Abstract
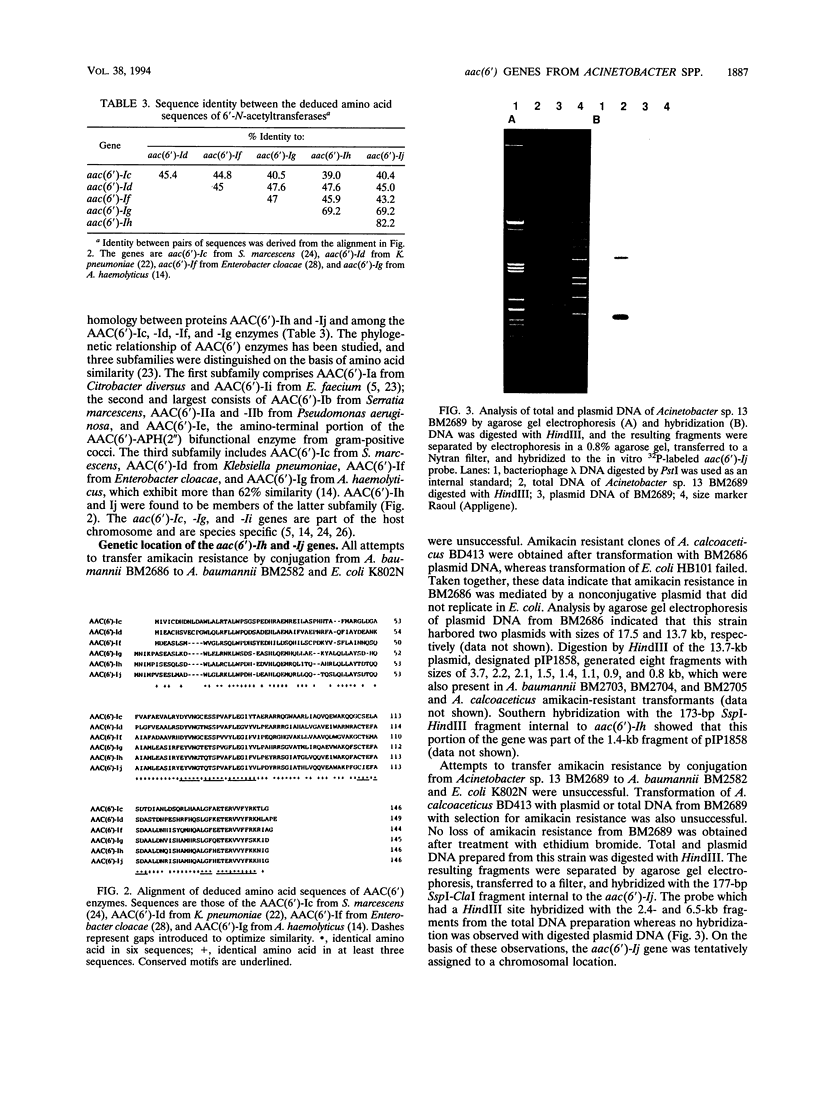
The amikacin resistance genes aac(6')-Ih of Acinetobacter baumannii BM2686 and aac(6')-Ij of Acinetobacter sp. 13 BM2689 encoding aminoglycoside 6'-N-acetyltransferases were characterized. The 441-bp coding sequences predict proteins with calculated masses of 16,698 and 16,677 Da, respectively. Analysis of the deduced amino acid sequences indicated that the proteins belonged to a subfamily of 6'-aminoglycoside acetyltransferase type I enzymes from gram-negative bacteria. The aac(6')-Ih gene of BM2686 was located on a 13.7-kb nonconjugative plasmid. The aac(6')-Ij gene from BM2689 was not transferable either by conjugation to Escherichia coli or A. baumannii or by transformation to Acinetobacter calcoaceticus. Plasmid DNA from BM2689 did not hybridize with an intragenic aac(6')-Ij probe. These results suggest a chromosomal location for this gene. The aac(6')-Ij gene was detected by DNA hybridization in all 28 strains of Acinetobacter sp. 13 tested but not in other Acinetobacter strains, including A. baumannii, proteolytic genospecies 4, 6, 14, 15, 16, and 17, and ungrouped strains. The aac(6')-Ih and -Ij probes did not hybridize in dot blot assays with DNA from members of the families Enterobacteriaceae and Pseudomonadaceae that produced 6'-N-acetyltransferases. These data suggest that the genes are confined to the Acinetobacter genus and that the aac(6')-Ij gene is species specific and may be used to identify Acinetobacter sp. 13.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouvet P. J., Jeanjean S. Delineation of new proteolytic genomic species in the genus Acinetobacter. Res Microbiol. 1989 May-Jun;140(4-5):291–299. doi: 10.1016/0923-2508(89)90021-1. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Y., Galimand M., Leclercq R., Duval J., Courvalin P. Characterization of the chromosomal aac(6')-Ii gene specific for Enterococcus faecium. Antimicrob Agents Chemother. 1993 Sep;37(9):1896–1903. doi: 10.1128/aac.37.9.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrlein M., Leying H., Cullmann W., Wendt S., Opferkuch W. Imipenem resistance in Acinetobacter baumanii is due to altered penicillin-binding proteins. Chemotherapy. 1991;37(6):405–412. doi: 10.1159/000238887. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Bouvet P., Vieu J. F., Courvalin P. Dissemination of amikacin resistance gene aphA6 in Acinetobacter spp. Antimicrob Agents Chemother. 1990 Jun;34(6):1244–1248. doi: 10.1128/aac.34.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Courvalin P. Characterization of transposon Tn1528, which confers amikacin resistance by synthesis of aminoglycoside 3'-O-phosphotransferase type VI. Antimicrob Agents Chemother. 1994 Apr;38(4):702–706. doi: 10.1128/aac.38.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988 Jan;32(1):15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Galimand M., Courvalin P. Characterization of Acinetobacter haemolyticus aac(6')-Ig gene encoding an aminoglycoside 6'-N-acetyltransferase which modifies amikacin. Antimicrob Agents Chemother. 1993 Oct;37(10):2093–2100. doi: 10.1128/aac.37.10.