Abstract
This decadal perspective summarizes novel, insightful observations achieved in exercise science. The topics span genomics and gene function, epigenetics, cell signaling, epidemiological phenomena and other important areas. A future strategy is presented along two parallel, integrated paths involving: 1) a continuance of genomic discovery and gene function; 2) classical biochemical/ physiological approaches toward solving biological- and health/disease-related phenomena.
Keywords: gene regulation, muscle as an endocrine organ, epigenetics, cell signaling, biochemistry, epidemiology, obesity
Introduction
In 2000, a perspective was provided concerning of the evolution of the “exercise sciences” in the 21st century (4). The article covered a wide range of topics such as: 1) emerging technologies and research initiatives; 2) new fields of research; 3) future funding trends and research priorities; 4) future challenges in exercise research-the building of a solid foundation; and 5) where the exercise sciences fit in health care.
Well, here we are 10 years later, and the current authors have been charged with the assignment of taking the pulse concerning the scope of progress that has been made in the exercise field in the last ten years along with projecting what impact such accomplishments bode for the future. Although the goal of this perspective will be essentially the same as before, we will take a slightly different tactic in formulating such a view. Rather than providing only our personal opinion, several experts in the field were contacted to provide their own insights. The responses received were quite insightful and there was a large degree of agreement in their respective viewpoints.
In the present article, the goal is to examine to what degree progress has been made on several fronts in the exercise field. Based on the dialogue presented below, it is not surprising that considerable advancements have occurred in skeletal muscle given the fact that it is the organ system of exercise/movement. Then, we will posture how the exercise field should evolve. Hopefully, such an approach will provide a reflection of what the science community has accomplished and how we can extend the knowledge base down the road. Furthermore, we apologize in advance for not providing more depth and breadth in covering topics beyond those highlighted in this perspective (see next section).
Selected Highlights and Novel Discoveries in the Last 10 Years
Before going into the details, it is important to point out some general background information. A Pub Med search with the word “exercise” retrieved ~82,826 peer reviewed articles published in the past 10 years. There were ~3836 papers linked to genetics; 45 to proteomics; 155 to genomics; 22 to epigenetics; 326 to signaling pathways. For additional links to the exercise theme: the term obesity retrieved 7357 articles; diabetes 6312; longevity 254; muscle 18,562; bone 4038; metabolism 24,033; nervous system 4505; hormones 6954; brain function 2644; circulation 3426; immune system 1281; respiratory 5734; and hematology 126. These data suggest a wide range of subject matter linked to the exercise sciences, and illustrate a vast group of investigators focusing on these important topics. Unfortunately, and as noted above, the authors cannot cover all these mentioned themes given the strict space constraints for this brief perspective.
Achievements in Gene Function and Regulation
Genomics, Genetic Factors and Exercise
The Human Genome Project was completed in 2003. No doubt, this milestone helped pave the way for genomic research exploring how genetic factors impact the responses and adaptations of health-related traits to exercise stimuli. Furthermore, studies have identified polymorphism in more than 239 genes and quantitative trait loci (QTL) and associated certain genotypes with cardiovascular responses, fitness phenotypes, as well as muscle strength and power adaptations (8). For example, there is a strong association between R577X genotype of the alpha actinin 3 gene (ACTN3) and performance in a variety of athletic endeavours. The R allele has been found to be associated with power-oriented performance, whereas the XX genotype may be linked with endurance ability (49). However, the collective data point to the fact that although some genotypes may be associated with certain phenotypes, the overall physiological significance is multifactorial and the result of interactions between the genome, the epigenome, and the environment, widely vary on an individual basis. Thus, this evolving field has generated a wide diversity of viewpoints as to how the field will evolve as pointed out by the excellent perspective provided by Stephen Roth, Ph.D., FACSM (42). He predicts that genomic studies will continue to enhance our understanding of the underlying biology of exercise responses. Genomic information can be useful for prescribing individualized exercise regimen especially when treating susceptible patients. No doubt, exercise genomics, applied in public health care, will be a hot area in the next decade.
Gene Knockouts
In the last 10 years the molecular manipulation of gene function, designed almost exclusively for the mouse, has exploded with a large number of the papers recently published in the last two years. The primary approached has been to null out a target gene and address the likely physiological and biochemical outcomes (phenotype) in the context of either acute or chronic exercise performance. The genes that have been studied span a broad scope, as illustrated by the following genes examined: triacylglycerol lipase, Insulin Like Growth Factor-1 (IGF-1), peroxisome proliferator-activated receptor gamma coactivator-1-alpha (PGC1-alpha), Adenosine Monophosphate (AMP) related kinases, desmin, Vascular Endothelial Growth Factor (VEGF), Tumor protein P53, carnitine functional interruption, Muscular Dystrophy (MDX), alpha1 AMP kinase, Uncoupling Protein-3 (UCP3), LOV keltch protein (LKP) kinase, thrombospondin-1 (TSP-1), myoglobin, myostatin, adenine nucleotide translocator, hypoxia inducible factor-1alpha to name a few.
To illustrate the power of this research approach the function of TSP-1 will be illustrated as reported by Malek and Olfert (28). This gene is a negative modulator of angiogenesis in several tissue types. Animals with this gene knocked out have greater muscle capillarity and a corresponding greater running endurance capacity than the wild type animals. This study thus provides a unique insight that the capillary-to-muscle interface is a critical factor that limits exercise capacity. Importantly, this paper points out that the negative consequences of the loss of TSP-1 must also be considered, as there are checks and balances to maintain optimal levels of physiological processes whether it is blood supply regulation or cell growth. In the context of the recent review article by Booth and Laye (7), this study would have been more complete if the animals were also studied under increased daily physical activity. Gene function may differ between inactive animals and those with high levels of daily physical activity (7). For example, with certain gene knockouts, animals fail to show the disease phenotype when the animals have access to voluntary wheel running (7). Thus for a full spectrum of gene function, knockout animals should be compared to wild type not only at rest but also under chronic exposure to physical activity and in response to an acute exercise stress stimulus (7).
