Abstract
Relaxin has been shown previously to stimulate cyclic AMP production and the activation of MAPK. We reported that phosphoinositide-3 kinase (PI3K) activity is required for biphasic stimulation of cAMP by relaxin and that relaxin treatment increased PI3K activity in THP-1 cells. A downstream target of PI3K is protein kinase C zeta (PKCζ). Relaxin stimulated translocation of PKCζ to the plasma membrane in THP-1, MCF-7, pregnant human myometrial (PHM1-31), and mouse mesangial (MMC) cells. PKCζ translocation is PI3K dependent and independent of cAMP production. Pharmacological and antisense approaches, utilized to inhibit or knock down PKCζ, resulted in a 40% inhibition of relaxin-stimulated cAMP production. The stimulation of PKCζ by relaxin therefore is downstream of PI3K leading to increased cAMP production. To determine the role of PI3K/PKCζ stimulation by relaxin on downstream-mediated events, we examined the increase in vascular endothelial growth factor (VEGF) gene expression by relaxin. Treatment of THP-1 or MMC cells with the PI3K inhibitor, LY294002, abolished the relaxin-mediated stimulation of VEGF transcript levels. In summary, relaxin has pleiotropic signaling effects in THP-1 cells activating ERK1/2, cAMP, PI3K, and PKCζ. We have described a novel bifurcated pathway by which relaxin stimulates Gs alpha and PI3K/PKCζ leading to increased cAMP production and increased VEGF gene expression. Some, but not all, of these pathways are detected in other cell lines which may cause the unique diversity of downstream responses from this interesting hormone.
Keywords: adenylyl cyclase, PI3K, PKC zeta, relaxin, cyclic AMP
Relaxin Increases cAMP
Early studies of the mechanism of relaxin action indicated that it increased cAMP and activated PKA, notably in a biphasic manner.1–6 In addition, a weak stimulation of myometrial adenylyl cyclase by relaxin was noted.7 Since that time, several studies have confirmed the involvement of cAMP and the activation of PKA in specific effects of relaxin, including those in human endometrial cells, the human monocytic line THP-1, and human endometrial stroma cells.8–12 The reversal of the following effects of relaxin by PKA inhibitors have been reported: inhibition of oxytocin-stimulated contractions of rat myometrial strips, oxytocin-stimulated phosphatidylinositide (PI) turnover and oxytocin-stimulated increases in intracellular calcium in rat myometrium and in the immortalized human myometrial cell line PHM1-41, and activation of maxi-K channels in PHM1-41 cells.9
We have also examined cAMP production in THP-1 cells. The stimulation of cAMP by relaxin in THP-1 cells is highly synergistic with low levels of forskolin, similar to the synergy exhibited in rat myometrial cells.5 Whole-cell treatment of THP-1 cells with relaxin produced a biphasic time course for cAMP accumulation, with a clear peak at 1 min and a second increase in cAMP accumulation at 10–20 min.13 This pattern was consistent with time courses in rat myometrial cells and uterine tissue in which small but reproducible increases were observed at 5 min and a clear peak in cAMP accumulation at 20 min.2,5 A similar biphasic curve was also noted for PKA activation.4 Although, phosphodiesterase (PDE) inhibitors increased the total cAMP levels in THP-1 cells, the stimulation of cAMP by relaxin was independent of changes in phosphodiesterase activity,13 ruling out a direct inhibition of PDE activity by relaxin. This biphasic time course suggests that multiple pathways may be involved in cAMP production. Functional studies of the relaxin receptors (LGR7 and LGR814) indicate that the rapid first peak is likely caused by Gsα-stimulated AC activity, because hormonal stimulation of cAMP via Gsα-coupled receptors is generally very fast (1–2 min).15 However, the second wave of cAMP is not consistent with the effects observed with most Gsα-coupled receptors.15,16
Relaxin Increases PI3K
The unique biphasic nature of cAMP accumulation observed in THP-1 and myometrial cells and uterine tissue suggested activation of AC by more than one mechanism.2,5,13 We showed that inhibitors of PI3K (LY294002 and wortmannin) partially blocked relaxin-mediated increases in cAMP in THP-1 cells but had no effect on the stimulation of AC by forskolin or isoproterenol (a β-adrenergic agonist),13 indicating potential unique properties of relaxin receptor signaling. The inhibition of cAMP production by LY294002 occurs across a wide range of relaxin concentrations (1–500 ng/mL13). Both LY294002 and wortmannin preferentially inhibited the second wave of cAMP production.13 The first peak of relaxin-stimulated cAMP accumulation was inhibited by 20–30%, whereas the second wave of cAMP production was inhibited by more than 70% by LY294002.