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather P. N., Orosz E., Shaw K. J., Hare R., Miller G. Characterization and transcriptional regulation of the 2'-N-acetyltransferase gene from Providencia stuartii. J Bacteriol. 1993 Oct;175(20):6492–6498. doi: 10.1128/jb.175.20.6492-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel A. F., Chabbert Y. A. Taxonomy and epidemiology of gram-negative bacterial plasmids studied by DNA-DNA filter hybridization in formamide. J Gen Microbiol. 1978 Feb;104(2):269–276. doi: 10.1099/00221287-104-2-269. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F. R., Nücken E. J., Henschke R. B. Nucleotide sequence analysis of 2''-aminoglycoside nucleotidyl-transferase ANT(2'') from Tn4000: its relationship with AAD(3'') and impact on Tn21 evolution. Mol Microbiol. 1988 Nov;2(6):709–717. doi: 10.1111/j.1365-2958.1988.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Rather P. N., Hare R. S., Miller G. H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993 Mar;57(1):138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Rather P. N., Sabatelli F. J., Mann P., Munayyer H., Mierzwa R., Petrikkos G. L., Hare R. S., Miller G. H., Bennett P. Characterization of the chromosomal aac(6')-Ic gene from Serratia marcescens. Antimicrob Agents Chemother. 1992 Jul;36(7):1447–1455. doi: 10.1128/aac.36.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Kumada T., Hsieh W. C., Chung H. Y., Chong Y., Hare R. S., Miller G. H., Sabatelli F. J., Howard J. Comparison of aminoglycoside resistance patterns in Japan, Formosa, and Korea, Chile, and the United States. Antimicrob Agents Chemother. 1985 Aug;28(2):282–288. doi: 10.1128/aac.28.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling A. M., Hawkey P. M., Heritage J., Downey P., Bennett P. M., Holmes B. The use of a DNA probe and PCR to examine the distribution of the aac(6')-Ic gene in Serratia marcescens and other gram-negative bacteria. J Antimicrob Chemother. 1993 Jun;31(6):841–854. doi: 10.1093/jac/31.6.841. [DOI] [PubMed] [Google Scholar]
- Terán F. J., Suárez J. E., Mendoza M. C. Cloning, sequencing, and use as a molecular probe of a gene encoding an aminoglycoside 6'-N-acetyltransferase of broad substrate profile. Antimicrob Agents Chemother. 1991 Apr;35(4):714–719. doi: 10.1128/aac.35.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjernberg I., Ursing J. Clinical strains of Acinetobacter classified by DNA-DNA hybridization. APMIS. 1989 Jul;97(7):595–605. doi: 10.1111/j.1699-0463.1989.tb00449.x. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Spohr M. Antimicrobial drug susceptibility of clinical isolates of Acinetobacter species (A. baumannii, A. haemolyticus, genospecies 3, and genospecies 6). Antimicrob Agents Chemother. 1989 Sep;33(9):1617–1619. doi: 10.1128/aac.33.9.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C., Go E., Mariano N., Berger B. J., Avraham I., Rubin D., Rahal J. J. Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J Infect Dis. 1993 Feb;167(2):448–451. doi: 10.1093/infdis/167.2.448. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zubay G. The isolation and properties of CAP, the catabolite gene activator. Methods Enzymol. 1980;65(1):856–877. doi: 10.1016/s0076-6879(80)65079-4. [DOI] [PubMed] [Google Scholar]