RNA Interference (RNAi)
RNA interference (RNAi) is the process of sequence-specific post-transcriptional gene silencing, initiated by double-stranded RNA that is identical in sequence to the target gene. The discovery that synthetic duplexes of 21 nucleotides (siRNAs) trigger gene-specific silencing in mammalian cells has made them a useful tool to study gene function in mammalian systems (16, 26). SiRNA technology involves the use of small interfering RNA fragments that can be delivered into cells either directly or via a plasmid vector delivery system. Once within the cell, siRNAs trigger the degradation of their cognate mRNA thereby reducing the substrate for translation. Thus, the target gene becomes significantly “knocked down” thereby reducing the effectiveness of the target gene’s regulation of physiological processes. This technology is theoretically simpler and more cost effective than genetically producing the ”knockout” approach and is particularly useful in studying function of genes that are lethal upon complete knockout. Another advantage of siRNA usage is that since the target protein is not completely eliminated, the knockdown perturbations are less likely to induce compensatory plasticity processes observed with complete knockouts. Consequently, in the future siRNA is predicted to be used in a broad range of experiments targeting the rat, because of its long standing use as an important animal model in the exercise field.
Epigenetics and Gene Regulation
Epigenetics is a new and rapidly growing research field that investigates heritable alterations in chromosome function/gene expression caused by mechanisms other than changes in DNA sequence. Epigenetic mechanisms are diverse but can be classified into three interacting areas involving: modulation of the chromatin/histone structure (methylation, acetylation, phosphorylation); DNA methylation; and noncoding RNA such as microRNA and antisense RNA; reviewed in (30). Recent studies have shown that epigenetic modulations can also be dynamically and rapidly occurring in response to environmental changes to alter gene expression. For example, our group, in carrying out recent studies on the plasticity of the myosin heavy chain (MHC) gene family in response to altered loading state, has discovered two types of epigenetic phenomena. The first involves the expression of antisense RNA in the fast MHC gene locus in which the MHC genes are organized in tandem on the same chromosome. These antisense RNAs allow adjacent genes to cross talk as well as to coordinate regulation of neighboring MHC genes (35, 41). Second, we have discovered that repression of slow MHC and activation of fast MHCs (and vice versa) in a given muscle involve altered patterns of acetylation and methylation of the histones that regulate expression of MHCs, e.g., slow to fast and fast to slow depending on the loading conditions (36).
Recently, epigenetic regulation was linked to rat behavior in response to exercise. It was shown that exercise causes epigenetic changes that lead to enhanced memory formation and better coping in response to stress. Significant increases in histone H3 phosphoacetylation and induction of the cFos gene were found in the brain of exercised rats (11). Another epigenetic inducer is diet. For example, recently, a high fat diet was shown to increase methylation of the leptin gene, thus reducing its expression in obese people (31). These findings raise the possibility that many of the adaptations that occur in muscle and in other organ systems in response to diet, exercise, chronic inactivity, aging, and many disease interventions could be regulated, in part, via epigenetic phenomena. These observations lead to an important topic for future investigation, which suggests the possibility that the beneficial effects of exercise is occurring via epigenetic reprogramming of gene expression. The notion that environmentally induced epigenetic traits have an impact on future generations has important ramifications for future research involving diet and exercise. For example, can diet and exercise induce specific epigenetic modulations which serve as countermeasure for many disorders, which helps in overcoming our genetic weakness and predisposition to certain diseases (7)?
Micro RNA
Micro-RNAs (miRNA) are small non-coding RNAs that regulate gene expression at the posttranscriptional level (44). These highly conserved, ~21-mer RNAs regulate the expression of genes by binding to the 3'-untranslated regions (3'-UTR) of specific mRNA. Each individual miRNA could act posttranscriptionally to target hundreds of mRNAs for translational repression, degradation or destabilization. They are involved in many aspects of cell function and play a significant role in disease development. Research suggests that miRNAs play major regulators of gene expression, and thus are part of the adaptive response (10). Computational analyses continue to identify gene targets for cellular miRNA; however, these targets must be validated with microarray data. MiRNAs together with transcription factors generate a complex combinatorial code regulating gene expression. There is speculation that in higher eukaryotes, the role of miRNAs in regulating gene expression could be as important as that of transcription factors. Thus, identifying and targeting miRNA-transcription factor gene networks may provide a potent approach in future research in Exercise Science as applied to therapy and disease prevention.
Genetic Selection and Maximal Exercise Performance
The relative contribution of genetic and environmental influences in terms of individual exercise capacity is difficult to determine in humans. In recent studies using self selected rodents after many generations (7 versus 15) it was possible to delineate key factors for determining maximal oxygen consumption rate (MOCR) in inherent high capacity runners (HCR) versus low capacity runners (LCR) independent of training stimuli. In generation 7 animals, MOCR was primarily differentiated between the two groups by the ability of the muscle system to extract and diffuse oxygen rather than the capacity to deliver oxygen. In generation 15 the opposite was apparent in that the HCR again had greater MOCR than LCR, but the difference in this generation was due to greater oxygen delivery rather than greater oxygen extraction. In both generations, HCR group had greater oxygen diffusion capacity. According to P. D. Wagner and associates (21), these unique studies are important in that they now allow researchers to dissect each step in the transport chain while also, eliminating the environmental factors contribution to these physiological phenomena.
