Abstract
The proapoptotic function of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) phosphatase has been linked to its capacity to antagonize the phosphatidylinositol-3-kinase–Akt signaling pathway. Previous studies have shown that the Forkhead transcriptional factor (FOXO3a) is a critical effector of the PTEN-mediated tumor suppressor. However, whether the PTEN–Akt– FOXO3a pathway is involved in neuronal apoptosis in developing rat brain after hypoxia–ischemia (HI) is unclear. In this study, we generated an HI model using postnatal day 10 rats. Immunohistochemistry and western blot were used to detect the expression of total and phosphorylated PTEN, Akt, and FOXO3a, as well as its target gene Bim. We found that dephosphorylation of PTEN was accompanied by dephosphorylation of Akt and FOXO3a, which induced FOXO3a translocation into the nucleus and upregulated the expression of Bim. Furthermore, we found that PTEN inhibition by bisperoxovanadium significantly increased the phosphorylation of Akt and FOXO3a, decreased the nuclear translocation of FOXO3a, and inhibited Bim expression after HI. Moreover, the downregulation of Bim caused by PTEN inhibition attenuated cellular apoptosis in developing rat brain. Our findings suggest that the PTEN–Akt–FOXO3a pathway is involved in neuronal apoptosis in neonatal rat brain after HI. Agents targeting PTEN may offer a promise to rescue neurons from HI brain damage.
Keywords: Akt, Bim, FOXO3a, hypoxia-ischemia, neuronal apoptosis, PTEN
Introduction
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was originally identified as a tumor suppressor because of its high frequency mutations in various types of tumors (Li et al, 1997). Its activity is regulated by the balance of phosphorylation and dephosphorylation at Ser residues at the C-terminal domain of the protein, which is important for the stability and function of the protein (Vazquez et al, 2000). Phosphorylation of the C-terminal domain of PTEN inactivates its phosphatase activity. Thus, dephosphorylated PTEN is an active form (Vazquez et al, 2001). The proapoptotic property of PTEN is mainly dependent on its lipid phosphatase activity, which antagonizes PI3K (phosphatidylinositol-3-kinase) –Akt and inhibits cell survival (Downes et al, 2001).
Activated Akt promotes cell survival by phosphorylating its substrates (Downward, 2004), including the mammalian members of the Forkhead transcription factors (FOXO). The FOXO family consists of three members, namely FOXO1a, FOXO3a, and FOXO4 (Hoekman et al, 2006). Among these members, FOXO3a has been shown to be important in the life span of tumor cells (Arden, 2006). While FOXO3a is phosphorylated by Akt, it will bind to the 14-3-3 protein and be retained in the cytoplasm (Van Der Heide et al 2004). Conversely, dephosphorylation of FOXO3a causes its translocation from the cytoplasm to the nucleus, inducing its target genes, such as the proapoptotic protein, Bcl-2-interacting mediator of cell death (Bim), and Fas ligand (Gilley et al, 2003; Brunet et al, 1999). The upregulation of Bim can promote cytochrome c release from the mitochondria and caspase-dependent apoptosis in tumor cells (Obexer et al, 2007). Whether the tumor suppressor PTEN is involved in the activation of Bim in developing the central nervous system is unknown.
Previous studies have shown that tumor cells lack apoptosis in PTEN-mutated cell lines (Stahl et al, 2003). These findings suggest a role for PTEN in regulating neuronal apoptosis. Evidence has shown that a suppression of PTEN expression may provide neuroprotection in neurodegenerative disorders (Griffin et al, 2005; Zhu et al, 2007). In PTEN-mutated cells (characterized by the constitutive activation of Akt and inactivation of FOXO3a), cellular apoptosis was significantly inhibited (Nakamura et al, 2000), which suggests that FOXO3a is a proapoptotic effector of PTEN-mediated tumor suppressor (Nakamura et al, 2000). However, the underlying mechanisms of PTEN in regulating neuronal apoptosis after hypoxia–ischemia (HI) remain unclear. Therefore, we hypothesized that the PTEN–Akt–FOXO3a pathway is involved in neuronal apoptosis in the developing rat brain after HI. To test this hypothesis, we generated neonatal HI brain damage using postnatal day 10 rats to study this pathway in HI-induced neuronal apoptosis.
Materials and methods
Animal Protocols
All animal research was approved by the Sichuan University Committee on Animal Research. Female Sprague –Dawley rats with litters of mixed gender were acquired from the animal center of the Sichuan University (Chengdu, China). The mother was given food and water and was housed in a temperature- and light-controlled facility until the pups were 10-days-old. For the HI model, we used a method described previously (Ferriero, 2004). Briefly, each pup was anesthetized with halothane. With the pup supine, the right common carotid artery was exposed and permanently ligated with a 7-0 silk suture through amid line cervical incision. After ligation of the common carotid artery, the pups were returned to the dam for 1 h to recover from anesthesia. Duration of 2.5 h of hypoxia (8% O2/92% N2) was used to induce HI injury. For the bisperoxovanadium (bpv)-treated group, pups received intraperitoneal injections of bpv (20 μg per 100 g) four times, with an interval of 3 h, according to a previous report (Zhang et al, 2007). Hypoxia–ischemia was induced 30 mins after the last injection of bpv. Control rats received intraperitoneal injections of normal saline (NS). Sham controls received halothane anesthesia and exposure of the common carotid artery without hypoxia, and ligation of the common carotid artery. Brains from sham controls and from control rats 0.5, 2, 4, 8, and 24 h after HI were collected for experiments.
