchuck A, Havrilesky LJ, deBruijn HW, van derZee AG, Woolas RP, Jacobs IJ, Zhang Z, Bast RC., Jr Preoperative sensitivity and specificity for early-stage ovarian cancer when combining cancer antigen CA-125II, CA 15-3, CA 72-4, and macrophage colony-stimulating factor using mixtures of multivariate normal distributions. J Clin Oncol. 2004;22:4059–4066. doi: 10.1200/JCO.2004.03.091. [DOI] [PubMed] [Google Scholar]
- 195.Metzgar RS, Gaillard MT, Levine SJ, Tuck FL, Bossen EH, Borowitz MJ. Antigens of human pancreatic adenocarcinoma cells defined by murine monoclonal antibodies. Cancer Res. 1982;42:601–608. [PubMed] [Google Scholar]
- 196.Mahvi DM, Seigler HF, Meyers WC, Kalthoff H, Schmiegel WH, Metzgar RS. DU-PAN-2 levels in serum and pancreatic ductal fluids of patients with benign and malignant pancreatic disease. Pancreas. 1988;3:488–493. doi: 10.1097/00006676-198808000-00020. [DOI] [PubMed] [Google Scholar]
- 197.Metzgar RS, Rodriguez N, Finn OJ, Lan MS, Daasch VN, Fernsten PD, Meyers WC, Sindelar WF, Sandler RS, Seigler HF. Detection of a pancreatic cancer-associated antigen (DU-PAN-2 antigen) in serum and ascites of patients with adenocarcinoma. Proc Natl Acad Sci USA. 1984;81:5242–5246. doi: 10.1073/pnas.81.16.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sawabu N, Toya D, Takemori Y, Hattori N, Fukui M. Measurement of a pancreatic cancer-associated antigen (DU-PAN-2) detected by a monoclonal antibody in sera of patients with digestive cancers. Int J Cancer. 1986;37:693–696. doi: 10.1002/ijc.2910370509. [DOI] [PubMed] [Google Scholar]
- 199.Bhargava AK, Petrelli NJ, Karna A, Parshall PL, Fitzpatrick JE, Douglass HO, Herrera L, Bray K, Gaur P. Serum levels of cancer-associated antigen CA-195 in gastrointestinal cancers and its comparison with CA19-9. J Clin Lab Anal. 1989;3:370–377. doi: 10.1002/jcla.1860030610. [DOI] [PubMed] [Google Scholar]
- 200.Magnani JL, Steplewski Z, Koprowski H, Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983;43:5489–5492. [PubMed] [Google Scholar]
- 201.McLaughlin R, O'Hanlon D, Kerin M, Kenny P, Grimes H, Given HF. Are elevated levels of the tumour marker CA19-9 of any clinical significance?--an evaluation. Ir J Med Sci. 1999;168:124–126. doi: 10.1007/BF02946481. [DOI] [PubMed] [Google Scholar]
- 202.Rhodes JM. Usefulness of novel tumour markers. Ann Oncol. 1999;10(Suppl 4):118–121. [PubMed] [Google Scholar]
- 203.Takezako Y, Okusaka T, Ueno H, Ikeda M, Morizane C, Najima M. Tumor markers for pancreatic and biliary tract cancer. Gan To Kagaku Ryoho. 2004;31:1443–1446. [PubMed] [Google Scholar]
- 204.MacLean GD, Reddish MA, Longenecker BM. Prognostic significance of preimmunotherapy serum CA27.29 (MUC-1) mucin level after active specific immunotherapy of metastatic adenocarcinoma patients. J Immunother. 1997;20:70–78. doi: 10.1097/00002371-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 205.Ritts RE, Pitt HA. CA 19-9 in pancreatic cancer. Surg Oncol Clin N Am. 1998;7:93–101. [PubMed] [Google Scholar]
- 206.Eskelinen M, Haglund U. Developments in serologic detection of human pancreatic adenocarcinoma. Scand J Gastroenterol. 1999;34:833–844. doi: 10.1080/003655299750025273. [DOI] [PubMed] [Google Scholar]
- 207.Yonezawa S, Sato E. Expression of mucin antigens in human cancers and its relationship with malignancy potential. Pathol Int. 1997;47:813–830. doi: 10.1111/j.1440-1827.1997.tb03713.x. [DOI] [PubMed] [Google Scholar]
- 208.Choudhury A, Moniaux N, Winpenny JP, Hollingsworth MA, Aubert JP, Batra SK. Human MUC4 mucin cDNA and its variants in pancreatic carcinoma. J Biochem. 2000;128:233–243. doi: 10.1093/oxfordjournals.jbchem.a022746. [DOI] [PubMed] [Google Scholar]
