Abstract
Traumatic brain injury (TBI) elicits acute inflammation that in turn exacerbates primary brain damage. A crucial part of innate immunity in the immune privileged central nervous system involves production of proinflammatory cytokines mediated by inflammasome signaling. Here we show that the nucleotide-binding, leucine-rich repeat pyrin domain containing protein 1 (NLRP1) inflammasome consisting of NLRP1, caspases-1 and -11, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), the X-linked inhibitor of apoptosis protein (XIAP) and pannexin 1 is expressed in neurons of the cerebral cortex. Moderate parasagittal fluid percussion injury (FPI) induced processing of interleukin-1β (IL-1β), activation of caspase-1, cleavage of XIAP and promoted assembly of the NLRP1 inflammasome complex. Anti-ASC neutralizing antibodies administered immediately after FPI to injured rats reduced caspase-1 activation, XIAP cleavage and processing of IL-1β resulting in a significant decrease in contusion volume. These studies show that the NLRP1 inflammasome constitutes an important component of the innate CNS inflammatory response after TBI and may be a novel therapeutic target for reducing the damaging effects of post-traumatic brain inflammation.
INTRODUCTION
Traumatic brain injury (TBI) is a complex and devastating clinical condition mediated by proinflammatory cytokines that produce neuronal loss, axonal destruction, and demyelination during the secondary injury cascade (Bramlett and Dietrich 2007; Povlishock 1992). Increased production of cytokines of the interleukin-1 (IL-1) family, such as IL-1β is well documented, providing clear evidence for a pivotal role of this cytokine in triggering TBI-induced inflammatory processes (Ciallella et al. 2002; DeKosky et al. 1994; Fan et al. 1995; Fassbender et al. 2000; Fink et al. 1999; Goss et al. 1995; Hutchinson et al. 2007; Kinoshita et al. 2002; Knoblach and Faden 2000; Morita-Fujimura et al. 1999; Utagawa et al. 2008). IL-1β and IL-18 are potent mediators of inflammation and initiate and/or amplify a wide variety of effects associated with innate immunity, host responses to tissue injury and microbial invasion (Bhat et al. 1996; Dinarello 2004; Dinarello 2005a; Dinarello 2005b; Dinarello 2006). Although the vast majority of studies indicate that inflammatory processes associated with the adaptive immune response contribute to secondary injury following TBI, little, if any, information is available about the innate CNS immune response following brain trauma.
In the innate immune response, activation and processing of proinflammatory cytokines IL-1β, IL-18 and IL-33 are controlled by inflammatory caspases-1 and -5 in cytoplasmic multiprotein complexes known as inflammasomes (Arend et al. 2008; Li et al. 2008; Martinon et al. 2002). Assembly of inflammasomes depends on the NLR (nucleotide binding domain, leucine-rich repeat containing) family of proteins (Ting et al. 2008). To date, more than 20 NLR proteins have been identified with more than 200 members predicted from recent genomic analysis (Rast et al. 2006). Inflammasomes are composed of three proteins: 1) an NLR family member; 2) the adaptor protein apoptosis speck-like protein with a caspase recruitment domain (ASC); and caspase-1. The exception is the NLRC4 (Ipaf) inflammasome that consists of NLRC4 and caspase-1 (Poyet et al. 2001). The inflammasome regulates caspase-1 processing and activity and, consequently, the levels of the active cytokines IL-1β and IL-18.
Our recent work shows that the NLRP1 inflammasome is present in spinal cord and cortical neurons and plays an important role in the innate CNS inflammatory response after injury and stroke (de Rivero Vaccari et al. 2008; Abulafia et al. 2009). In these studies the NLRP1 inflammasome was shown to be an important therapeutic target to reduce caspase-1 activation and tissue damage leading to improved functional outcomes. Here we extend this approach to determine whether TBI would also induce inflammasome activation in vulnerable brain regions. The cell type specific distribution of inflammasome proteins was evaluated in control and traumatized brains. To establish the importance of inflammasome activation in the innate CNS immune response in pathophysiology of TBI, we therapeutically neutralized the inflammasome that resulted in reduced caspase-1 and IL-1β activation, leading to significant improvement in tissue sparing.
METHODS
Animals and Traumatic Brain Injury
Male Sprague–Dawley rats (250–350 g) were anaesthetized using 3% halothane and a gas mixture of 70% N2O and a balance of O2 to achieve deep sedation. Injury was produced using a fluid-percussion injury device that consisted of a saline-filled Plexiglas cylindrical reservoir bent at one end with a rubber-covered piston and with the opposite end fitted with a transducer housing and injury screw adapted for the rat's skull as previously described (Dietrich et al. 1994). The metal screw was firmly connected to the plastic injury tube of the intubated anaesthetized rat and the injury was induced by the descent of a metal pendulum that struck the piston. A power lab system (CB Sciences, Dover, NH, USA) was used to measure the atmospheric level of the fluid percussion impact. After TBI, all rats were returned to their cages and allowed to recover from the surgical procedures. Injury was moderate (1.7 to 2.2 atmospheres) and animals were sacrificed at different times following TBI. Sham and naive animals were used as controls. Naive rats were anaesthetized and killed. Tissue samples were snap-frozen in liquid nitrogen and stored at −80 °C until the time of assay. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami.
Antibodies
Rabbit anti-Rattus-novegicus ASC and NALP1 antisera were prepared by Bethyl Laboratories as described (de Rivero Vaccari et al. 2008). Other antibodies were purchased from commercial sources and include: anti-IL-1β (Cell Signaling), anti-caspase-1 (Upstate); anti-caspase-1 (Santa Cruz), anti-caspase-11 (Alexis Biochemicals), anti-caspase-11 (Santa Cruz), anti-XIAP (BD Transduction Laboratories); anti-caspase-3 (Upstate), anti-pannexin-1 (Zymed) and anti-MAP2 (Chemicon).