Immunohistochemistry
Paraffin-embedded sections were used for immunohistochemistry as described previously (Li et al, 2007). Briefly, the sections were deparaffinized in xylene and dehydrated through 100% to 70% graded ethanol to distilled water, immersed in diluted antigen unmasking solution (Vector Laboratories, Foster City, CA, USA), and boiled for 30 mins in a microwave. After cooling, the sections were washed with phosphate-buffered saline. Endogenous peroxidase was inhibited with 0.3% hydrogen peroxide in methanol at room temperature for 20 mins. The sections were incubated using Clean Vision blocking solution (ImmunoVision Technologies, CA, USA) for 1 to 2 h. The following primary antibodies were used: rabbit anti-PTEN (Cell Signaling, Beverly, MA, USA, 1:100), rabbit anti-p-PTEN (ser 380) (Cell Signaling, 1:150), rabbit anti-p-FOXO3a (ser 253) (Cell Signaling, 1:100), and rabbit anti-Bim (Abcam, Cambridge, UK, 1:200). The sections were treated overnight at 4°C with primary antibodies in blocking solution. After washing in 0.05 mol/L phosphate-buffered saline, the sections were incubated with goat anti-rabbit IgG as a secondary antibody for 30 mins and then placed in a horseradish peroxidase complex solution for 30 mins. Peroxidase activity was shown by dipping the sections in a mixture containing 0.05% DAB (3-3′-diaminobenzidine, Dako, Carpinteria, CA, USA) and 0.03% H2O2 for 5 mins. The sections were then counterstained with hematoxylin, coverslipped, and observed under a microscope (Leica, CM 2000, Nussloch, Germany). Application of a control serum instead of the primary antibody on alternative sections of the same brain provided negative controls.
Western Blot Analysis
Isolated cortices at different time points as described above were homogenized in ice-cold lysis buffer containing cytosol extraction buffer that contained HEPES (pH 7.9; 10 mmol/L), KCL (10 mmol/L), EDTA (ethylenediaminetetraacetic acid; 0.1 mmol/L), EGTA (ethylene glycol tetraacetic acid; 0.1 mmol/L), DTT (dithiothreitol; 1 mmol/L), PMSF (phenylmethanesulfonyl fluoride; 0.5 mmol/L), protease inhibitor aprotinin (5 μg/mL), leupeptine (5 μg/mL), and phosphokinase inhibitor (10 μg/mL). Lysates were centrifuged at 14,000 r.p.m. for 30 mins at 4°C. Cytosol and nuclear proteins were purified as described earlier (Li et al, 2007). Protein concentration was determined by a BCA protein assay kit (Pierce, Rockford, IL, USA) using bovine serum albumin as the standard. Protein samples (100 μg per lane) were separated on an 8% SDS-polyacrylamide gel as described previously (Mao et al, 2006.). The protein was then transferred to polyvinylidene fluoride membranes. The membranes were blocked in 5% bovine serum albumin in Tris-buffered saline containing 0.05% Tween-20 for 1 h at room temperature with rotation. The membranes were incubated for 1 h at room temperature with the following antibodies: rabbit anti-PTEN monoclonal antibody (Cell Signaling, 1:1,000), rabbit anti-p-PTEN (ser 380) polyclonal antibody (Cell Signaling, 1:1,000), rabbit anti-Akt polyclonal antibody (Cell Signaling, 1:400), rabbit anti-p-Akt (Ser 473) monoclonal antibody (Cell Signaling, 1:400), rabbit anti-p- FOXO3a (ser 253) polyclonal antibody (Cell Signaling, 1:800), and rabbit anti-Bim polyclonal antibody (Abcam, 1:200) membranes were then incubated overnight at 4°C. Rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polyclonal antibody (Sigma, St Louis, MO, USA, 1:2,000) was used as an internal loading control. Membranes were then incubated with peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:3,000) in blocking solution for 1 h. Signals of bound antibodies were developed by enhanced chemiluminescence (Pierce). NIH image was used to measure the densities of protein signals on X-ray films after scanning. Protein levels were normalized to GAPDH as a loading control. Relative optical density of protein bands was measured after subtracting the film background.
Reverse-Transcriptase PCR and Real-Time PCR
Rat cortices from sham controls, 0.5, 2, 4, 8 and 24h after HI, as well as from bpv (pic) and NS-treated rats were removed and frozen for reverse transcriptase-PCR analysis as described previously (Li et al, 2008). Briefly, frozen samples were homogenized and total RNA was isolated using Trizol reagent (Gibco BRL, Gaithersburg, MD, USA). cDNAs were generated from 1 μg of total RNA using a reverse transcriptase reagent kit (TaKaRa Bio, Dalian, China), which were then amplified with primers designed on the basis of the NCBI Genebank. The primer sequences were as follows:
Bim: 5′-CACCCATGAGTTGTGACA-3′ (forward), 5′-TGGAAGCCATTGCACTGAG-3′ (reverse);
GAPDH: 5′-CCTCAAGATTGTCAGCAAT-3′ (forward), 5′-CCATCCACAGTCTTCTGAGT-3′ (reverse).