We postulated that the partial block of cAMP is a consequence of the stimulation of multiple pathways by relaxin. Activation of Gsα by relaxin is rapid and insensitive to the effects of PI3K inhibitors. The second pathway leading to an increase in cAMP requires PI3K activation. Additional evidence for these two mechanisms is derived from in vitro studies of relaxin with purified plasma membrane preparations. Relaxin generated a 25% increase in AC activity in isolated plasma membrane preparations from rat myometrium.7 Relaxin also stimulated cAMP production in plasma membrane preparations from THP-1 cells which was relatively insensitive to the PI3K inhibitor LY294002.13 PI3K is mainly cytosolic and is translocated to the plasma membrane upon activation.17 The presence of PI3K therefore was not expected in our membrane preparations. The increase in cAMP in membranes must be caused by an activation of Gsα by the LGR7/8 relaxin receptor. If we added back cytosol derived from THP-1 cells to our membrane preparations, we reconstituted an LY294002-sensitive relaxin response. The addition of cytosol to membranes had no effect on isoproterenol-stimulated cAMP production. In reconstituted membranes and cytosol, inhibitors of PI3K only partially blocked the increase in cAMP production by relaxin as was previously observed in whole cells. Thus, we proposed two potential pathways for relaxin: a Gsα membrane–delimited pathway and a second pathway that we hypothesized is mediated via the βγ subunits of Gs, leading to activation of PI3K.
Relaxin stimulation of THP-1 cells also increased the activity of PI3K for the substrate phosphoinositide by 1.6-fold.13 This stimulation was completely reversed by treatment with the PI3K inhibitor wortmannin. It is not completely surprising that relaxin can stimulate PI3K. Phosphoinositide-3 kinases are lipid kinases that phosphorylate the 3′-OH group of the inositol ring in phospholipids.18 Several members of class I PI3Ks can be stimulated by G-protein–coupled receptors, including Gs-coupled receptors,19–21 presumably by recruitment to the plasma membrane upon binding Gβγ.20 The preferred substrate in intact cells for class I PI3Ks is phosphatidylinositol 4,5-bisphosphate leading to the generation of phosphatidylinositol 3,4,5-trisphosphate (commonly known as PIP3), an important cellular second messenger.
Relaxin Increases PKCζ
A key link between PI3K and AC activation may be protein kinase C zeta (PKCζ). The PI3K product, PIP3, stimulates the activity of several downstream signaling molecules, including PKCζ which was shown previously to directly phosphorylate and activate adenylyl cyclase.22 More recently,23 we used pharmacological and antisense approaches to inhibit or knock down PKCζ activity, resulting in a 40% inhibition of relaxin stimulation of cAMP. Immunofluorescence microscopy was used to examine relaxin-mediated PKCζ translocation to the plasma membrane.23 Relaxin stimulated translocation of PKCζ to the plasma membrane in THP-1 cells, a breast cancer cell line (MCF-7), pregnant human myometrial (PHM1-3124), and mouse mesangial cells (MMCs25). These cell lines all respond to relaxin in a variety of ways. Relaxin increased cAMP and vascular endothelial growth factor (VEGF) mRNA in THP-1 cells26,27; differentiated MCF-7 cells28; inhibited oxytocin-stimulated increase in Ca2+ and PI turnover and activated maxi-K channels in PHM1-31 cells9; and degraded fibronectin and collagen in MMC cells.29 PKCζ translocation was confirmed by confocal microscopy and was PI3K dependent and independent of cAMP production. Thus, relaxin stimulates PKCζ, downstream of PI3K, to enhance cAMP production.
Relaxin Increases MAPK
Cyclic AMP, PI3K, and PKCζ are certainly not the only pathways stimulated by relaxin. Previously, Unemori's group showed that relaxin stimulates ERK activation in THP-1, pulmonary and coronary artery cells, and human endometrial stromal cells.30 In addition, Dschietzig et al. demonstrated ERK1/2 activation in human umbilical vein endothelical cells and HeLa cells.31 Both groups demonstrate downstream consequences as a result of MAPK activation by relaxin. In THP-1 cells, MEK inhibitors block relaxin-mediated increases in VEGF transcript levels. In human umbilical vein endothelical cells and HeLa cells, inhibition of the ERK pathway blocked NF-κB translocation and upregulation of the endothelin type-B receptor by relaxin. Thus, the ability of relaxin to activate pathways in addition to cAMP is important to downstream-mediated events.