ASC neutralization
To dissect the contribution of the NLRP1 inflammasome to TBI-induced inflammation, we blocked the activity of the inflammasome with antibodies against the inflammasome adaptor protein ASC. Antibody treatment was started immediately after trauma. One group of animals received 15 µg of anti-ASC intracerebroventricularly into the right ventricle after injury, whereas control groups received a similar treatment regimen, but using IgG of the same isotype corresponding to anti-ASC. Cortices were removed at 24 h after treatment and lysates were prepared and immunoblotted for caspase-1 and XIAP. For lesion volume analysis rats were subjected to moderate FPI and then treated with 15 µg of anti-ASC intracerebroventricularly and then injected with 50 µg of the anti-ASC antibody intraperitoneally at 24 and 48 h after injury. Another group of rats was treated with IgG and served as control. At 3 days after TBI animals were perfusion-fixed for quantitative analysis of contusion areas and volumes.
Immunoblotting
A 2 mm section of cortex was homogenized in PTN50 extraction buffer (50 mM NaPi, pH: 7.4, 50 mM NaCl, 1% Triton X-100) with proteases (1 µg/ml pepstatin A, 1 µM aprotinin, 1 mM phenylmethylsulfonyl fluoride, 5 µg/ml leupeptin). Proteins were resolved in 10–20% Tris-HCl Criterion precasted gels (Bio-Rad) as described (de Rivero Vaccari et al. 2008).
Coimmunoprecipitation
To assess the protein composition and association of proteins in the inflammasome, 500 µg of cortical lysates from sham animals and traumatized animals at 4 h were immunoprecipitated with anti-ASC or anti-NALP1 antibodies using TrueBlot™ anti-Rabbit Ig immunoprecipitation beads. Cortical lysates were precleared by adding 50 µl of anti-rabbit TrueBlot™ beads to 500 µg of lysate in a microcentrifuge tube as described (de Rivero Vaccari et al. 2008).
Immunohistochemistry
Immunostained brain sections of uninjured and injured rats at 4 h were examined with a Zeiss laser scanning confocal microscope (Zeiss, Inc.). Rats were perfused with 4% paraformaldehyde as described, and processed for cryostat sectioning (Leica SM 2000R Sliding Microtome). Sections (50 µm) were blocked by treatment with normal goat serum (Vector Laboratories). Tissue sections were rinsed with 0.1 M phosphate-buffered saline (PBS; pH 7.4) and incubated overnight at 4°C with primary antibodies against caspase-1 (1:500), caspase-11 (1:500), ASC (1:500 dilution), and NALP-1 (1:500). To determine the precise cellular distribution of inflammasome proteins, sections were double stained with the neuronal marker anti-microtubule associated protein-2 (MAP2, neurons - Chemicon) as described (de Rivero Vaccari et al. 2008).
Contusion volume analysis
At 3 days after TBI were sacrificed and perfusion fixed. For calculating contusion areas and volume TBI, 6 coronal sections were chosen for morphometric study. Quantification of lesion volume in the injured brain was calculated using computer-assisted microscopy and Neurolucida software (MicroBrightfield, Colchester, VT). Following perfusion, brains were removed and fixed in 4% paraformaldehyde (n = 5 animals per group), transverse sectioned at 10 µm, and then stained with hematoxylin and eosin (H&E) for histological assessment of contusion areas by an individual blinded to the experimental groups. Coronal sections spaced at every 780 µm from bregma levels 1.8 to 7.3 were used for contusion area and volume measurements (Zilles, 1985). Volumes were determined by tracing the area of cortical damage that was demarcated in H&E stained sections. The contused area consisted of pyknotic neurons, reactive astrocytes and an area of shearing at the gray/white matter interface of the lateral cerebral cortex. The extracellular space also appeared edematous relative to regions outside of the contused area. The Neurolucida, Microbrighfield software program for lesion volume calculations, made numerical integration of successive areas.
Statistical Analysis
Data are expressed as standard error of the mean (+/−s.e.m.). Statistical comparisons between uninjured and injured groups were made using Student’s t-test, one-way ANOVA or two-way paired comparisons ANOVA followed by Tukey’s multiple comparison tests. P-values of significance used were *P < 0.05, and #P < 0.10.
RESULTS
TBI induces processing of IL-1β in the cerebral cortex
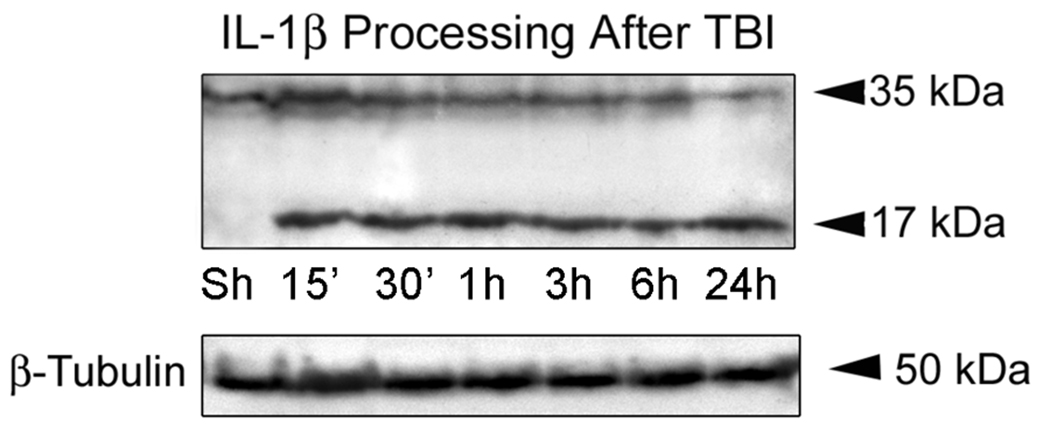
In order to determine whether TBI induces the processing of IL-1β from a precursor into a mature secreted form, we performed quantitative immunoblot analysis on cortical lysates from sham-operated and traumatized animals at 15, 30 min, 1, 3, 6 and 24 hr after TBI. As shown in Figure 1, within 15 min after TBI, pro-IL-1β (35 kDa) is rapidly processed to the mature form (17 kDa) in cortices of traumatized rats. These results indicate that increased levels of the mature form of IL-1β are induced rapidly within the cortex by moderate TBI and are consistent with the rapid processing and secretion observed by the vast majority of investigations studying secretion of IL-1β from primary macrophages or cell lines (Dinarello 2005a).