The reaction mixture for Bim real-time PCR contained 2 μL of the reverse transcriptase reaction product, using SYBR Green (Invitrogen, Carlsbad, CA, USA), which underwent 45 cycles at 94°C for 20 secs, at 54°C for 20 secs, at 72°C for 30 secs, and at 80°C for 30 secs. The relative amount of Bim transcripts in each sample was determined using the standard-curve method and by normalizing for GAPDH mRNA expression levels.
TUNEL Staining
Apoptotic cell death was detected using an In Situ Cell Death Detection Kit (Roche, Mannheim, Germany), according to the manufacturer’s instructions. Briefly, sections were deparaffinized in xylene, rehydrated through graded ethanol, treated with 0.1 mol/L citrate solution and 3% hydrogen peroxide at room temperature for 10 mins, and then treated with proteinase K (20 μg/mL) for 7 mins. Sections were washed in phosphate-buffered saline and incubated at 37°C for 1 h with biotinylated nucleotide and the terminal deoxynucleotidyl transferase, recombinant (rTdT) enzyme. After washes, sections were incubated with a converter-AP solution at 37°C for 30 mins and detected with a fast red substrate solution. As negative controls, alternate sections were processed in parallel without the rTdT enzyme.
Results
Expression and Distribution of PTEN, p-PTEN, p-FOXO3a, and Bim after HI
To determine the expression of PTEN and its phosphorylation status (p-PTEN) in the P10 rat cortex after HI, we performed immunohistochemistry using paraffin-embedded sections from sham controls, as well as from control rats at 0.5, 2, 4, 8, and 24 h after HI (each group n = 5). We found that the expression of PTEN and p-PTEN decreased at 2 h (data not shown) and reached the lowest at 4 h after HI (Figures 1B and 1D) compared with sham controls (Figures 1A and 1C). The decreased p-PTEN started to recover but still remained at a low level at 8 and 24 h (data not shown).
Figure 1.
Immunoreactivity of PTEN, p-PTEN, p-FOXO3a, and Bim in P10 rat cortices was detected using immunohistochemistry (n=5). We found that the expression of PTEN, p-PTEN, and p-FOXO3a significantly decreased at 4 h in the ischemic cortex after HI (B, D, and F). However, a strong immunoreactivity for PTEN, p-PTEN, and p-FOXO3a was observed in the sham cortex (A, C, and E). We also found that expression of Bim evidently increased at 4 h in the ischemic cortex after HI (H). However, a weak immunoreactivity for Bim was detected in the sham cortex (G). Immunoreactivity for PTEN, p-PTEN, p-FOXO3a, and Bim was mainly observed in the cytoplasm in the ischemic cortex. Arrows show positive staining cells. Magnification, ×400 (HI, hypoxia– ischemia).
To determine whether the phosphorylated form of FOXO3a (p-FOXO3a), a downstream transcriptional factor of PTEN (Nakamura et al, 2000), is regulated in this model, alternate slides from the brains as described above were used for immunohistochemistry detection. We found that the expression of p- FOXO3a was decreased at 0.5 and 2 h (data not shown), and reached the lowest at 4 h after HI (Figure 1F) compared with that of sham controls (Figure 1E). p-FOXO3a started to recover but still remained at a low level at 8 and 24 h (data not shown).
We further examined the expression of Bim, a target gene of FOXO3a, using immunohistochemistry. We found that the expression of Bim evidently increased from 0.5 h, (data not shown), and maintained at 4 h after HI (Figure 1H) compared with that of sham controls (Figure 1G). However, Bim expression declined fast to baseline at 8 h (data not shown).
Expression and Phosphorylation of PTEN, Akt, and FOXO3a Protein after HI
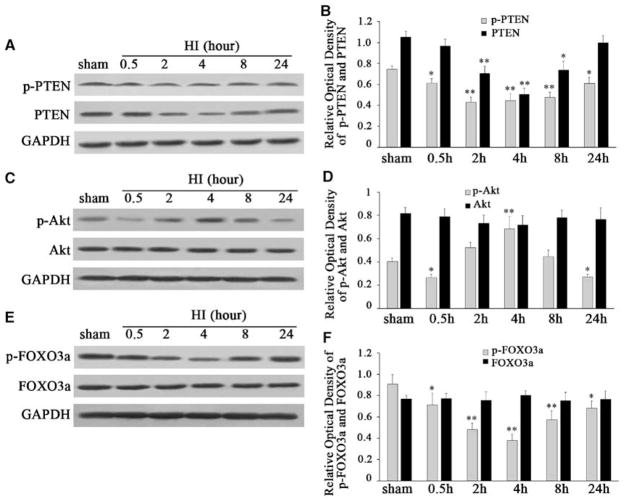
To quantify p-PTEN and PTEN expression after HI in this model, we measured the p-PTEN and PTEN protein expression using western blot analysis. Total protein was isolated using brain cortices from sham controls, as well as from control rats at 0.5, 2, 4, 8, and 24 h after HI (each group n = 5). We found that p- PTEN significantly decreased at 0.5 h, and reached the lowest at 2 to 4 h (Figures 2A and 2B). p-PTEN started to recover but still remained at a low level at 8 and 24 h (Figures 2A and 2B). After normalization with GAPDH, there was an ~18% p-PTEN decrease at 0.5 h and a 19% decrease at 24 h after HI than in sham controls (F = 21.861, P < 0.05, Figure 2B). In comparison with the findings that p-PTEN was decreased at 0.5 h after HI, total PTEN was not changed at 0.5 h, but significantly decreased at 2 h and reached the lowest at 4 h than in sham controls after HI (Figures 2A and 2B). After normalization with GAPDH, ~52% of PTEN decreased at 4 h after HI compared with that of sham controls (F = 39.451, P < 0.01, Figure 2B). Total PTEN started to at 8 h and returned to baseline at 24 h (Figures 2A and 2B).