Is Activation of PI3K or PKCζ Required for Downstream Signaling by Relaxin?
Although relaxin stimulates PI3K and PKCζ in a number of cell types, it is unknown whether these pathways are required for relaxin-mediated effects. We have focused on regulation of VEGF and matrix metalloprotease (MMP) gene expression by relaxin. Relaxin upregulates VEGF mRNA levels at wound sites27 and in human endometrial32 and THP-127 cells. Relaxin stimulates MMP-1, -3, and -9,33 increasing MMP-9 expression in uterine and cervical tissues and breast cancer cell lines.34,35 We also observe an enhancement of VEGF and MMP-9 transcription in THP-1 cells (Fig. 1).
Figure 1.
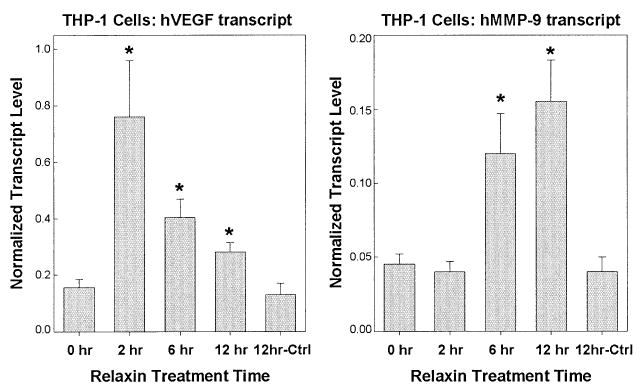
Relaxin stimulates VEGF and MMP-9 gene expression in THP-1 cells. THP-1 cells were serum-starved for 12 hours with 1% fetal bovine serum and then treated with 0.5 mg/mL relaxin or control buffer (Ctrl) in duplicate for 0, 2, 6, or 12 hours. RNA was isolated using RNeasy mini kits (Qiagen, Valencia, CA) and incubated with RNAse-free DNAse for 30 min at 37°C followed by heat inactivation at 75°C for 10 min. Samples (10 ng total RNA) then were analyzed in triplicate by quantitative real-time PCR (Q-RT-PCR) performed by amplification of samples in 96-well plates in an ABI Prism 7700 (Applied Biosystems, Norwalk, CT). Data were analyzed by the use of the Sequence Detection Application software, and the absolute values of human VEGF and MMP-9 transcripts were generated using a standard curve of a known amount of single-stranded DNA run in parallel on the same 96-well plate. All values were corrected for RNA input by normalization to the level of β-actin transcripts. Data are expressed as the mean ± SD from a single experiment and are representative of two different experiments. Significant differences (*P <.05) between groups are designated (t test).
PI3K and PKCζ are also implicated in the transcriptional activation of VEGF and MMPs. PKCζ is essential in smooth muscle cells for the activation of MMP-1,-3, and -9,36 and, in conjunction with PI3K, PKCζ is required for the transcriptional activation of VEGF in renal cell carcinomas.37 ERK pathways have also been implicated in VEGF gene transcription and, as discussed previously, inhibition of ERK activity partially blocks relaxin stimulation of VEGF in THP-1 cells.38,39 The VEGF promoter does not contain a consensus sequence for the cAMP response element; therefore, stimulation by relaxin may potentially occur via activation of ERK and/or PI3K. MMP-9 is regulated by the cAMP response element40 and both PI3K and PKA are important in MMP-9 gene transcription in epithelial breast cells.40,41
Inhibition of PI3K Blocks Relaxin-Stimulated Increases in VEGF
We examined the regulation of VEGF by relaxin in THP-1 and MMC cells. Treatment of THP-1 or MMC cells with the PI3K inhibitor, LY294002, abolished relaxin-stimulation of VEGF transcript levels in both cell lines (Fig. 2). This result suggests that stimulation of VEGF requires PI3K activation in these cell lines. If ERK or cAMP pathways are involved, they either act in conjunction with or downstream of PI3K or represent only a small component of the stimulation by relaxin. Thus, PI3K and potentially PKCζ are an important aspect of the diverse actions of relaxin.