- 209.Jhala N, Jhala D, Vickers SM, Eltoum I, Batra SK, Manne U, Eloubeidi M, Jones JJ, Grizzle WE. Biomarkers in Diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 210.Gold DV, Modrak DE, Ying Z, Cardillo TM, Sharkey RM, Goldenberg DM. New MUC1 serum immunoassay differentiates pancreatic cancer from pancreatitis. J Clin Oncol. 2006;24:252–258. doi: 10.1200/JCO.2005.02.8282. [DOI] [PubMed] [Google Scholar]
- 211.Chakraborty S, Jain M, Sasson AR, Batra SK. MUC4 as a diagnostic marker in cancer. Exp Opin Med Diagn. 2008;2:891–910. doi: 10.1517/17530059.2.8.891. [DOI] [PubMed] [Google Scholar]
- 212.Denardo SJ, Richman CM, Albrecht H, Burke PA, Natarajan A, Yuan A, Gregg JP, O'Donnell RT, Denardo GL. Enhancement of the therapeutic index: from nonmyeloablative and myeloablative toward pretargeted radioimmunotherapy for metastatic prostate cancer. Clin Cancer Res. 2005;11:7187s–7194s. doi: 10.1158/1078-0432.CCR-1004-0013. [DOI] [PubMed] [Google Scholar]
- 213.Walsh MD, Hohn BG, Thong W, Devine PL, Gardiner RA, Samaratunga ML, McGuckin MA. Mucin expression by transitional cell carcinomas of the bladder. Br J Urol. 1994;73:256–262. doi: 10.1111/j.1464-410x.1994.tb07514.x. [DOI] [PubMed] [Google Scholar]
- 214.Tamada S, Goto M, Nomoto M, Nagata K, Shimizu T, Tanaka S, Sakoda K, Imai K, Yonezawa S. Expression of MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: its relationship with tumor progression and prognosis. Pathol Int. 2002;52:713–723. doi: 10.1046/j.1440-1827.2002.01414.x. [DOI] [PubMed] [Google Scholar]
- 215.Anderson KS. Tumor vaccines for breast cancer. Cancer Investig. 2009;27:361–368. doi: 10.1080/07357900802574421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci USA. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ding L, Lalani EN, Reddish M, Koganty R, Wong T, Samuel J, Yacyshyn MB, Meikle A, Fung PY, Taylor-Papadimitriou J. Immunogenicity of synthetic peptides related to the core peptide sequence encoded by the human MUC1 mucin gene: effect of immunization on the growth of murine mammary adenocarcinoma cells transfected with the human MUC1 gene. Cancer Immunol Immunother. 1993;36:9–17. doi: 10.1007/BF01789125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Finn OJ. Premalignant lesions as targets for cancer vaccines. J Exp Med. 2003;198:1623–1626. doi: 10.1084/jem.20031787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, Moynahan M, Houghton A, Norton L, Livingston PO. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 220.Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- 221.Ho JJ. Mucins in the diagnosis and therapy of pancreatic cancer. Curr Pharm Des. 2000;6:1881–1896. doi: 10.2174/1381612003398609. [DOI] [PubMed] [Google Scholar]
- 222.Ioannides CG, Fisk B, Jerome KR, Irimura T, Wharton JT, Finn OJ. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol. 1993;151:3693–3703. [PubMed] [Google Scholar]
- 223.Jerome KR, Barnd DL, Bendt KM, Boyer CM, Taylor-Papadimitriou J, McKenzie IF, Bast RC, Jr, Finn OJ. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991;51:2908–2916. [PubMed] [Google Scholar]
- 224.Oei AL, Moreno M, Verheijen RH, Sweep FC, Thomas CM, Massuger LF, von Mensdorff-Pouilly S. Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. Int J Cancer. 2008;123:1848–1853. doi: 10.1002/ijc.23725. [DOI] [PubMed] [Google Scholar]
- 225.Pecher G, Haring A, Kaiser L, Thiel E. Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol Immunother. 2002;51:669–673. doi: 10.1007/s00262-002-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Snijdewint FG, von Mensdorff-Pouilly S, Karuntu-Wanamarta AH, Verstraeten AA, Livingston PO, Hilgers J, Kenemans P. Antibody-dependent cell-mediated cytotoxicity can be induced by MUC1 peptide vaccination of breast cancer patients. Int J Cancer. 2001;93:97–106. doi: 10.1002/ijc.1286. [DOI] [PubMed] [Google Scholar]