Figure 2.
Western blot analysis of the expression and phosphorylation of PTEN, Akt, and FOXO3a in the hypoxic–ischemic cortex of P10 rats after HI (A, C, and E). One band at 54 kDa corresponding to the p-PTEN protein significantly decreased at 0.5 h, reached the lowest at 2 h, and maintained at 4 h, started to recover but still remained at a low level at 8 and 24 h compared with that of sham controls (panel A). PTEN was not changed at 0.5 h, but remarkably decreased at 2 h, reached the lowest at 4 h, started to recover at 8 h, and returned to baseline at 24 h after HI, compared with that of sham controls (panel A). One band at ~60 kDa corresponding to the p-Akt protein decreased at 0.5 h, transiently induced at 4 h, returned to baseline at 8 h, and declined again at 24 h after HI, compared with that of sham controls (panel C). However, total Akt remained unchanged at different time points (panel C). One band at ~97 kDa corresponding to p-FOXO3a significantly decreased at 0.5 and 2 h, reached the lowest at 4 h, started to recover, but still remained at a low level at 8 and 24 h compared with that of sham controls (panel E). However, total FOXO3a was not obviously changed at the indicated time points (panel E). Quantification of PTEN, p-PTEN, Akt, p-Akt, FOXO3a, and p-FOXO3a expression in the HI group and in sham controls (B, D, and F). Data were obtained by densitometry and were normalized using GAPDH as loading control. Values are expressed in relative optical density and are represented as mean±s.d. For each column, n=5, *P<0.05, **P<0.01 versus that of sham controls. (HI, hypoxia–ischemia).
As Akt is downstream of PTEN (Downes et al, 2001), we speculated whether the regulation of PTEN after HI would influence the activity of Akt. To investigate the changes of Akt activity after HI, we analyzed both phosphorylation of Akt (p-Akt) and total Akt protein expression using western blot analysis. We found that p-Akt significantly decreased at 0.5 h, but transiently increased at 4 h, and then decreased again at 24 h after HI (Figure 2C and 2D). After normalization with GAPDH, there was an ~35% p-Akt decrease at 0.5 h, and a 33% decrease at 24 h after HI, compared with that in sham controls (F = 31.843, P < 0.05, Figures 2D). However, total Akt protein remained unchanged at different time points after HI (F = 1.066, P > 0.05, Figures 2C and 2D).
As FOXO3a has been identified as a principal substrate of Akt in neurons (Van Der Heide et al, 2004), we quantified both p-FOXO3a and the total FOXO3a protein expression after HI. We found that p-FOXO3a was significantly decreased at 0.5 and 2 h, and reached the lowest at 4 h (Figures 2E and 2F). p-FOXO3a started to recover but still remained at a low level at 8 and 24 h (Figures 2E and 2F). After normalization with GAPDH, there was an approximately 21% p-FOXO3a decrease at 0.5 h and a 25% decrease at 24 h after HI compared with that in sham controls (F = 21.215, P < 0.05, Figure 2F). However, total FOXO3a was not markedly changed at the indicated time points (F = 0.316, P > 0.05, Figures 2E and 2F). The decreased p-FOXO3a level with HI means an increase in the dephosphorylation of FOXO3a.
HI Promotes FOXO3a Translocation from the Cytoplasm to the Nucleus and Induction of Bim
Dephosphorylation of FOXO3a has been reported to induce Bim expression and to lead to cell death in cultured cerebellar granule neurons deprived of growth factors (Brunet et al, 1999; Gilley et al, 2003). We speculated whether dephosphorylation of FOXO3a induced by HI would promote FOXO3a translocation into the nucleus and upregulate Bim transcription in this model. To answer this question, we extracted nuclear and cytosolic proteins from cortices and quantified FOXO3a expression separately in the nucleus and cytoplasm using western blot analysis (Figure 3A). We found that the nuclear protein of FOXO3a was obviously increased from 0.5 to 24 h in a time-dependent manner (Figures 3A and 3B). After normalization with GAPDH expression, we found an ~1.7- and 2.9-fold nuclear protein of FOXO3a increase at 0.5 and 24 h, respectively, after HI compared with sham controls (F = 32.071, P < 0.01, Figure 3B). On the contrary, cytoplasmic protein was evidently decreased from 0.5 to 24 h (Figure 3A and 3B). After normalization with GAPDH expression, there was an ~42 and 64% of FOXO3a decrease at 0.5 and 24 h, respectively, after HI compared with sham controls (F = 54.898, P < 0.01, Figure 3B).
Figure 3.