Cell Signaling: and Regulatory Molecules Impacting Metabolism and Muscle Mass
Muscle metabolism
One of the long standing questions in all fields of biomedical science involves filling in the gap between the stimulus and response to a given perturbation, e.g., exercise (aerobic and/or resistance loading). In the last decade major strides have occurred in filling in such gaps and this is illustrated by a couple of examples, even though there are numerous signaling pathways that control physiological and immune homeostasis. In the metabolic fields relative to exercise, the signaling pathway centered on adenosine monophosphosphate kinase (AMPK) has shown that by activation of this so called “fuel gauge” or “metabolic regulator” (pharmacological and contraction induced activation), a number of outcomes occur in association with AMPK activation (47). These include increases in glucose disposal; fatty acid oxidation; activating transcriptional regulators of mitochondrial biogenesis; mediating actions of hormones such as leptin, adiponectin, and glucocorticoids. Interestingly, AMPK also serves as a negative modulator of anabolic processes (glycogen, fatty acid, and protein synthesis). Thus, AMPK is a powerful regulatory molecule and in the future it will likely be the target of various pharmacological interventions for treating various disorders centered on diabetes and obesity. In this context, we have witnessed the controversy of using pharmacological manipulation of AMPK function and its downstream targets (PPAR-delta and PGC1-alpha) as presented in the findings of Evans and associates on the improvement of exercise performance via an exercise “pill” (34). This paper has created several counter viewpoints as to the physiological impact and merit of such an approach as reviewed in the excellent article by Booth and Laye (7).
Mitochondrial Biogenesis
Forty two years ago John Holloszy, M.D., made a seminal discovery that programmed running exercise carried out over several weeks induces a doubling of the mitochondria in the leg muscles of rodents not normally accustomed to physical activity (18). Since that time hundreds of studies have focused on this important phenomenon, which serves as one of the key lynchpins that define the field of muscle plasticity. Fast forward to this decade, several studies as reviewed by Holloszy and Hood, respectively (19, 20), provide the mechanism(s) driving this important discovery. For example, studies have shown that a single bout of exercise induces a rapid increase in mitochondrial biogenesis that is mediated by PGC1-alpha and other factors, which induce transcription of both nuclear and mitochondrial genes that combine to encode the protein comprising the mitochondria (3, 25). This important discovery has rejuvenated the science community such that mitochondrial research in health, disease, and aging will be a major focus in the next decade. In fact a new field of science referred to as “mitochondrial medicine” has emerged.
Muscle mass
Another important discovery/advancement of similar importance involves the IGF-1- protein kinase B/AKT-mTOR (mammalian target of rapamycin) signaling pathway, which has been linked to anabolic processes, particularly in skeletal muscle in response to exercise (6). This pathway has been shown to activate a number of down stream effectors that act on enhancing expression of the ribosomal translation machinery, as well as increasing activity of activators and enzyme systems in the processes governing protein translation as well as immune function. Further, this system is linked to inhibiting processes Forkhead box 01 (Foxo1-Atrogin-1 MAFbx system) governing the catabolic processes of protein degradation (29, 32, 43). There are several other signaling pathways that have been dissected and worthy of being mentioned, but lack of space prevents such a dialogue in this brief review.
In the context of the above topics concerning signaling for metabolic regulation and the regulation of anabolic pathways for controlling muscle mass, a perspective has evolved suggesting that if the signaling pathway for metabolic control is activated, the pathway(s) for anabolic outcomes is down regulated and vice versa (2, 12). This incompatibility seems counter intuitive to some degree since many athletes train for enhanced functional capacity for both properties. Clearly research in the future is needed to address this important topic.
Muscle as an Endocrine Organ System
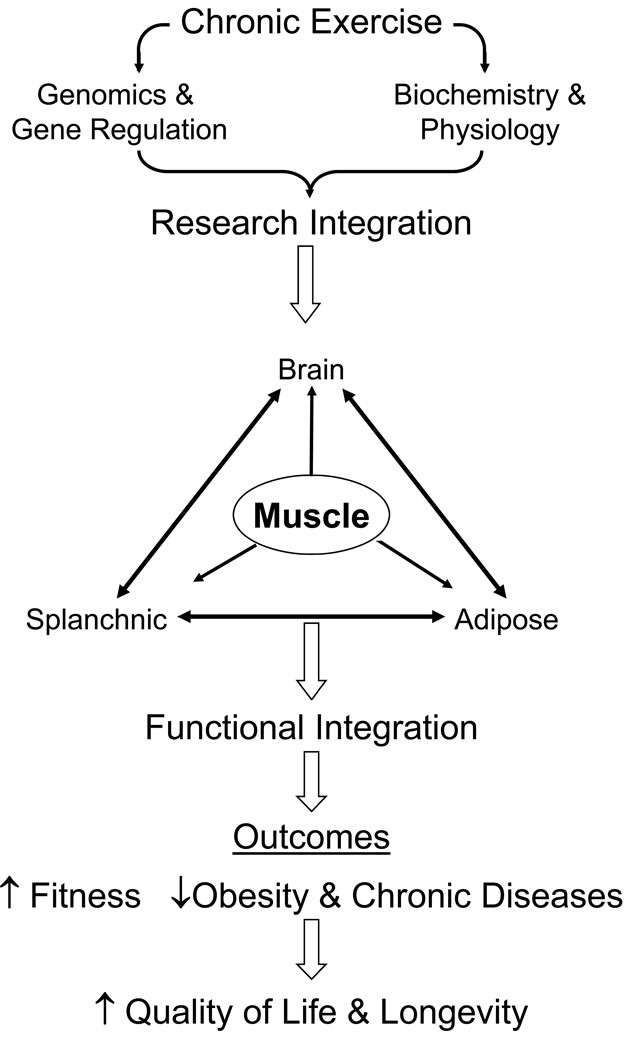
In recent years Pedersen and associates (37) coined the term “myokine” for any factor apparently expressed/synthesized in skeletal muscle in response to physical activity, which in turn can act either locally or released into the blood to regulate function in other tissues. Three myokines have been identified and partially characterized. Interleukin-6 (IL-6) appears to act both locally on carbohydrate metabolism and distally on hormone activity in the pancreas/liver and in lypolysis in adipose tissue. IL-8, acts locally and may play a key role on angiogenic processes. IL-15, which is released during resistance exercise (40), appears to regulate anabolic processes in skeletal muscle. Interestingly, individuals expressing certain single nucleotide polymorphism in the IL-15 receptor-alpha, demonstrated more muscle hypertrophy than other subjects in response to resistance exercise training (40). These collective observations illustrate that this emerging field is ready to explode and will have a major impact on how we view the role of skeletal muscle in terms of being a regulator of function in other organ systems (see Fig. 1).