Figure 2.
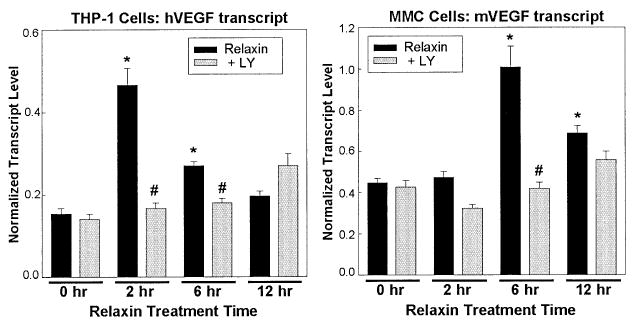
PI3K activity is required for relaxin stimulation of VEGF in THP-1 and MMC cells. THP-1 and MMC cells were serum-starved for 12 hours in 1% fetal bovine serum and pretreated for 15 min with 50 mM LY294002 or vehicle, followed by addition of 0.1 mg/mL relaxin. Triplicate samples were harvested for RNA isolation after 0, 2, 6, or 12 hours. RNA was isolated, DNAse-treated, and subjected to Q-RT-PCR as described in the legend to Figure 1, using β-actin to normalize transcript levels for human (THP-1) and mouse (MMC) VEGF. Significant differences (*,#P < 0.05) between groups are designated (t-test).
Conclusion
In summary, relaxin has pleiotropic signaling effects in THP-1 cells activating ERK1/2, cAMP, PI3K, and PKCζ. We have described a novel bifurcated pathway by which relaxin stimulates Gsα and PI3K/PKCζ leading to increased cAMP production. The stimulation of multiple pathways is an important aspect of relaxin-mediated actions, in that the stimulation of PI3K is required for increased VEGF transcript levels in THP-1 and MMC cells. Some, but not all, of these pathways are detected in other cell lines which may cause the unique diversity of downstream responses from this interesting hormone.
Acknowledgments
This work was supported by National Institutes of Health Grant GM60419 and the Texas Advanced Research Program No. 011618-00590-1999. We thank Drs. Barbara Sanborn and Chun-Ying Ku for all their help and support.
References
- 1.Braddon SA. Relaxin-dependent adenosine 6′,5′-monophosphate concentration changes in the mouse pubic symphysis. Endocrinology. 1978;102:1292–1299. doi: 10.1210/endo-102-4-1292. [DOI] [PubMed] [Google Scholar]
- 2.Sanborn BM, Weisbrodt NW, Kuo HS, Sherwood OD. The interaction of relaxin with the rat uterus. I. Effect of cyclic nucleotide levels and spontaneous contractile activity. Endocrinology. 1980;106:1210–1215. doi: 10.1210/endo-106-4-1210. [DOI] [PubMed] [Google Scholar]
- 3.Cheah SH, Sherwood OD. Target tissues for relaxin in the rat: tissue distribution of injected 125I-labeled relaxin and tissue changes in adenosine 3′,5′-monophosphate levels after in vitro relaxin incubation. Endocrinology. 1980;106:1203–1209. doi: 10.1210/endo-106-4-1203. [DOI] [PubMed] [Google Scholar]
- 4.Sanborn BM, Sherwood OD. Effect of relaxin on bound cAMP in rat uterus. Endocrinol Res Commun. 1981;8:179–192. doi: 10.3109/07435808109045738. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CJ, McCormack SM, Sanborn BM. The effect of relaxin on cyclic AMP concentrations in rat myometrial cells in culture. Endocrinology. 1985;116:2029–2035. doi: 10.1210/endo-116-5-2029. [DOI] [PubMed] [Google Scholar]
- 6.Sherwood OD. Relaxin. In: Knobil E, Neill JD, editors. The Physiology of Pregnancy. Raven Press Ltd.; New York: 1994. pp. 861–1009. [Google Scholar]
- 7.Sanborn BM, Kuo HS, Weisbrodt NW, Sherwood OD. Effect of porcine relaxin on cyclic nucleotide levels and spontaneous contractions of the rat uterus. In: Anderson RR, editor. Relaxin. Plenum Press; New York: 1982. pp. 273–287. [DOI] [PubMed] [Google Scholar]
- 8.Sanborn BM, Anwer K, Monga M, et al. Mechanisms controlling the acute effects of relaxin on the myometrium. In: MacLennan AH, Traegear GW, Bryant-Greenwood GD, editors. Progress in Relaxin Research. Global Publication Services; Sinapore: 1995. pp. 289–297. [Google Scholar]
- 9.Sanborn BM, Dodge K, Ku CY, Yue C. Relaxin and scaffolding proteins in signalling crosstalk. In: Tragaer GW, Ivell R, Bathgate RA, Wade JD, editors. Relaxin 2000. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. pp. 279–283. [Google Scholar]