HI promotes FOXO3a translocation from the cytoplasm to the nucleus as well as the induction of Bim (A, C, and D). The nuclear protein of FOXO3a was induced from 0.5 to 24 h compared with that of sham controls (panel A). On the contrary, cytosolic protein decreased at the indicated time points (panel A). Real-time PCR and western blot analysis of Bim mRNA and protein expression (panels C and D). Bim mRNA was induced at 0.5 h, peaked at 2 h, started to decline at 4 h, and returned to baseline at 24 h after HI (panel C). Bim protein was induced at 0.5 h, peaked at 2 h, maintained a high level at 4 h, and then declined to baseline at 8 and 24 h after HI (panel D and E). Quantification of the nuclear and cytosolic protein of FOXO3a and Bim expression in the HI group and in sham controls (panels B and E). Data were obtained by densitometry and were normalized using GAPDH as loading control. Values are expressed in relative optical density and represented as mean±s.d. For each column, n=4, *P<0.05, **P<0.01 versus that of sham controls. (HI, hypoxia–ischemia).
To determine whether Bim, a downstream target gene of FOXO3a, was regulated by FOXO3a translocation into the nucleus, we quantified Bim mRNA and protein expression using real-time PCR and western blot analysis. Total RNA and protein were isolated using brain cortices from sham controls, as well as from control rats 0.5, 2, 4, 8, and 24 h after HI (each group n = 4). We found that Bim mRNA was obviously induced at 0.5 h, peaked at 2 h, started to decline at 4 h, and returned to baseline at 24 h after HI (Figure 3C). There was an ~2.6- and 4.0-fold Bim mRNA increase at 0.5 and 2 h, respectively, after HI compared with that in sham controls (P < 0.01, Figure 3C).
Similarly, Bim protein was significantly induced after HI (Figure 3D and 3E). After normalization with GAPDH expression, there was an ~1.3- and 2.1-fold Bim protein increase at 0.5 and 2 h, respectively, after HI compared with sham controls (F = 47.992, P < 0.01, Figure 3E).
Bisperoxovanadium Significantly Enhanced Phosphorylation of PTEN, Akt, and FOXO3a after HI
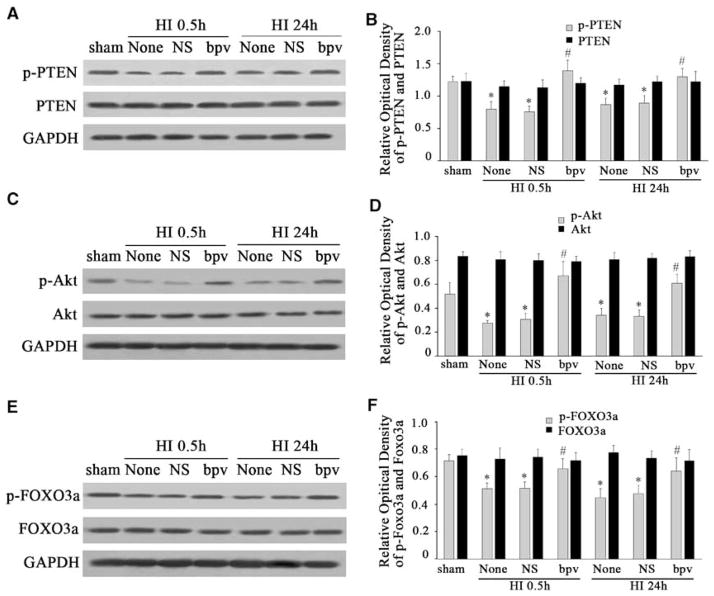
To further determine whether PTEN was involved in the regulation of Akt and FOXO3a after HI, we used a potent PTEN phosphatase inhibitor, bpv, which preferentially inhibits PTEN phosphatase activity in the nanomolar range without affecting cell viability (Schmid et al, 2004). In this study, 100 nmol/L of bpv was injected intraperitoneally before HI. We found that bpv pretreatment increased the expression of p- PTEN, p-Akt, and p-FOXO3a at 0.5 and 24 h after HI (Figures 4A, 4C, and 4E). After normalization with GAPDH, there was an ~1.8- and 1.4-fold p-PTEN increase at 0.5 and 24 h (F = 20.454, P < 0.05, Figure 4B), a 2.2- and 1.8-fold p-Akt increase at 0.5 and 24 h (F = 18.565, P < 0.05, Figure 4D), and a 1.4- and 1.3- fold p-FOXO3a increase at 0.5 and 24 h (F = 16.393, P < 0.05, Figure 4F), respectively, compared with the corresponding values in NS or nontreated cortex. However, total PTEN, total Akt, and total FOXO3a were not changed at 0.5 and 24 h in the bpv-treated cortex compared with similar values in NS-treated or nontreated cortex (Figures 4A, 4C, and 4E).