Figure 1.
depicts two parallel tracts of research focus in exercise science: 1) genomics and gene regulation, and 2) basic biochemistry and physiology. These priorities should move synergistically with one another to insure that integrated function across several organ systems will unfold to derive the important outcomes of fitness, reduced disease potential, and longevity. The rationale for this scheme is depicted in the text material.
The Dynamics of Connective Tissue and Bone Adaptation With Exercise
The study of connective tissue-both in regards to tendon and the intramuscular connective tissue, recently has blossomed to the forefront due, in part, to the efforts of Kjaer’s group (27). It has been demonstrated that the metabolism, blood flow, and turnover of collagen in connective tissue is rapid and that regulatory factors are up regulated in relation to exercise (IGF-1, Transforming Growth Factor (TGF-beta, Il-6). By use of microdialysis and stable isotopes, ultrasonography, atomic force microscopy, these various approaches have made it possible to determine the structure and function of this dynamic tissue along with examining the adaptive regulation in response to various activity paradigms. The consensus of these findings is that the connective tissue infrastructure responds to exercise stimuli as rapidly as the myofiber complex.
With regard to physical activity and the anabolic responses of bone it appears that the response varies among different skeletal elements and across different regions of the same bone according to the recent findings of Hamrick et al. (15). Their findings on treadmill running mice for only 30 min per day suggest that the osteogenic responses of cortical bone to exercise varies significantly along the length of a bone, and more distal regions appear most likely to exhibit morphological changes when loading conditions are altered. The mechanisms for this heterogeneity have not been elucidated.
On the front of genetic and environmental factors contributing to bone mass, Suuriniemi and coworkers (45) investigated the role of PvuII polymorphism in the estrogen receptor (ER)—alpha gene concerning the activity profiles on 245-pre and early pubertal girls given that impairment of bone mass at puberty is an important risk factor for osteoporosis in later life. Their findings suggest that the PvuII polymorphism in the ER alpha gene may modulate the effect of exercise on bone mineral density at loaded sites. The heterozygotes appear to benefit most from the exercise effect; whereas, neither of the homozygote group received any significant improvement from physical activity. The findings further suggest that physical activity may hide the genetic effect on bone, e.g., one may compensate one’s less favorable Pp genotype by increasing physical activity at early puberty. It would be interesting to determine in future research whether these exercise- induced effects are occurring via epigenetic reprogramming.
The Role of Progenitor (satellite) Cells in Muscle Adaptation
Skeletal muscle fibers (cells) are unique in that they are multinucleated and also maintain satellite cell pools in the basal lamina. Whether myonuclear addition from the satellite cell pool is a prerequisite for marked skeletal muscle fiber enlargement to occur in response to loading stimuli is the subject of ongoing inquiries in the muscle biology field. Marcus Bamman and his colleagues (38) addressed this topic by using cluster analyses of 66 subjects that underwent a rigorous quadriceps resistance exercise training program. The subjects were subsequently classified following training into 3 groups of fiber enlargement: 1) extreme responders; 2) moderate responders, and 3) nonresponders. Extreme responders had more nuclei per fiber prior to training and showed the greatest level of satellite cell expansion and incorporation into the enlarged myofibers as compared to both the moderate and non responders. These observations provide strong evidence that myonuclear proliferation/differentiation is a prerequisite for load-induced fiber enlargement in human muscle. These findings on human muscle essentially corroborate previous studies on rodent skeletal muscle that were overloaded for long duration following irradiated to prevent satellite cell proliferation/differentiation. In that study irradiation prevented the marked hypertrophy that was observed in non-irradiated muscle as well as the incorporation of satellite cells in the muscle’s nuclear domain (1). Moreover there is additional evidence that injury repair processes are also dependent on satellite cell proliferation in the repair of injured/ regenerating fibers.
Exercise and Endothelial Cardiovascular Biology
The crucial role played by the endothelium (the lining cells of blood vessels) in cardiovascular biology is becoming increasingly appreciated as endothelial dysfunction appears to have detrimental consequences and long term effects. For example, endothelial injury has been implicated in atherosclerosis, thrombosis, and hypertension. During the last ten years it has become evident that endothelial progenitor cells (EPCs), released from bone marrow, may play an important role in maintaining an intact endothelial cell layer (33). Earlier reports from animal experiments suggest that circulating EPCs bind to the activated dysfunctional epithelium via specific receptors and reconstitute the endothelial cell layer by secretion of mediators of proliferation (39). Recent research also suggests that acute exercise stimulates release of EPCs in steady state strenuous exercise along with other regulatory factors such as vascular endothelial growth Factor (VEGF) and Interleukin 6. Additionally, reports by Brehm and associates (9) provide strong evidence that physical activity predisposes the mobilization and enhanced functional activity of circulating progenitor cells that may lead to improved cardiovascular function in patients with recently acquired myocardial infarct. Finally, Hagberg and associates (48) report that chronic long duration exercise training in aging male subjects demonstrated greater hyperemic forearm blood flow compared to less active subjects even though the EPC counts were not different between the two groups. Additionally, detraining of the active subjects resulted in both a large decrease in reactive forearm blood flow and circulating EPCs and VEGF receptor number. These alterations were correlated to changes in antioxidant capacity. These collective findings clearly point to the important role that exercise plays in maintain the homeostasis of the vascular endothelial system and the dynamic nature of its response to inactivity.