- 10.Fei DT, Gross MC, Lofgren JL, et al. Cyclic AMP response to recombinant human relaxin by cultured human endometrial cells–a specific and high throughput in vitro bioassay. Biochem Biophys Res Commun. 1990;170:214–222. doi: 10.1016/0006-291x(90)91262-q. [DOI] [PubMed] [Google Scholar]
- 11.Parsell DA, Mak JY, Amento EP, Unemori EN. Relaxin binds to and elicits a response from cells of the human monocytic cell line, THP-1. J Biol Chem. 1996;271:27936–27941. doi: 10.1074/jbc.271.44.27936. [DOI] [PubMed] [Google Scholar]
- 12.Telgmann R, Maronde E, Tasken K, Gellersen B. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology. 1997;138:929–937. doi: 10.1210/endo.138.3.5004. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen BT, Yang L, Sanborn BM, Dessauer CW. Phosphoinositide 3-kinase activity is required for biphasic stimulation of cyclic adenosine 3′,5′-monophosphate by relaxin. Mol Endocrinol. 2003;17:1075–1084. doi: 10.1210/me.2002-0284. [DOI] [PubMed] [Google Scholar]
- 14.Hsu SY, Nakabayashi K, Nishi S, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 15.Barber R. Discrimination between intact cell desensitization and agonist affinity changes. Mol Cell Endocrinol. 1986;46:263–270. doi: 10.1016/0303-7207(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 16.Bhalla RC, Sanborn RM, Korenman SG. Hormonal interactions in the uterus: inhibition of isoproterenol-induced accumulation of adenosine 3′:5′-cyclic monophosphate by oxytocin and prostaglandins. Proc Natl Acad Sci USA. 1972;69:3761–3764. doi: 10.1073/pnas.69.12.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- 18.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 19.Yart A, Roche S, Wetzker R, et al. A function for phosphoinositide 3-kinase beta lipid products in coupling beta gamma to Ras activation in response to lysophosphatidic acid. J Biol Chem. 2002;277:21167–21178. doi: 10.1074/jbc.M110411200. [DOI] [PubMed] [Google Scholar]
- 20.Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105:125–136. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombo F, Noel J, Mayers P, et al. beta-Adrenergic stimulation of rat cardiac fibroblasts promotes protein synthesis via the activation of phosphatidylinositol 3-kinase. J Mol Cell Cardiol. 2001;33:1091–1106. doi: 10.1006/jmcc.2001.1381. [DOI] [PubMed] [Google Scholar]
- 22.Kawabe J, Iwami G, Ebina T, et al. Differential activation of adenylyl cyclase by protein kinase C isoenzymes. J Biol Chem. 1994;269:16554–16558. [PubMed] [Google Scholar]
- 23.Nguyen BT, Dessauer CW. Relaxin stimulates protein kinase C zeta translocation: requirement for cyclic adenosine 3′,5′-monophosphate production. Mol Endocrinol. 2005;19 doi: 10.1210/me.2004-0279. In press. [DOI] [PubMed] [Google Scholar]
- 24.Monga M, Ku CY, Dodge K, Sanborn BM. Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol Reprod. 1996;55:427–432. doi: 10.1095/biolreprod55.2.427. [DOI] [PubMed] [Google Scholar]
- 25.Wolf G, Haberstroh U, Neilson EG. Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol. 1992;140:95–107. [PMC free article] [PubMed] [Google Scholar]
- 26.Bartsch O, Bartlick B, Ivell R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol Hum Reprod. 2001;7:799–809. doi: 10.1093/molehr/7.9.799. [DOI] [PubMed] [Google Scholar]
- 27.Unemori EN, Lewis M, Constant J, et al. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen. 2000;8:361–370. doi: 10.1111/j.1524-475x.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- 28.Sacchi TB, Bani D, Brandi ML, et al. Relaxin influences growth, differentiation and cell-cell adhesion of human breast-cancer cells in culture. Int J Cancer. 1994;57:129–134. doi: 10.1002/ijc.2910570123. [DOI] [PubMed] [Google Scholar]