- 227.Reddish M, MacLean GD, Koganty RR, Kan-Mitchell J, Jones V, Mitchell MS, Longenecker BM. Anti-MUC1 class I restricted CTLs in metastatic breast cancer patients immunized with a synthetic MUC1 peptide. Int J Cancer. 1998;76:817–823. doi: 10.1002/(sici)1097-0215(19980610)76:6<817::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 228.Karanikas V, Hwang LA, Pearson J, Ong CS, Apostolopoulos V, Vaughan H, Xing PX, Jamieson G, Pietersz G, Tait B, Broadbent R, Thynne G, McKenzie IF. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Investig. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Apostolopoulos V, Pietersz GA, Tsibanis A, Tsikkinis A, Drakaki H, Loveland BE, Piddlesden SJ, Plebanski M, Pouniotis DS, Alexis MN, McKenzie IF, Vassilaros S. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835] Breast Cancer Res. 2006;8:R27. doi: 10.1186/bcr1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Scholl SM, Balloul JM, Le Goc G, Bizouarne N, Schatz C, Kieny MP, von Mensdorff-Pouilly S, Vincent-Salomon A, Deneux L, Tartour E, Fridman W, Pouillart P, Acres B. Recombinant vaccinia virus encoding human MUC1 and IL2 as immunotherapy in patients with breast cancer. J Immunother. 2000;23:570–580. doi: 10.1097/00002371-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 231.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 232.O'Boyle KP, Zamore R, Adluri S, Cohen A, Kemeny N, Welt S, Lloyd KO, Oettgen HF, Old LJ, Livingston PO. Immunization of colorectal cancer patients with modified ovine submaxillary gland mucin and adjuvants induces IgM and IgG antibodies to sialylated Tn. Cancer Res. 1992;52:5663–5667. [PubMed] [Google Scholar]
- 233.Adluri S, Helling F, Ogata S, Zhang S, Itzkowitz SH, Lloyd KO, Livingston PO. Immunogenicity of synthetic TF-KLH (keyhole limpet hemocyanin) and sTn-KLH conjugates in colorectal carcinoma patients. Cancer Immunol Immunother. 1995;41:185–192. doi: 10.1007/BF01521345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, Kim DW, Marshall J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Trans Med. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, Remondo C, Cereda V, Jones JL, Pazdur MP, Higgins JP, Hodge JW, Steinberg SM, Kotz H, Dahut WL, Schlom J. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Raez LE, Fein S, Podack ER. Lung cancer immunotherapy. Clin Med Res. 2005;3:221–228. doi: 10.3121/cmr.3.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.West HJ. Novel targeted agents for lung cancer. Clin Lung Cancer. 2009;10(Suppl 1):S41–S46. doi: 10.3816/CLC.2009.s.007. [DOI] [PubMed] [Google Scholar]
- 238.Palmer M, Parker J, Modi S, Butts C, Smylie M, Meikle A, Kehoe M, MacLean G, Longenecker M. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer. 2001;3:49–57. doi: 10.3816/clc.2001.n.018. [DOI] [PubMed] [Google Scholar]
- 239.Soulieres D, Murray N, Maksymiuk A, Marshall E, Goss G, Butts C. A liposomal MUC1 vaccine for treatment of non-small cell lung cancer (NSCLC): differences in QOL assessments for Stage IIIBLR and IV patients. J Clin Oncol 2005 ASCO Meeting Proceedings. 2005;23:7209. [Google Scholar]
- 240.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res. 2007;13:s4652–s4654. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]
- 241.Richards ER, Devine PL, Quin RJ, Fontenot JD, Ward BG, McGuckin MA. Antibodies reactive with the protein core of MUC1 mucin are present in ovarian cancer patients and healthy women. Cancer Immunol Immunother. 1998;46:245–252. doi: 10.1007/s002620050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, Hensley ML, Spassova MK, Ouerfelli O, Spriggs DR, Tew WP, Konner J, Clausen H, bu Rustum N, Dansihefsky SJ, Livingston PO. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13:4170–4177. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 243.Diamantidis M, Tsapournas G, Kountouras J, Zavos C. New aspects of regulatory signaling pathways and novel therapies in pancreatic cancer. Current Mol Med. 2008;8:12–37. doi: 10.2174/156652408783565586. [DOI] [PubMed] [Google Scholar]