Figure 1. TBI induces IL-1β processing in the injured cortex.
Immunoblot analysis of IL-1β in cortical lysates of sham-operated animals (Sh) and traumatized rat cortices at 15, 30 min, 1, 3, 6 and 24 hr after injury. β-tubulin was used as internal standard and control for protein loading. N = 5 per group.
TBI induces expression of inflammasome proteins
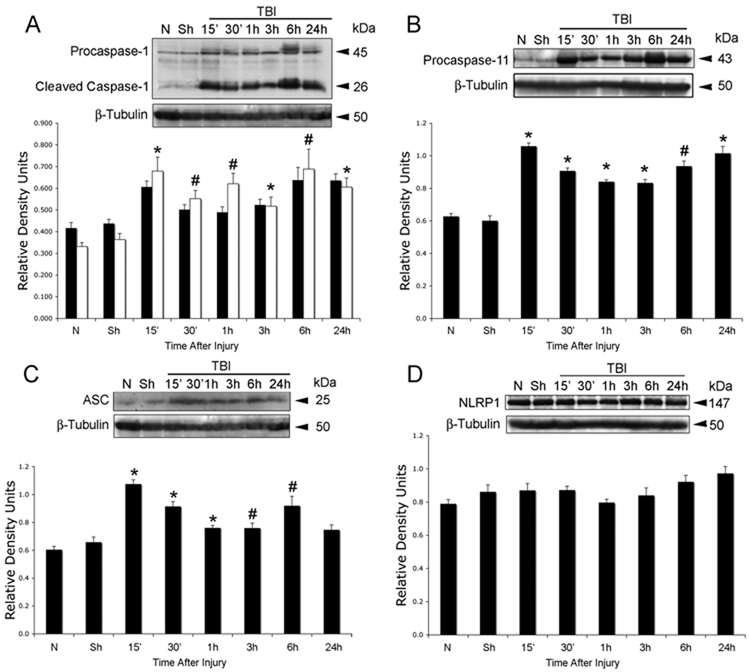
Processing of pro-IL-1β and IL-18 involves the activation of a caspase-1-activating platform, termed the inflammasome (Martinon et al. 2002; Martinon and Tschopp 2006; Ogura et al. 2006). In order to provide direct evidence for involvement of the inflammasome in TBI-induced inflammation, we analyzed traumatized cortices for the time course of expression of key inflammatory caspases and inflammasome proteins (Fig. 2). Antibody specificity for the reagents used in preliminary studies have been rigorously documented in our previous manuscript (de Rivero Vaccari et al. 2008). TBI rapidly activated caspase-1 (Fig. 2A) and upregulated caspase-11, the rodent ortholog of human caspase-5 (Fig. 2B). Proteolytic processing of procaspase-1 was detected at 15 min after trauma. Accordingly, there were significant increases in the levels of the adaptor protein ASC within 1 h after TBI (Fig. 2C), whereas no significant changes in the levels of NLRP1 were observed (Fig. 2D). NLRP3 was not detected in lysates from sham and traumatized animals at any time point examined (data not shown) and served as a negative control. These results demonstrate that TBI rapidly stimulates expression of NLRP1 inflammasome signaling molecules.
Figure 2. TBI induces activation and processing of caspase-1 and increases levels of ASC and caspase-11, but not NLRP1.
Representative immunoblot analysis of caspase-1 (A), caspase-11 (B), ASC (C) and NLRP1 (D) in cortical lysates of sham (Sh), naïve (N) and traumatized cortices at indicated times after injury. β-tubulin was used as internal standard and control for protein loading. (A) caspase-1 proform (solid bars), caspase-1 cleaved form (open bars). Data are presented as mean ± s.e.m. *p < 0.05; #p < 0.10; compared to sham. N = 5 per group.