Figure 4.
bpv pretreatment significantly enhanced the phosphorylation of PTEN, Akt, and FOXO3a after HI. bpv, a PTEN phosphatase inhibitor, was used to inhibit the activity of PTEN in this study. bpv can inhibit dephosphorylation of PTEN, the active form of PTEN. While the dephosphorylation form of PTEN was inhibited by bpv, the nonactive form of PTEN, the p-PTEN level increased. When p-PTEN increased, it promoted p-Akt expression and then phosphorylated FOXO3a. We found that bpv pretreatment increased the expression of p-PTEN, p-Akt, and p-FOXO3a at 0.5 and 24 h after HI. However, total PTEN, Akt, and FOXO3a protein remained unchanged (A, C, and E). Quantification of the expression of PTEN, p-PTEN, Akt, p-Akt, FOXO3a, and p-FOXO3a in sham controls, in HI alone (None), in NS (normal saline), and in bpv-treated cortices (B, D, and F). Data were obtained by densitometry and were normalized using GAPDH as loading control. Values are expressed in relative optical density and represented as mean±s.d. For each column, n=5, *P<0.05 versus that of sham control, #P<0.05 versus that of NS and nontreatment groups. (HI, hypoxia–ischemia; NS, normal saline).
Bisperoxovanadium Attenuated FOXO3a Translocation into the Nucleus and the Induction of Bim after HI
As bpv treatment rescued the expression of p-PTEN, p-Akt, and p-FOXO3a, we further investigated whether PTEN was involved in the regulation of FOXO3a translocation from the cytoplasm to the nucleus after HI. We found that FOXO3a was upregulated in the nucleus but downregulated in the cytoplasm at 0.5 and 24 h after HI (Figures 5A and 5B). However, bpv obviously blocked the upregulation of FOXO3a in the nucleus and the downregulation of FOXO3a in the cytoplasm (Figures 5A and 5B). After normalization with GAPDH expression, we found that bpv blocked ~31 and 54% of the nuclear FOXO3a protein (F = 32.768, P < 0.01, Figure 5B). Meanwhile, there was an ~1.5- and 1.6-fold increase in FOXO3a cytosolic protein at 0.5 and 24 h after HI (F = 28.958, P < 0.05, Figure 5B), respectively, in the bpv-treated cortex compared with that in the NStreated cortex or in the nontreated cortex.
Figure 5.
bpv pretreatment significantly attenuated FOXO3a translocation from the cytoplasm to the nucleus and the induction of Bim after HI (A, C, and D). We have proven that bpv increased p-PTEN expression, which promoted p-Akt expression and led to an increase in p-FOXO3a. While FOXO3a is phosphorylated by Akt, it will bind to the 14-3-3 protein and will be retained in the cytoplasm. In this study, we found that the nuclear protein of FOXO3a reduced in the bpv-treated cortex compared with that in the NS and nontreated cortex (panel A). On the contrary, cytosolic protein was evidently increased in the bpv-treated cortex compared with the NS and nontreated cortex (panel A). Bim mRNA and protein obviously reduced at 0.5 and 24 h in the bpv (pic)-treated cortex than in the NS and nontreated cortex (panels C and D). Quantification of the nuclear and cytosolic protein of FOXO3a and Bim expression in sham controls, in HI alone (None), in NS, and in bpv treated-cortex (B, panel C, and E). Data were obtained by densitometry and were normalized using GAPDH as loading control. Values are expressed in relative optical density and represented as mean±s.d. For each column, n=4, *P<0.05, **P<0.01 versus that of sham control, #P<0.05, ##P<0.01 versus that of NS and nontreatment group. (HI, hypoxia–ischemia; NS, normal saline).
To further understand whether PTEN blockage could regulate a proapoptotic gene, Bim, one of the FOXO3a target genes in this model, we detected the expression of Bim mRNA and protein after PTEN was blocked with bpv using real-time PCR and western blot analyses. We found that Bim mRNA and protein levels significantly reduced at 0.5 and 24 h in the bpv-treated cortex compared with the those in the NS-treated cortex or in the nontreated cortex. (Figures 5C and 5D). There was an ~ 73 and 62% of Bim mRNA decrease at 0.5 and 24 h, respectively, by real-time PCR quantification (P < 0.01, Figure 5C). After normalization with GAPDH expression, we found that there was an ~78 and 75% Bim protein decrease at 0.5 and 24 h (F = 135.819, P < 0.01, Figure 5E), respectively, in the bpv-treated cortex compared with that in the NS-treated or nontreated cortex.
Bisperoxovanadium Reduced Neuronal Apoptosis in the P10 Rat after HI
As PTEN activity blocked with bpv decreased the expression of the proapoptotic protein, Bim, we investigated whether cellular apoptosis could be blocked with the downregulation of Bim. Brain sections from sham controls and from control rats 0.5, 2, 4, 8, and 24 h after HI, as well as from bpv-treated sections were used to detect cellular apoptosis using TUNEL (terminal deoxynucleotidyl transferase- mediated dUTP-biotin nick end labeling; each group n = 5). We found that TUNEL-positive cells were neither detected in sham controls (Figure 6A), nor at 0.5 and 2 h (data not shown) after HI. However, positive cells increased at 4 h (Figure 6B) and 8 h (Figure 6C), and peaked at 24 h (Figure 6D) after HI. Meanwhile, we found that bpv pretreatment obviously reduced cellular apoptosis at 24 h (Figure 6F) compared with that in the NS-treated brain cortex (Figure 6E).
Figure 6.

bpv pretreatment reduced neuronal apoptosis in P10 rat brain after HI. TUNEL-positive cells were hardly detected in the sham controls (A). However, the positive cells were increased at 4 h (B) and 8 h (C), and peaked at 24 h (D) after HI. bpv treatment obviously reduced cellular apoptosis at 24 h after HI (F). (HI, hypoxia–ischemia; NS, normal saline).