Epidemiological Studies on Physical Activity and Longevity
In the last decade there have been many articles published pointing to the positive impact that physical activity plays in the evolution of several degenerative diseases such as cardiovascular dysfunction, diabetes, metabolic syndrome, and osteoporosis to name a few. In fact, studies show that physical exercise is “more protective” than might be predicted on exercise-induced changes in risk factors (23). However, the critical question is whether exercise plays a positive role in extending one’s life span. In 2001, Blair and Colleagues (5) set the tone by trying to sort out whether it was physical activity per se or the level of fitness that contributed to health benefits leading to longevity, since both were linked to reducing morbidity from coronary heart disease, stroke, cardiovascular disease, certain types of cancer, and all-cause mortality. It was recommended that future studies define more precisely the shape of the dose-response gradient across activity and the level of fitness groups with a primary focus on musculoskeletal fitness relative to additional health outcomes. While ongoing research suggests that activity level is an important contributor to longevity for both males and females, only recently did new insight occur on this important topic by focusing more-so on elite athletes. Recently, Teramonto and Bungum (46) analyzed mortality and longevity of elite athletes using a variety of standardized tests. Their findings show that elite endurance (aerobic) athletes and mixed-sport (aerobic and anaerobic) athletes survive longer than the general population, indicated by lower mortality and higher longevity. Further the results point to lower cardiovascular disease as the primary factor for these lower mortality rates. On the other hand there are inconsistent results among studies on power (anaerobic) athletes. Thus, there is some truth to the term “survival of the fittest.”
To put this important issue into a broader perspective, Fraser and Shavlik (13) studied 34,192 California Seventh-Day Adventists and found that this subject pool has higher life expectancy than other white Californians by ~7.28 years, giving them the perhaps highest expectancy of any formally described population. Additional analyses attributed this life extension to diet (leaning toward more vegetarian), exercise, lower body mass index, less dependency on hormone replacement, and lack of smoking. It might turn out that in the long run it is the behavioral choices that individuals make that contribute to one’s longevity.
Biomedical Informatics
Biomedical informatics (BMI) is an expanding field that is playing an ever growing role in health care and biomedical research. BMI now encompasses sub-disciplines such as bioinformatics, imaging informatics, clinical informatics, and public health informatics (14). Indeed, electronic medical record systems (EMRs) and numerous NIH initiatives like the Clinical Translational Science Award place a heavy emphasis on biomedical informatics. A fundamental component of biomedical informatics is the so-called “ontology,” which provides a controlled vocabulary and set of terminologies that can be used to model a domain of knowledge or discourse. Currently, an exercise/physical activity/physical inactivity specific ontology does not exist. Consequently, it is our recommendation that recognized organizations in the field of exercise science like the American College of Sports Medicine take the lead in developing ontologies that will play an essential role in accelerating breakthroughs in the field of exercise science.
Moving Forward On Two Paths
Building on A Solid Foundation
Based on the topics covered above, it is clear that research initiatives in the last decade were focused heavily on gene discovery, gene expression, along with their manipulation and regulation. These new areas of study were enhanced further by the emergence of the epigenetic field. New technologies blossomed and became commonly available such that it became easier to perform molecular/biochemical analyses via kits purchased off the shelf from a large variety of vendors (which, in turn, generated a lot of junk mail). Furthermore, a wide variety of high throughput analyses systems became available in many areas in the biological sciences to include genomics, proteomics, and epigenetics. These in turn generated a “mountain” of data that required advances in the bioinformatics field to design software for better analyses and integration of the large volume of data being generated. Thus, it is safe to say that the research centered around “gene expression and function” will only get bigger and better as more information is generated and integrated in different fields, including the exercise sciences. However, is this path the only way to go?
Back To the Future: The Essence of Fundamental Biochemistry
In a recent opinion/OP-ED article in the New York Times (www.nytimes.com/2009/08/06/opinion/06watson.html?_r=1) Nobel Laureate, James D. Watson, provided a deep rooted perspective on the topic: “To Fight Cancer, Know the Enemy.” Watson opined that over the years since 1971, the NIH National Cancer Institute squandered the assault on fighting cancer by putting more resources into comprehensive cancer centers rather than putting needed money into basic cancer research. While the death rates for cancer have dropped over time, the cure for cancer is nowhere on the horizon.
Watson points out that a comprehensive overview of how cancer biology works did not begin to emerge until about 2000, with more extensive details about specific cancers beginning to pour forth only after the completion of the Human Genome Project in 2003. At present, while there are promising drugs in the pipeline, these “powerful attackers” may not be effective for every case and for a life long cure. Watson postulates that the time has come to turn the focus away from decoding the genetic instructions behind cancer and to a greater degree toward understanding the “chemical reactions within cancer cells.” This concept is based on the long standing discoveries of biochemists that cancer cells, in order to grow and replicate, are almost exclusively dependent on the metabolic processes of carbohydrate metabolism which, “over-drive” reactions that lead to increase glucose transportation into these rapid growing cells to fuel the signaling driving proliferation differentiation processes. Thus, Watson argues the need to return to performing studies on the biochemistry of cells to ascertain the function and mechanisms of gene products. In the authors’ view, there are potential lessons learned from the Watson opinion piece that can be translated to the exercise sciences in dealing with a number of degenerative diseases and health epidemics.