TBI induces dramatic changes in the composition of the inflammasome
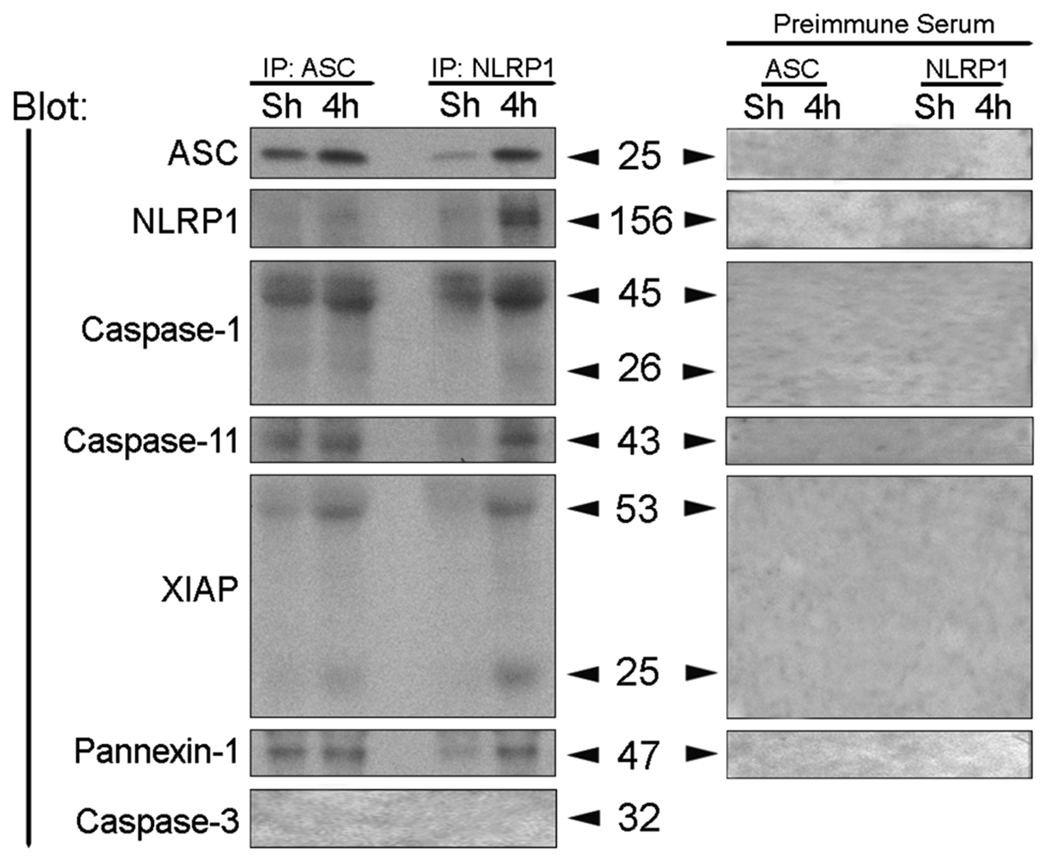
We next determined whether the increased expression levels of inflammasome proteins lead to formation of an inflammasome complex. To characterize the associations of inflammasome proteins after TBI, coimmunoprecipitations of cortical lysates from injured animals at 4 h after trauma were performed using anti-ASC antibody, anti-NLRP1and preimmune serum as control (Fig. 3). In sham cortices, ASC was immunoprecipitated with anti-ASC, caspase-1, caspase-11 and pannexin-1; however very low levels of NLRP1 and XIAP were present in this signaling complex (Fig. 3). At 4 h after TBI, the composition of the signaling complex changed within the traumatized cerebral cortex. Notably, there was increased association of caspase-1, caspase-11, NLRP1, XIAP and pannexin 1 with ASC. However, the levels of full-length 53-kDa XIAP protein was cleaved to generate a 25-kDa fragment. Anti-ASC did not immunoprecipitate caspase-3, whereas preimmune serum did not immunoprecipitate the inflammasome-associated proteins, demonstrating antibody specificity, and thus serving as negative controls. In reciprocal coimmunoprecipitation experiments, anti-NLRP1 immunoprecipitated ASC, caspases-1, and -11, pannexin-1 as well as XIAP, but it did not immunoprecipitate caspase-3, thus providing additional evidence for formation of the inflammasome complex after TBI. Thus, although the levels of NLRP1 did not change at different time points after TBI (Fig. 3B), the proportion of NLRP1 recruited into the inflammasome complex increased. These findings indicate that TBI activates the NLRP1 inflammasome that consists of NLRP1, ASC, caspase-1, caspase-11, XIAP and pannexin-1, leading to activation of caspase-1 and cleavage of XIAP. This finding is the first report that the NLRP1 inflammasome is present in traumatized cortex and the first demonstration that pannexin-1 is part of the NLRP1 inflammasome complex.
Figure 3. TBI induces association of NLRP1 inflammasome proteins, processing of caspase-1, and cleavage of XIAP.
Coimmunoprecipitation with anti-ASC, anti-NLRP1 and preimmune serum of cortical lysates obtained from sham animals (Sh) and traumatized animals at 4 h after TBI. Immunoprecipitates were blotted for ASC, NLRP1, caspase-1, caspase-11, XIAP, pannexin 1 and caspase-3 (control). Anti-NLRP1 and anti-ASC immunoprecipitated NLRP1, processed caspase-1, caspase-11, pannexin 1, and the pro- and cleaved forms of XIAP, thus indicating association of these proteins in a multiprotein complex. Preimmune serum did not immunoprecipitate inflammasome proteins and was used as control.
NLRP1 Inflammasome proteins are present in cortical neurons, and TBI induces alterations in protein expression pattern
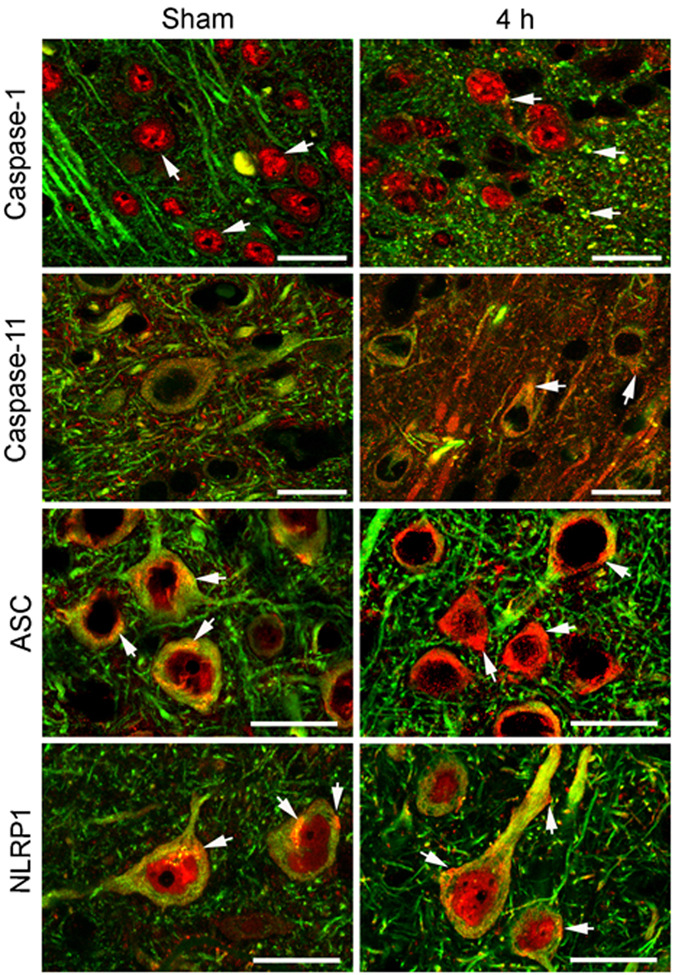
Figure 4 shows confocal images of the cell type expression and regional distribution of NLRP1 inflammasome proteins in cortical neurons of sham animals and proteins near the cortical injury epicenter at 4 h after injury. Sections were stained for caspase-1, caspase-11, ASC and NLRP1 (red), and the neuronal marker MAP2 (Fisher et al.). Caspase-1 immunoreactivity was seen in MAP2 positive cells, indicating that caspase-1 is expressed in neurons in the cerebral cortex of sham animals. Intense caspase-1 immunoreactivity was seen in the nucleus (arrow), and patchy staining was present in the cell cytoplasm (arrow) and processes. In contrast, caspase-11 immunoreactivity demonstrated diffuse punctate staining confined to the neuronal soma and processes. Intense ASC and NLRP1 staining was detected in the soma of cortical neurons and exhibited a patchy distribution pattern (arrows), while weak NLRP1 immunoreactivity was detected in the nucleus.