- 29.McDonald GA, Sarkar P, Rennke H, et al. Relaxin increases ubiquitin-dependent degradation of fibronectin in vitro and ameliorates renal fibrosis in vivo. Am J Physiol. 2003;285:F59–F67. doi: 10.1152/ajprenal.00157.2002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Liu SH, Erikson M, et al. Relaxin activates the MAP kinase pathway in human endometrial stromal cells. J Cell Biochem. 2002;85:536–544. doi: 10.1002/jcb.10150. [DOI] [PubMed] [Google Scholar]
- 31.Dschietzig T, Bartsch C, Richter C, et al. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist: attenuation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-kappaB. Circ Res. 2003;92:32–40. doi: 10.1161/01.res.0000051884.27117.7e. [DOI] [PubMed] [Google Scholar]
- 32.Unemori EN, Erikson ME, Rocco SE, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999;14:800–806. doi: 10.1093/humrep/14.3.800. [DOI] [PubMed] [Google Scholar]
- 33.Palejwala S, Stein DE, Weiss G, et al. Relaxin positively regulates matrix metalloproteinase expression in human lower uterine segment fibroblasts using a tyrosine kinase signaling pathway. Endocrinology. 2001;142:3405–3413. doi: 10.1210/endo.142.8.8295. [DOI] [PubMed] [Google Scholar]
- 34.Lenhart JA, Ryan PL, Ohleth KM, et al. Relaxin increases secretion of matrix metalloproteinase-2 and matrix metalloproteinase-9 during uterine and cervical growth and remodeling in the pig. Endocrinology. 2001;142:3941–3949. doi: 10.1210/endo.142.9.8387. [DOI] [PubMed] [Google Scholar]
- 35.Binder C, Hagemann T, Husen B, et al. Relaxin enhances in-vitro invasiveness of breast cancer cell lines by up-regulation of matrix metalloproteases. Mol Hum Reprod. 2002;8:789–796. doi: 10.1093/molehr/8.9.789. [DOI] [PubMed] [Google Scholar]
- 36.Hussain S, Assender JW, Bond M, et al. Activation of protein kinase Czeta is essential for cytokine-induced metalloproteinase-1, -3, and -9 secretion from rabbit smooth muscle cells and inhibits proliferation. J Biol Chem. 2002;277:27345–27352. doi: 10.1074/jbc.M111890200. [DOI] [PubMed] [Google Scholar]
- 37.Pal S, Datta K, Khosravi-Far R, Mukhopadhyay D. Role of protein kinase Czeta in Ras-mediated transcriptional activation of vascular permeability factor/vascular endothelial growth factor expression. J Biol Chem. 2001;276:2395–2403. doi: 10.1074/jbc.M007818200. [DOI] [PubMed] [Google Scholar]
- 38.Milanini-Mongiat J, Pouyssegur J, Pages G. Identification of two p42/p44 mitogen activated kinases phosphorylation sites on Sp1: Their implication in vascular endothelial growth factor gene transcription. J Biol Chem. 2002;277:20631–20639. doi: 10.1074/jbc.M201753200. [DOI] [PubMed] [Google Scholar]
- 39.Jung YD, Liu W, Reinmuth N, et al. Vascular endothelial growth factor is upregulated by interleukin-1 beta in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162. doi: 10.1023/a:1012291524723. [DOI] [PubMed] [Google Scholar]
- 40.Alper O, Bergmann-Leitner ES, Abrams S, Cho-Chung YS. Apoptosis, growth arrest and suppression of invasiveness by CRE-decoy oligonucleotide in ovarian cancer cells: protein kinase A downregulation and cytoplasmic export of CRE-binding proteins. Mol Cell Biochem. 2001;218:55–63. doi: 10.1023/a:1007205205131. [DOI] [PubMed] [Google Scholar]
- 41.Price DJ, Avraham S, Feuerstein J, et al. The invasive phenotype in HMT-3522 cells requires increased EGF receptor signaling through both PI 3-kinase and ERK 1,2 pathways. Cell Commun Adhes. 2002;9:87–102. doi: 10.1080/15419060214147. [DOI] [PubMed] [Google Scholar]