- 244.Wei J, Gao W, Wu J, Meng K, Zhang J, Chen J, Miao Y. Dendritic cells expressing a combined PADRE/MUC4-derived polyepitope DNA vaccine induce multiple cytotoxic T-cell responses. Cancer Biother Radiopharm. 2008;23:121–128. doi: 10.1089/cbr.2007.0427. [DOI] [PubMed] [Google Scholar]
- 245.Li Y, Cozzi PJ. MUC1 is a promising therapeutic target for prostate cancer therapy. Current Cancer Drug Targets. 2007;7:259–271. doi: 10.2174/156800907780618338. [DOI] [PubMed] [Google Scholar]
- 246.Pantuck AJ, vanOphoven A, Gitlitz BJ, Tso CL, Acres B, Squiban P, Ross ME, Belldegrun AS, Figlin RA. Phase I trial of antigen-specific gene therapy using a recombinant vaccinia virus encoding MUC-1 and IL-2 in MUC-1-positive patients with advanced prostate cancer. J Immunother. 2004;27:240–253. doi: 10.1097/00002371-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 247.North SA, Graham K, Bodnar D, Venner P. A Pilot Study of the Liposomal MUC1 Vaccine BLP25 in Prostate Specific Antigen Failures After Radical Prostatectomy. J Urol. 2006;176:91–95. doi: 10.1016/S0022-5347(06)00494-0. [DOI] [PubMed] [Google Scholar]
- 248.Loveland BE, Zhao A, White S, Gan H, Hamilton K, Xing PX, Pietersz GA, Apostolopoulos V, Vaughan H, Karanikas V, Kyriakou P, McKenzie IF, Mitchell PL. Mannan-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–877. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- 249.Wierecky J, Muller MR, Wirths S, Halder-Oehler E, Dorfel D, Schmidt SM, Hantschel M, Brugger W, Schroder S, Horger MS, Kanz L, Brossart P. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910–5918. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]
- 250.Ferreira CS, Cheung MC, Missailidis S, Bisland S, Gariepy J. Phototoxic aptamers selectively enter and kill epithelial cancer cells. Nucleic Acids Res. 2009;37:866–876. doi: 10.1093/nar/gkn967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Albrecht H, Denardo GL, Denardo SJ. Development of anti-MUC1 di-scFvs for molecular targeting of epithelial cancers, such as breast and prostate cancers. Q J Nucl Med Mol Imaging. 2007;51:304–313. [PubMed] [Google Scholar]
- 252.Epenetos AA, Hird V, Lambert H, Mason P, Coulter C. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int J Gynecol Cancer. 2000;10:44–46. doi: 10.1046/j.1525-1438.2000.99510.x. [DOI] [PubMed] [Google Scholar]
- 253.Barratt-Boyes SM, Vlad A, Finn OJ. Immunization of chimpanzees with tumor antigen MUC1 mucin tandem repeat peptide elicits both helper and cytotoxic T-cell responses. Clin Cancer Res. 1999;5:1918–1924. [PubMed] [Google Scholar]
- 254.Julien S, Picco G, Sewell R, Vercoutter-Edouart AS, Tarp M, Miles D, Clausen H, Taylor-Papadimitriou J, Burchell JM. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100:1746–1754. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mukherjee P, Pathangey LB, Bradley JB, Tinder TL, Basu GD, Akporiaye ET, Gendler SJ. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25:1607–1618. doi: 10.1016/j.vaccine.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chen X, Gao W, Gambotto A, Finn OJ. Lentiviral vectors encoding human MUC1-specific, MHC-unrestricted single-chain TCR and a fusion suicide gene: potential for universal and safe cancer immunotherapy. Cancer Immunol Immunother. 2009;58:977–987. doi: 10.1007/s00262-008-0624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Reinartz S, Hombach A, Kohler S, Schlebusch H, Wallwiener D, Abken H, Wagner U. Interleukin-6 fused to an anti-idiotype antibody in a vaccine increases the specific humoral immune response against CA125+ (MUC-16) ovarian cancer. Cancer Res. 2003;63:3234–3240. [PubMed] [Google Scholar]
- 258.Xu Y, Zhang L, Hu G. Potential application of alternatively glycosylated serum MUC1 and MUC5AC in gastric cancer diagnosis. Biologicals. 2009;37:18–25. doi: 10.1016/j.biologicals.2008.08.002. [DOI] [PubMed] [Google Scholar]