Figure 4. NLRP1 inflammasome proteins are present in cortical neurons and TBI induces alterations in protein expression patterns.
Confocal images of cortical neurons in sham and injured brains at 4 h post-trauma. Sections were stained for caspase-1, caspase-11, ASC and NLRP1 (red) and the neuronal marker MAP2 (Fisher et al.). In sham animals, caspase-1 immunoreactivity was seen in the nucleus (arrow). By 4 h after injury, increased caspase-1 staining was present in neuronal nuclei and patchy staining was present in the cell cytoplasm and processes near the plasma membrane. Caspase-11 immunoreactivity showed diffuse punctate staining confined to the neuronal soma and processes (arrow). Increased caspase-11 staining was present by 4 h post-trauma in the neuronal soma in a patchy distribution (arrow). Intense ASC and NLRP1 staining was detected in the soma of cortical neurons and exhibited a patchy distribution pattern in the cytoplasm (arrow). Both inflammasome proteins showed increased expression as evidenced by intense patchy staining located near or associated with the plasma membrane (arrows) by 4 h post-trauma. Bar = 20 µm.
Moderate FPI resulted in altered staining patterns of inflammasome proteins in cortical neurons (Fig. 4). At 4 h after injury, increased caspase-1 immunoreactivity was present in neuronal nuclei, while intense caspase-1 staining was seen in the cell cytoplasm as large patches (arrow) near the plasma membrane. Increased caspase-11 staining was present in the neuronal soma that was localized in a patchy distribution (arrow). A more striking alteration was observed in the immunostaining of ASC and NLRP1 after TBI. By 4 h, immunoreactivity of both inflammasome proteins was markedly enhanced, and intense patchy staining was seen in the neuronal soma near or associated with the plasma membrane (arrows). As shown previously, XIAP was present in the perinuclear region and cell processes of cortical neurons and TBI induced alterations in the expression pattern (Lotocki et al. 2003). The cellular distribution and location of NLRP1 inflammasome proteins near the plasma membrane of neurons after trauma is consistent with their role in the processing and secretion of IL-1β. Anti-ASC and anti-NLRP1 antibody specificity was evaluated by preabsorption of antiserum with immunogen peptides to remove specific antibody binding (de Rivero Vaccari et al. 2008). Antigen-depleted antiserum did not stain sections of sham and traumatized brains and served as a negative control (de Rivero Vaccari et al. 2008). Of importance is the fact that the intensity and pattern of inflammasome protein expression in neurons was altered by TBI and is consistent with the idea that neurons process and secrete IL-1β via activation of the inflammasome complex.
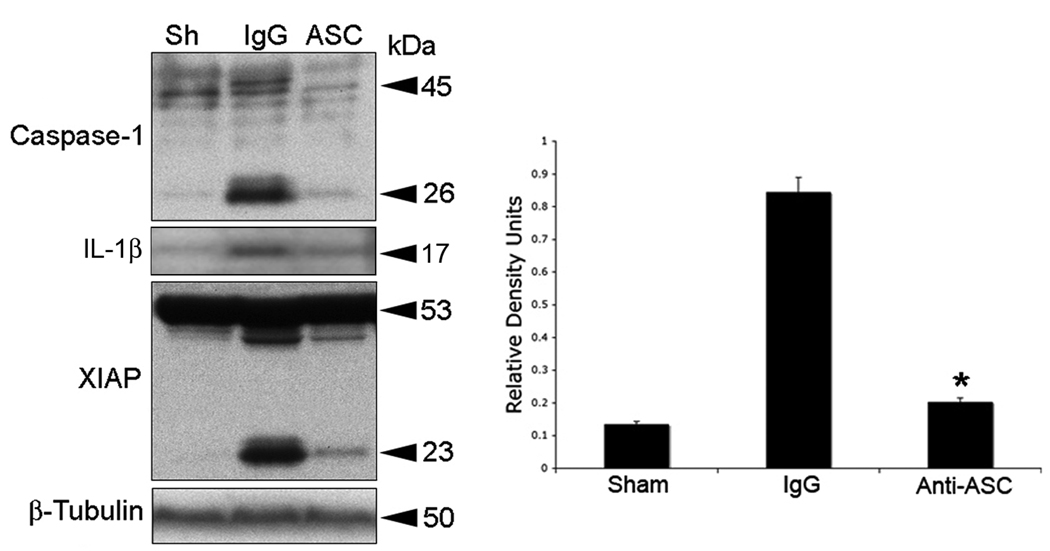
ASC neutralization reduces TBI-induced activation and processing of caspase-1 and XIAP cleavage
To dissect the contribution of the NLRP1 inflammasome to TBI-induced inflammation, we blocked the activity of the NLRP1 inflammasome with antibodies against the inflammasome adaptor protein ASC. Antibody treatment was started immediately after trauma. One group of animals received 15 µg of anti-ASC delivered intracerebroventricularly (Fig. 5, ASC). Control groups received a similar treatment regimen, but using IgG of the same isotype corresponding to anti-ASC. Cortices were removed at 24 h after treatment and lysates were prepared and immunoblotted for caspase-1 and XIAP (Fig. 5). As shown previously, anti-inflammasome antibodies cross the blood-brain barrier and are taken up by neurons in the traumatized CNS (de Rivero Vaccari et al. 2008; Abulafia et al. 2008). Moreover, neutralization of ASC after TBI interfered with inflammasome signaling and significantly reduced processing of caspase-1, IL-1β, and decreased XIAP cleavage (Fig. 5).