For example, we are well aware of the critical problems associated with the problem of obesity, which seems to keep growing in spite of a lot of attention by the science community. Perhaps it is time to get back to basics. This is illustrated by the unique studies recently published by Huber and associates (22). They have made the unique observation that by under nourishing (caloric restriction) pregnant rats, that the development of the fetus is imprinted with a biochemical footprint favoring the economy of energy balance. After birth the animals grow normally in the neonatal state, but as they proceed into adulthood they become obese even though they do not consume more food than their normal sibling counterparts. Also, these animals prefer exercise relative to food intake if presented with the choice; and exercise proves to be useful in preventing the development of obesity in this model. Further, the biochemical cascade of this process is much different in its biochemical mechanism as compared to normal animal littermates that were fed high fat diets and also became obese.
To put these above findings into a human context a report by Kyle and Pichard (24) involving the Dutch famine of 1944–45, involving prenatal famine due to marked food reduction in pregnant mothers resulted in significant alterations in physiological homeostasis of the offspring. These included: increases in impaired glucose tolerance, obesity, coronary heart disease, atherogenic lipid profiles, antisocial personality and other related disorders. These unique findings point to the importance of using modern tools to dissect the signaling processes and the biochemical frame work for understanding obesity and other disorders in different types of animal models and potentially in humans with different prenatal, neonatal developmental, genetic and epigenetic imprints. Furthermore, the above phenomena raises important questions for maternal fetal programming of exercise effects, e.g., will physically active mothers have more physically active off spring? Or will such offspring have some protection against inactivity related disorders?
In Figure 1, we present a conceptual framework of integrating the physiology/biochemistry with genomic data as an approach for better interpretation of data in exercise physiology and potential outcomes.
Some Key Themes Driving the Exercise Science Field in the Future
The following topics listed below were provided by investigators that responded to the inquiry. They are by no means the end-all of where the science should be heading.
Exercise mimetics: The controversial paper of Ron Evans et al (34) has sparked keen interest into whether there are a wide range of pharmacological agents (exercise pills) that can activate certain pathways linked to enhancing running capacity and/or muscle growth. The key question is whether exercise stimuli are essential requirements to enhancing physical fitness and improved metabolic outcomes. See the reviews of Booth and Laye on this controversial topic (7) as well as the article by Hawley and Holloszy (17), the latter of which puts exercise mimetics in proper perspective.
Studies are already unfolding to search for large numbers of single nucleotide polymorphisms (SNPs) and invariant genomic probes (IGPs) to unlock genomic variation contributing to fitness, performance, and trainability. These probing breakthroughs are made possible by both human and mouse genotyping arrays generated by collaborations between Jackson Laboratories and Affymetrix (note that the authors have no financial conflicts of interest on these technology advancements).
Reactive oxygen species: The focus will be to understand the underlying biology of these species, including their role in regulating muscle mass under different impacting loading state and as signaling molecules for organelle, organ, and organismal adaptations.
Genomics: the genomic basis of muscle function is already expanding (due to new technologies) to gain insights on athletic performance, general health, and the exercise impact on different diseases.
The muscle from inside and out: The role of myokines, cytokines, and adipokines are thought to impact both organ systems and organism homeostasis; the new emphasis should focus on mechanisms driving such synergism.
The processing of substrate fuels during acute and chronic exercise in athletic, sedentary, and obese lifestyles.
Experiments need to be designed to ascertain the mechanism(s) of cell signaling regulation when aerobic and anabolic training paradigms are simultaneously imposed on animal and human subjects.
Muscle fiber, connective tissue, bone and satellite cell integration: each of these systems is dynamic and the challenge is to understand their integrative role in responses to various mechanical stimuli.
Mechanical sensos and signaling regulators that control muscle size: this area is largely unexplored.
Discovering biomarkers for predicting exercise and altered health settings: it is accepted that there is a large variability in how humans respond to different types of training stimuli; is it possible to predict who are the responders versus non responders.
Extreme Environments: there are many challenges to frame the underlying mechanisms as to how individuals perform in stressful environments of heat, cold, hypoxia, and insufficient nutrition.
The link between exercising muscle and brain plasticity: This is possibly the key to the real quality of life in the aging population.
Exercise and disease prevention: probably the biggest challenge for impacting the health industry in the next decade and beyond.
Mechanisms regulating aging and exercise induced longevity: The real bottom line to exercise research endeavors.
In the context of the above topics, it is important to note that several of the previous possible future research topics may involve epigenetic research to answer some critical questions which could not be solved with basic genomic approaches.
Budget Trends: Are the Necessary Resources Available To Complete the Mission?—Not
In 2000, the NIH’s Operational Budget was $22 Billion (B). It increased further to $30 B by 2003 as part of the “budget doubling package” initiated previously by Congress in the late ’90s. From that point on to the present time the budget has remained flat; and with corrections for inflation, the actual operating dollars has steadily fallen in excess of 10%. This budget profile has had a marked negative effect on NIH funding for investigator initiated R0-types of grant applications. In some of the NIH Institutes the pay line percentages are approaching single digits.