Discussion
Despite the progress in understanding the roles of tumor suppressor PTEN in normal brain function (Li et al, 2002), the roles and mechanisms of PTEN in the pathologic processes of neuronal apoptosis are not clear. In this study, we showed for the first time that the PTEN–Akt–FOXO3a pathway is involved in neuronal apoptosis in the developing rat brain after HI.
Evidence supporting the involvement of the PTEN–Akt–FOXO3a pathway in cellular apoptosis after HI is provided in this study. First, the phosphorylation level of PTEN, Akt, and FOXO3a decreased at an early stage of 0.5 h after HI, which was before cellular apoptosis, suggesting that the dephosphorylation of PTEN and its downstream components might be involved in mediating HI brain damage. Second, the HI-induced dephosphorylation of FOXO3a was accompanied by an increase in FOXO3a translocation from the cytoplasm to the nucleus. Nuclear translocation of FOXO3a induced Bim expression, indicating that FOXO3a dephosphorylation might contribute to cellular apoptosis. Third, pretreatment of rats with bpv, which is a potential inhibitor of PTEN activity, improved p-PTEN, p-Akt, and p-FOXO3a, leading to a decrease in FOXO3a translocation from the cytoplasm to the nucleus. Finally, PTEN inhibition with bpv decreased early cellular apoptosis after HI. Therefore, PTEN might have a critical role in regulating the Akt–FOXO3a pathway involved in cellular apoptosis in the developing rat brain after HI.
Phosphatase and tensin homolog deleted on chromosome 10 is a negative regulator of the PI3K–Akt pathway that prevents the recruitment of Akt to the cellular membrane for phosphorylation (Fresno Vara et al, 2004). However, when PTEN was phosphorylated, its interaction with phosphatidylinositolphosphate (PIP), a substance of PTEN, will be decreased and will lead to a decrease in its activity (Vazquez et al, 2001). Therefore, dephosphorylation of PTEN indicates an increase in its activity, which might have a role in cell growth. We found that p-PTEN but not total PTEN decreased early at 0.5 h, which indicates that PTEN was dephosphorylated at 0.5 h after HI (Figure 2). Dephosphorylation of PTEN is a signal for PTEN degradation, as dephosphorylated PTEN is degraded by proteasome (Torres and Pulido, 2001). Our findings that p-PTEN decreased after HI are consistent with previous findings in an adult global ischemic rat model (Zhang et al, 2007). Although there are controversial reports that p-PTEN increased at 24 h after focal ischemia (Omori et al, 2002), we believe that these different findings may be due to the difference in animal age and the model of ischemia.
Akt is a downstream factor of PTEN, which has a critical role in controlling the balance between survival and apoptosis by regulating the phosphorylation of its downstream components (Franke et al, 2003). In this study, p-Akt decreased at an early stage at 0.5 h and at a later stage at 24 h after HI, which is consistent with the changes of p-PTEN. This suggests that the decrease in p-Akt after HI may be related to the decrease in p-PTEN. However, it is not clear whether the decrease in p-Akt will have a role in the pathologic changes of neonatal brain after HI. We detected the regulation of transcriptional factor FOXO3a, a downstream event of p-Akt. FOXO3a is known to have an important role in cellular apoptosis (Huang and Tindall, 2007). We found that p-FOXO3a decreased starting at 0.5 h and maintained at a low level at 24 h after HI, which is consistent with the changes of p-Akt. As p-FOXO3a, the inactive form of FOXO3a, decreased and total FOXO3a was not changed, dephosphorylation of FOXO3a, the active form of FOXO3a, increased. We then investigated whether dephosphorylation of FOXO3a leads to its translocation from the cytoplasm to the nucleus and activates its target gene Bim, a proapoptotic protein of the Bcl-2 family. We found that nuclear translocation of FOXO3a was induced starting at 0.5 h after HI (Figure 3). Meanwhile, Bim expression was significantly induced at 0.5 h and was maintained at 4 h, whereas apoptotic cells were obviously detected at 24 h after HI. Our findings suggest that p-Akt decrease resulted in an increase in FOXO3a translocation to the nucleus, promoting its target gene Bim transcription, leading to apoptosis. We also found that Bim expression was not entirely in accordance with the nuclear translocation of FOXO3a at 24 h after HI, as the nuclear protein of FOXO3a remained at a high level, whereas the Bim protein returned to baseline. The possible reason for this discrepancy may be due to the degradation of Bim by the ubiquitin–proteosome pathway as previously reported (Meller et al, 2006).
Next, we tried to investigate whether the PTEN signaling pathway was involved in cellular apoptosis in this model. The PTEN phosphatase inhibitor, bpv, was used before HI treatment. Bisperoxovanadium can inhibit the dephosphorylation of PTEN, the active form of PTEN, and can lead to an increase in the p-PTEN level. We found that bpv increased the expression of p-PTEN, p-Akt, and p-FOXO3a at 0.5 and 24 h after HI (Figures 4A, 4C, and 4E). Meanwhile, we found that bpv significantly attenuated the expression of FOXO3a in the nucleus but increased it in the cytoplasm, which indicates that bpv attenuated FOXO3a translocation to the nucleus (Figure 5A). The reduction of FOXO3a translocation by PTEN inhibition might be mediated through increased p-PTEN, leading to the enhancement of p-Akt and p-FOXO3a, as the increase in p-FOXO3a will lead to FOXO3a retention in the cytoplasm (Van Der Heide et al, 2004). We further showed whether the reduction of FOXO3a translocation by bpv could downregulate its target gene, Bim, and decrease cellular apoptosis in the developing rat brain after HI. We found that bpv could significantly inhibit not only the expression of Bim (Figures 5C and 5D) but also cellular apoptosis at 24 h after HI (Figure 6). Our findings are consistent with a recent report that PTEN inhibition may provide neuroprotection by the upregulation of p-Akt after adult ischemic brain injury (Zhang et al, 2007).