Figure 5. ASC neutralization decreases TBI-induced activation and processing of caspase-1, IL-1β, and XIAP cleavage.
Representative immunoblots of injured cortices from animals subjected to TBI and treated intracerebroventricularly with antibodies to ASC (ASC), IgG controls (IgG) or left untreated (Sh) after injury. Animals were sacrificed 24 h after treatment. Treatment resulted in inhibition of inflammasome activation as detected by a decrease in the processing of procaspase-1, IL-1β, and cleavage of XIAP. Anti-ASC treatment also resulted in a significant reduction in caspase-1 activity (right panel). *p < 0.05 vs sham, **p < 0.05 vs IgG.
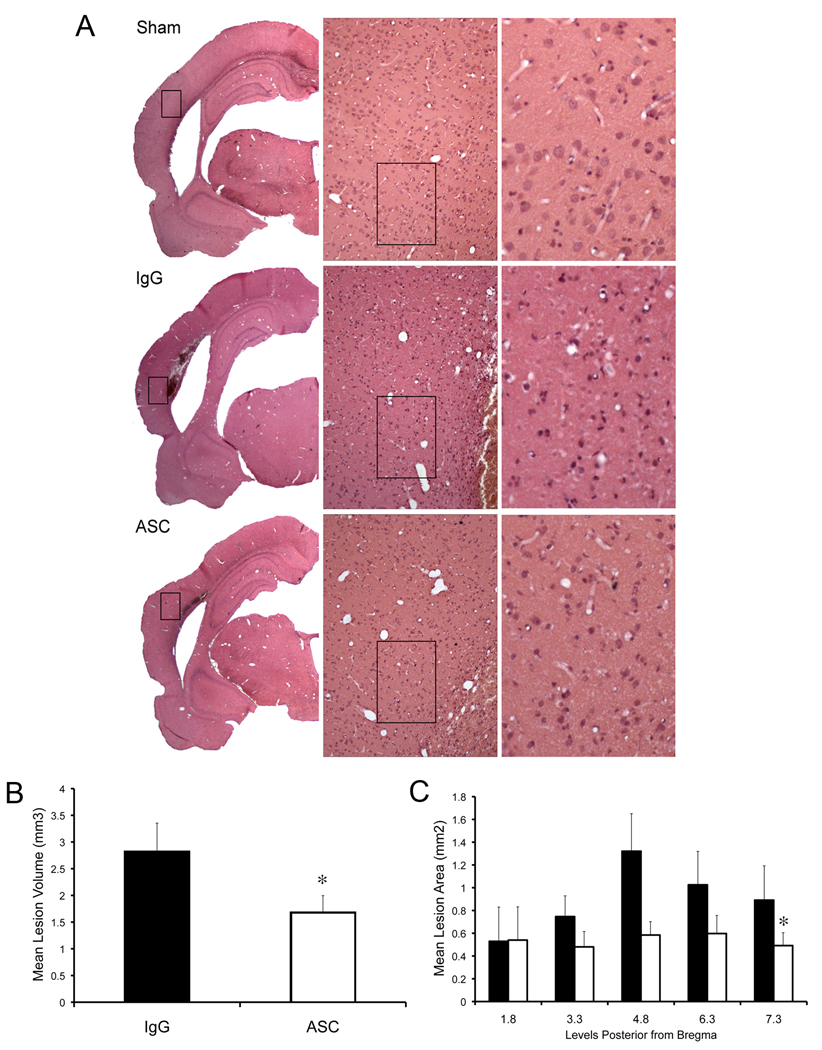
ASC neutralization decreases brain contusion volume
To determine whether inflammasome signaling was causally linked to tissue damage during TBI in vivo, we blocked the activity of ASC with neutralizing antibodies and measured the lesion volumes at 3 d after injury. Rats were subjected to moderate FPI, treated immediately with 15 µg of anti-ASC intracerebroventricularly and then injected with 50 µg of the anti-ASC antibody intraperitoneally at 24 and 48 h after injury. Another group of rats was treated with IgG and served as control. Figure 6 shows representative coronal brain sections of the lesion at 3 d after trauma. Brains from animals treated with anti-ASC after the FPI demonstrated significantly reduced contusion volume compared to vehicle treated rats.
Figure 6. ASC neutralization decreases brain contusion volume.
(A) Representative coronal sections of brains of antibody-treated (anti-ASC), IgG treated and sham animals at 3 d after TBI. Hematoxylin & eosin stained sections at bregma level 4.8 represent the injury epicenter. (B) Administration of anti-ASC significantly reduced the lesion volume at 3 d after injury. Significance was determined by comparing average contusion volume of antibody-treated animals to control groups using Student’s t-test. (C) Effect of anti-ASC on lesion size. Solid bars represent mean lesion area of rats that received IgG, whereas white bars represents areas of rats that received anti-ASC. Significance was determined by two-way paired comparisons ANOVA followed by Tukey’s multiple comparison tests (N=5 per group). Data are presented as mean ± s.e.m. *p<0.05 compared to nontreated animals. Bar = 50 µm.