In 2009 the Obama Administration, as part of the stimulation package initiative, infused $8.4 B into the NIH budget for scientific priorities in the form of Challenge Grants in Health Science Research (these grants are supposed to provide 2 yr of funding, but with no opportunity for renewal). Also, in many of the NIH Institutes there was an infusion of money into the typical R01 type of grant, which has the potential to elevate transiently the funding level for a short period time (2009 and 2010). Presently, as this article is being written, there is no assurance that the funding profile will be enhanced to a higher steady state level in real dollars beyond the 2010 budget. Also, to the authors’ knowledge, with so many applications being submitted in the Challenge grant initiative (~20,000), it is highly unlikely that many individuals in the exercise sciences field benefited from the stimulus package initiative. This is punctuated by the fact that the pay line for most of the grants was in the second to third percentile! Thus, unless there is a dedicated stimulus to the NIH budget down the road that provides a continuous increase in the operational budget that exceeds the cost of inflation with a primary target toward RO grant applications, the authors are pessimistic concerning the potential of enhancing the research mission well beyond that which has occurred up until the present time.
Summary
From our perspective, investigators working in the field of exercise science and its related fields, have made outstanding strides on many fronts as illustrated by the examples delineated in this perspective. This occurred in spite of funding limitations during the latter half of this decade. In spite of this funding fiasco, there is available an amazing database and an assortment of state-of-the-art technologies, analytical tools and sets of resources that posture the community for bigger and better things to come. However, unless appropriate stimulating packages and stable budget profiles return to viable levels, 10 yr from now the report card or progress report will not reach its true potential.
Acknowledgments
The authors are indebted to the following individuals who contributed their insights and suggestions to the composition of this article: Greg Adams, Marcas Bamman, Claude Bouchard, Vince Caiozzo, Michael Kjaer, Mark Olfert, Bente Klarlund Pedersen, Steve Roth, Michael Sawka, Stefano Schiaffino, Espen Spangenburg, Ron Terjung, Peter Wagner, William Winder.
This article was supported in part by NIH grants AR- 30348 and HL-73473
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002;283:C1182–C1195. doi: 10.1152/ajpcell.00173.2002. [DOI] [PubMed] [Google Scholar]
- 2.Atherton PJ, Babraj JA, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- 3.Baar K, Wende AR, Jones TE, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin KM. Research in the exercise sciences: Where do we go from here? J Appl Physiol. 2000;88:332–336. doi: 10.1152/jappl.2000.88.1.332. [DOI] [PubMed] [Google Scholar]
- 5.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33 Suppl 6:S379–S399. doi: 10.1097/00005768-200106001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bodine SC. mTOR Signaling and the Molecular Adaptation to Resistance Exercise. Med Sci Sports Exerc. 2006;38:1950–1957. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- 7.Booth FW, Laye MJ. Lack of adequate appreciation of physical exercise's complexities can pre-empt appropriate design and interpretation in scientific discovery. J Physiol. 2009 doi: 10.1113/jphysiol.2009.179507. (In Press), doi: 10.1113/jphysiol.2009.179507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray MSHJ, Pérusse L, Rankinen T, Roth SM, Wolfarth B, Bouchard C. The Human Gene Map for Performance and Health-Related Fitness Phenotypes:The 2006–2007 Update. Med Sci Sports Exerc. 2009;41:35–73. doi: 10.1249/mss.0b013e3181844179. [DOI] [PubMed] [Google Scholar]
- 9.Brehm M, Picard F, Ebner P, et al. Effects of exercise training on mobilization and functional activity of blood-derived progenitor cells in patients with acute myocardial infarction. Eur J Med Res. 2009;14:393–405. doi: 10.1186/2047-783X-14-9-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callis TE, Deng Z, Chen J-F, Wang D-Z. Muscling Through the microRNA World. Experimental Biology and Medicine. 2008;233:131–138. doi: 10.3181/0709-MR-237. [DOI] [PubMed] [Google Scholar]
- 11.Collins A, Hill LE, Chandramohan Y, Whitcomb D, Droste SK, Reul JMHM. Exercise Improves Cognitive Responses to Psychological Stress through Enhancement of Epigenetic Mechanisms and Gene Expression in the Dentate Gyrus. PLoS ONE. 2009;4:e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favier F, Benoit H, Freyssenet D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflungers Archiv European J Physiol. 2008;456:587–600. doi: 10.1007/s00424-007-0423-z. [DOI] [PubMed] [Google Scholar]
- 13.Fraser GE, Shavlik DJ. Ten Years of Life: Is It a Matter of Choice? Arch Intern Med. 2001;161:1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- 14.Greenes RAMDP, Shortliffe EHMDP. Commentary: Informatics in Biomedicine and Health Care. Acad Med. 2009;84:818–820. doi: 10.1097/ACM.0b013e3181a81f94. [Editorial]. [DOI] [PubMed] [Google Scholar]
- 15.Hamrick M, Skedros J, Pennington C, McNeil P. Increased osteogenic response to exercise in metaphyseal versus diaphyseal cortical bone. J Musculoskelet Neuronal Interact. 2006;6:258–263. [PubMed] [Google Scholar]
- 16.Hassan A. Potential applications of RNA interference-based therapeutics in the treatment of cardiovascular disease. Recent Pat Cardiovasc Drug Discov. 2006;1:141–149. doi: 10.2174/157489006777442540. [DOI] [PubMed] [Google Scholar]
- 17.Hawley JA, Holloszy JO. Exercise: it's the real thing! Nutrition Reviews. 2009;67:172–178. doi: 10.1111/j.1753-4887.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 18.Holloszy JO. Biochemical Adaptations in Muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 19.Hollozsy J. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol. 2008;59 Suppl.7:5–18. [PubMed] [Google Scholar]