In this study, we also found that p-Akt was increased transiently at 4 h after HI. However, p-FOXO3a was not increased accordingly. The possible reason for this incompatibility may be a temporal increase in p-Akt expression but not the increase in its activity, as the p-Akt level may not entirely represent its kinase activity (Hill et al, 2001). This transient increase in the p-Akt level was also founded in an adult rat ischemic model by Zhao et al (2005) who found that Akt activity was actually decreased after ischemia.
In summary, we have shown that the PTEN–Akt–FOXO3a pathway is involved in neuronal apoptosis in the developing rat brain after HI. Agents targeting PTEN might help to study the protective mechanisms in neonatal HI.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No.30825039, No.30770748), the China Medical Board of New York (00-722), the Ministry of Education of China (2006331-11-7 and 20070610092), and from the Science and Technology Bureau of Sichuan province (JY029-067).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41:709–17. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Downes CP, Bennett D, McConnachie G, Leslie NR, Pass I, MacPhee C, Patel L, Gray A. Antagonism of PI 3-kinase-dependent signalling pathways by the tumour suppressor protein, PTEN. Biochem Soc Trans. 2001;29:846–51. doi: 10.1042/0300-5127:0290846. [DOI] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–82. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–98. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93:105–17. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- Hill MM, Andjelkovic M, Brazil DP, Ferrari S, Fabbro D, Hemmings BA. Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a staurosporine-insensitive kinase. J Biol Chem. 2001;276:25643–6. doi: 10.1074/jbc.C100174200. [DOI] [PubMed] [Google Scholar]
- Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6:134–40. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Li L, Liu F, Salmonsen RA, Turner TK, Litofsky NS, Di Cristofano A, Pandolfi PP, Jones SN, Recht LD, Ross AH. PTEN in neural precursor cells: regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci. 2002;20:21–9. doi: 10.1006/mcne.2002.1115. [DOI] [PubMed] [Google Scholar]
- Li L, Qu Y, Li J, Xiong Y, Mao M, Mu D. Relationship between HIF-1alpha expression and neuronal apoptosis in neonatal rats with hypoxia-ischemia brain injury. Brain Res. 2007;1180:133–9. doi: 10.1016/j.brainres.2007.08.059. [DOI] [PubMed] [Google Scholar]
- Li L, Qu Y, Mao M, Xiong Y, Mu D. The involvement of phosphoinositid 3-kinase/Akt pathway in the activation of hypoxia-inducible factor-1alpha in the developing rat brain after hypoxia-ischemia. Brain Res. 2008;1197:152–8. doi: 10.1016/j.brainres.2007.12.059. [DOI] [PubMed] [Google Scholar]
- Mao M, Hua Y, Jiang X, Li L, Zhang L, Mu D. Expression of tumor necrosis factor alpha and neuronal apoptosis in the developing rat brain after neonatal stroke. Neurosci Lett. 2006;403:227–32. doi: 10.1016/j.neulet.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Meller R, Cameron JA, Torrey DJ, Clayton CE, Ordonez AN, Henshall DC, Minami M, Schindler CK, Saugstad JA, Simon RP. Rapid degradation of Bim by the ubiquitin-proteasome pathway mediates short-term ischemic tolerance in cultured neurons. J Biol Chem. 2006;281:7429–36. doi: 10.1074/jbc.M512138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–82. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–47. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- Omori N, Jin G, Li F, Zhang WR, Wang SJ, Hamakawa Y, Nagano I, Manabe Y, Shoji M, Abe K. Enhanced phosphorylation of PTEN in rat brain after transient middle cerebral artery occlusion. Brain Res. 2002;954:317–22. doi: 10.1016/s0006-8993(02)03366-8. [DOI] [PubMed] [Google Scholar]
- Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–8. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- Stahl JM, Cheung M, Sharma A, Trivedi NR, Shanmugam S, Robertson GP. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res. 2003;63:2881–90. [PubMed] [Google Scholar]
- Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome- mediated degradation. J Biol Chem. 2001;276:993–8. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–8. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–30. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Wu DN, Han D, Zhang GY. Critical role of PTEN in the coupling between PI3K/Akt and JNK1/2 signaling in ischemic brain injury. FEBS Lett. 2007;581:495–505. doi: 10.1016/j.febslet.2006.12.055. [DOI] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hoell P, Ahlemeyer B, Sure U, Bertalanffy H, Krieglstein J. Implication of PTEN in production of reactive oxygen species and neuronal death in in vitro models of stroke and Parkinson’s disease. Neurochem Int. 2007;50:507–16. doi: 10.1016/j.neuint.2006.10.010. [DOI] [PubMed] [Google Scholar]