DISCUSSION
In this study, we have shown for the first time a role for the NLRP1 inflammasome system in the inflammatory response induced by TBI. Our data show that moderate fluid percussion brain injury initiates activation of the NLRP1 inflammasome, resulting in processing of caspase-1 and upregulation of caspase-11 and the adaptor protein ASC, leading to maturation of IL-1β. The NLRP1 inflammasome was detected in cerebral cortical neurons in the normal and traumatized brain. The neuronal NLRP1 inflammasome is a multiprotein complex that consists of inflammatory caspase-1, caspase-11, NLRP1, ASC, the inhibitor of apoptosis protein XIAP, and pannexin 1. Neutralization of ASC interferes with NLRP1 inflammasome signaling and leads to a significant reduction in processing of caspase-1, resulting in a significant reduction in contusion volume. These results suggest a protective response by this novel anti-inflammatory treatment. Thus, the NLRP1 inflammasome plays an important role in innate CNS immunity after TBI.
Increased production of cytokines of the IL-1 family, such as IL-1β is well documented in human TBI and in animal models of brain injury, providing clear evidence for a pivotal role of this cytokine in triggering TBI-induced inflammatory processes (Bhat et al. 1996; Dinarello 2004; Dinarello 2005a; Dinarello 2005b; Dinarello 2006). IL-1β and IL-18 are potent mediators of inflammation and initiate and/or amplify a wide variety of effects associated with innate immunity, host responses to tissue injury and microbial invasion (Bhat et al. 1996; Dinarello 2004; Dinarello 2005a; Dinarello 2005b; Dinarello 2006). Upon cleavage of their proforms by caspase-1, these cytokines become active and are secreted. Thus, caspase-1 activity is critical for the inflammatory response. The generation of pro-inflammatory cytokines induces the subsequent recruitment of circulating immune cells that may amplify the immune response at the site of injury.
Assembly of the inflammasome depends on NOD-like receptors (NLR) family members such as NLRPs. Our data show that the inflammasome in cortical neurons is a protein complex containing NLRP1 as a scaffolding protein that activates caspase-1 to promote IL-1β maturation. Although the total levels of NLPR1 in lysates did not change significantly after TBI (Fig. 2), the proportion of NLRP1 that forms the inflammasome increases (Fig. 3). The NLRP1 inflammasome in cortical neurons is similar in composition to the NLRP1 inflammasome in spinal cord motor neurons (de Rivero Vaccari et al. 2008). In addition, we report that the NLRP inflammasome contains pannexin 1. Recent evidence indicates that pannexin 1 plays a crucial role in inflammation, explicitly through its association with the P2X7 receptor (Kanneganti et al. 2007; Pelegrin and Surprenant 2006). The pannexin 1 channel as well as the inflammasome can be activated by high extracellular K+ (unpublished data). Following TBI, high concentrations of K+ ions required for channel activation may occur in the vicinity of dying cells and the subsequent release of cytoplasmic contents into a confined space. Alternatively, efflux of K+ through pannexin 1 channels could create a locally high K+ concentration sufficient to activate the channel. Thus, TBI-induced high extracellular K+ may serve as a stimulus to activate pannexin 1 in the neuronal NLRP1 inflammasome and may serve as a novel upstream target for therapeutic interventions targeting TBI-induced inflammatory responses. Additional studies with pannexin-1 deficient animals are needed to determine the precise role of pannexin 1 in TBI-induced inflammasome signaling to test this hypothesis.
Although several studies have investigated the role of NLR family members using biochemical or genetic approaches, data on the cell types and tissues that express these proteins are still lacking. Our recent work demonstrates that the NLRP1 inflammasome is present in spinal cord neurons in rats (de Rivero Vaccari et al. 2008) and cortical neurons in mice (Abulafia et al. 2009) and plays an important role in the innate CNS inflammatory response after experimental SCI and thromboembolic stroke. In this study, we show that the NLRP1 inflammasome is also present in cortical neurons of the rat. A recent report using monoclonal antibodies against NLRP1 and NLRP3 revealed distinct tissue distribution patterns of these NLR family members, where NLRP1 is expressed in human neurons (Kummer et al. 2007). These findings indicate that microglial cells, which share properties with tissue macrophages, are not the main source of IL-1β in the brain. Indeed, IL-1β has been implicated in the pathogenesis of several neurological diseases including TBI, Alzheimer’s disease, epilepsy, Parkinson’s disease and stroke (Allan et al. 2005). Given the large number of NLR family members and their distinct but separate expression profiles in tissue, we hypothesize that CNS cells contain a number of yet undiscovered inflammasomes that contribute to a site-specific role in the inflammatory response.
Specific IL-1 antagonizing drugs (e.g., IL-1Ra or anakinra) have been successfully introduced in clinical treatments of autoinflammatory diseases such as rheumatoid arthritis or gout (Braddock and Quinn 2004; Dinarello 2005a; Fisher et al. 1994; Liao et al. 1984; So et al. 2007) and recent results of a phase II study of IL-1Ra treatment in stroke patients are encouraging (Emsley et al. 2005). Moreover, inhibitors of caspase-1 have been shown to attenuate mechanical neuronal injury (Fink et al. 1999) and improve survival of transplanted neurons in rats (Marchionini et al. 2004). However, despite the prominent role of IL-1β in acute brain injury, there is a paucity of specific drugs that block inflammation and improve functional outcomes after TBI. Our recent work on SCI (de Rivero Vaccari et al. 2008) and ischemic stroke (Abulafia et al. 2009) shows that neurons are a source of IL-1β in the CNS and provides in vivo evidence for a direct link between activation of the innate immune response and secondary injury. We also demonstrate that blocking IL-1β activation after injury results in tissue sparing and functional improvement. In this study, we extended this approach and tested a novel upstream anti-inflammatory therapeutic strategy that involves inhibition of caspase-1 activation by blocking inflammasome signaling. We show that anti-ASC treatment blocks the innate CNS inflammatory response, which is critical for brain homeostasis and injury-induced inflammatory processes. Thus, neutralizing antibodies against the inflammasome are successful in inhibiting inflammation in multiple CNS injury models (Abulafia et al. 2008; de Rivero Vaccari et al. 2008).