- 20.Hood D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–472. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 21.Howlett RA, Kirkton SD, Gonzalez NC, et al. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J Appl Physiol. 2009;106:1819–1825. doi: 10.1152/japplphysiol.00914.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber K, Miles JL, Norman AM, Thompson NM, Davison M, Breier BH. Prenatally Induced Changes in Muscle Structure and Metabolic Function Facilitate Exercise-Induced Obesity Prevention. Endocrinology. 2009;150:4135–4144. doi: 10.1210/en.2009-0125. [DOI] [PubMed] [Google Scholar]
- 23.Joyner MJ, Green DJ. Excersise protects the cardiovascular system: effects beyond traditional risk factors. The Journal of Physiology. 2009 doi: 10.1113/jphysiol.2009.179432. (in press). doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyle UG, Pichard C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Current Opinion in Clinical Nutrition & Metabolic Care. 2006;9:388–394. doi: 10.1097/01.mco.0000232898.74415.42. [DOI] [PubMed] [Google Scholar]
- 25.Leick L, Wojtaszewski JFP, Johansen ST, et al. PGC-1{alpha} is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–E474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- 26.Lu PY, Xie F, Woodle MC. Non-Viral Vectors for Gene Therapy, Second Edition: Part 2. Volume 54 ed. Leaf Huang M-CH, and Ernst Wagner: Academic Press; 2005. In Vivo Application of RNA Interference: From Functional Genomics to Therapeutics. Advances in Genetics; pp. 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackey AL, Heinemeier KM, Anneli Koskinen SO, Kjaer M. Dynamic Adaptation of Tendon and Muscle Connective Tissue to Mechanical Loading. Connective Tissue Research. 2008;49:165–168. doi: 10.1080/03008200802151672. [DOI] [PubMed] [Google Scholar]
- 28.Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol. 2009;94:749–760. doi: 10.1113/expphysiol.2008.045989. [DOI] [PubMed] [Google Scholar]
- 29.Mammucari C, Schiaffino D, Sandri M. FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- 30.Mehler MF. Epigenetics and the nervous system. Annals of Neurology. 2008;64:602–617. doi: 10.1002/ana.21595. [DOI] [PubMed] [Google Scholar]
- 31.Milagro FICJ, García-Díaz DF, Goyenechea E, Paternain L, Martínez JA. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem. 2009;65:1–9. doi: 10.1007/BF03165964. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki M, Esser KA. Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J Appl Physiol. 2009;106:1367–1373. doi: 10.1152/japplphysiol.91355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moebius-Winkler S, Hilberg T, Menzel K, et al. Time Dependent Mobilization of Circulating Progenitor Cells During Strenuous Exercise in Healthy Individuals. J Appl Physiol. 2009;107(6):1943–1950. doi: 10.1152/japplphysiol.00532.2009. [DOI] [PubMed] [Google Scholar]
- 34.Narkar VADM, Yu RT, Embler E, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandorf CE, Haddad F, Roy RR, Qin AX, Edgerton VR, Baldwin KM. Dynamics of Myosin Heavy Chain Gene Regulation in Slow Skeletal Muscle: Role of Natural Antisense RNA. J Biol Chem. 2006;281:38330–38342. doi: 10.1074/jbc.M607249200. [DOI] [PubMed] [Google Scholar]
- 36.Pandorf CE, Haddad F, Wright C, Bodell PW, Baldwin KM. Differential epigenetic modifications of histones at the myosin heavy chain genes in fast and slow skeletal muscle fibers and in response to muscle unloading. Am J Physiol Cell Physiol. 2009;297:C6–C16. doi: 10.1152/ajpcell.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen BK, Akerstrom TCA, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 38.Petrella JK, Kim J-s, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 39.Rehman J, Li J, Orschell CM, March KL. Peripheral Blood "Endothelial Progenitor Cells" Are Derived From Monocyte/Macrophages and Secrete Angiogenic Growth Factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 40.Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol. 2004;97:2214–2219. doi: 10.1152/japplphysiol.00491.2004. [DOI] [PubMed] [Google Scholar]
- 41.Rinaldi C, Haddad F, Bodell PW, Qin AX, Jiang W, Baldwin KM. Intergenic Bidirectional Promoter and Cooperative Regulation of the IIx and IIb MHC Genes in Fast Skeletal Muscle. Am J Physiol Regul Integr Comp Physiol. 2008;295:208–218. doi: 10.1152/ajpregu.00134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth SM. Perspective on the future use of genomics in exercise prescription. J Appl Physiol. 2008;104:1243–1245. doi: 10.1152/japplphysiol.01000.2007. [DOI] [PubMed] [Google Scholar]
- 43.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunders M, Lim L. (micro)Genomic medicine: microRNAs as therapeutics and biomarkers. RNA Biol. 2009;6:324–328. doi: 10.4161/rna.6.3.8871. [DOI] [PubMed] [Google Scholar]
- 45.Suuriniemi M, Mahonen A, Kovanen V, et al. Association Between Exercise and Pubertal BMD Is Modulated by Estrogen Receptor alpha Genotype. Journal of Bone and Mineral Research. 2004;19:1758–1765. doi: 10.1359/JBMR.040918. [DOI] [PubMed] [Google Scholar]
- 46.Teramoto M, Bungum TJ. Mortality and longevity of elite athletes. Journal of Science and Medicine in Sport. 2009 doi: 10.1016/j.jsams.2009.04.010. (In Press) doi: 10.1016/j.jsams.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Winder W, Thomson D. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochemistry and Biophysics. 2007;47:332–347. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- 48.Witkowski S, Lockard MM, Jenkins NT, Obisesan TO, E.Spangenburg E, Hagberg JM. Relationship between circulating progenitor cells to vascular function and oxidative stress with long term training and short term detraining in older men. Clin Sci. 2010;118:303–311. doi: 10.1042/CS20090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang N, Garton F, North K. alpha-Actinin-3 and Performance. Med Sport Sci. 2009;54:88–101. doi: 10.1159/000235698. [DOI] [PubMed] [Google Scholar]