Treatment with the neutralizing antibody was also tested on its effect on contusion volume in the present model. In rats receiving anti-ASC, a significant difference was shown between the treated and nontreated groups in terms of lesion volume. The fact that anti-ASC treatment in the present model reduced caspase-1 activation and improved tissue sparing emphasizes the importance of this targeted treatment strategy on limiting inflammatory processes that contribute to pathogenesis of TBI. Our findings suggest a novel upstream target for therapeutic interventions for the inhibition of TBI-induced inflammatory responses. We show that the NLRP1 inflammasome in neurons of the cerebral cortex after TBI is critical for the activation of the innate CNS inflammatory response. Since the innate immune response impacts on outcomes of various neurodegenerative diseases, the continued discovery of novel treatment strategies that interfere with the activation of the inflammatory response after CNS injury remains an important research goal.
ACKNOWLEDGEMENTS
This work was supported by a grant from NIH/NINDS, NS30291 and NN42133.
REFERENCES
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Bhat RV, DiRocco R, Marcy VR, Flood DG, Zhu Y, Dobrzanski P, Siman R, Scott R, Contreras PC, Miller M. Increased expression of IL-1beta converting enzyme in hippocampus after ischemia: selective localization in microglia. J Neurosci. 1996;16:4146–4154. doi: 10.1523/JNEUROSCI.16-13-04146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov. 2004;3:330–339. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- Ciallella JR, Ikonomovic MD, Paljug WR, Wilbur YI, Dixon CE, Kochanek PM, Marion DW, DeKosky ST. Changes in expression of amyloid precursor protein and interleukin-1beta after experimental traumatic brain injury in rats. J Neurotrauma. 2002;19:1555–1567. doi: 10.1089/089771502762300229. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Goss JR, Miller PD, Styren SD, Kochanek PM, Marion D. Upregulation of nerve growth factor following cortical trauma. Exp Neurol. 1994;130:173–177. doi: 10.1006/exnr.1994.1196. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol. 2004;4:378–385. doi: 10.1016/j.coph.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005a;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1beta. Crit Care Med. 2005b;33:S460–S462. doi: 10.1097/01.ccm.0000185500.11080.91. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces expression of interleukin-1 beta mRNA in the rat brain. Brain Res Mol Brain Res. 1995;30:125–130. doi: 10.1016/0169-328x(94)00287-o. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Schneider S, Bertsch T, Schlueter D, Fatar M, Ragoschke A, Kuhl S, Kischka U, Hennerici M. Temporal profile of release of interleukin-1beta in neurotrauma. Neurosci Lett. 2000;284:135–138. doi: 10.1016/s0304-3940(00)00977-0. [DOI] [PubMed] [Google Scholar]
- Fink KB, Andrews LJ, Butler WE, Ona VO, Li M, Bogdanov M, Endres M, Khan SQ, Namura S, Stieg PE, Beal MF, Moskowitz MA, Yuan J, Friedlander RM. Reduction of post-traumatic brain injury and free radical production by inhibition of the caspase-1 cascade. Neuroscience. 1999;94:1213–1218. doi: 10.1016/s0306-4522(99)00345-0. [DOI] [PubMed] [Google Scholar]
- Fisher CJ, Jr., Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, et al. Phase III rhIL-1ra Sepsis Syndrome Study Group. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Jama. 1994;271:1836–1843. [PubMed] [Google Scholar]
- Goss JR, Styren SD, Miller PD, Kochanek PM, Palmer AM, Marion DW, DeKosky ST. Hypothermia attenuates the normal increase in interleukin 1 beta RNA and nerve growth factor following traumatic brain injury in the rat. J Neurotrauma. 1995;12:159–167. doi: 10.1089/neu.1995.12.159. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, O'Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, Timofeev I, Al-Rawi PG, Menon DK, Pickard JD. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery. 2002;51:195–203. doi: 10.1097/00006123-200207000-00027. discussion 203. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Cortical interleukin-1 beta elevation after traumatic brain injury in the rat: no effect of two selective antagonists on motor recovery. Neurosci Lett. 2000;289:5–8. doi: 10.1016/s0304-3940(00)01263-5. [DOI] [PubMed] [Google Scholar]
- Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Grimshaw RS, Rosenstreich DL. Identification of a specific interleukin 1 inhibitor in the urine of febrile patients. J Exp Med. 1984;159:126–136. doi: 10.1084/jem.159.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotocki G, Alonso OF, Frydel B, Dietrich WD, Keane RW. Monoubiquitination and cellular distribution of XIAP in neurons after traumatic brain injury. J Cereb Blood Flow Metab. 2003;23:1129–1136. doi: 10.1097/01.WCB.0000086938.68719.E0. [DOI] [PubMed] [Google Scholar]
- Marchionini DM, Collier TJ, Pitzer MR, Sortwell CE. Reassessment of caspase inhibition to augment grafted dopamine neuron survival. Cell Transplant. 2004;13:273–282. doi: 10.3727/000000004783983972. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2006 doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- Morita-Fujimura Y, Fujimura M, Kawase M, Murakami K, Kim GW, Chan PH. Inhibition of interleukin-1beta converting enzyme family proteases (caspases) reduces cold injury-induced brain trauma and DNA fragmentation in mice. J Cereb Blood Flow Metab. 1999;19:634–642. doi: 10.1097/00004647-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X(7) receptor. Embo J. 2006 doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- Rast JP, Smith LC, Loza-Coll M, Hibino T, Litman GW. Genomic insights into the immune system of the sea urchin. Science. 2006;314:952–956. doi: 10.1126/science.1134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utagawa A, Truettner JS, Dietrich WD, Bramlett HM. Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Exp Neurol. 2008;211:283–291. doi: 10.1016/j.expneurol.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]