Abstract
It has become increasingly common for parents of children with autism to supplement behavior analytic interventions with therapies that have not yet been subjected to adequate scientific scrutiny. When caregivers elect to use unproven therapies despite advice to the contrary, practitioners should employ the methods of applied behavior analysis to experimentally evaluate the outcomes. Controlled evaluations of unproven therapies can be challenging, however, particularly when ongoing behavioral services are supplemented with biomedical interventions. This paper describes the methods and results of a behavior analytic evaluation of hyperbaric oxygen therapy, an unproven intervention that has been growing in popularity over the past several years. Three young children with autism participated. No benefits of the therapy were evident beyond those obtained through the behavioral intervention alone. Considerations for conducting this type of research are highlighted, along with suggestions for practitioners.
Descriptors: Autism, biomedical interventions, hyperbaric oxygen therapy, unproven therapies
There has been a proliferation of therapies for autism over the past several decades, fueled in large part by the increased focus on the diagnosis, prevention, and treatment of this disorder (Jacobson, Foxx, & Mulick, 2005). The urgency to find an effective intervention after a child has been diagnosed with autism can lead parents and professionals to implement so-called “alternative” therapies that are not yet supported by science (Goin-Kochel, Myers, & Mackintosh, 2007; Hanson et al., 2007; Wong & Smith, 2006). For example, some physicians have begun to prescribe various medical treatments, such as chelation therapy, dietary restrictions, and large doses of vitamins, based solely on anecdotal information or uncontrolled case studies (Jacobson et al.; Simpson, 2005).
As a result, many practicing behavior analysts are working with clients who are receiving multiple forms of intervention, including those that are currently unproven (Smith & Antolovich, 2000). Caregivers and professionals also might question behavior analysts about the likely effectiveness of alternative treatments. According to the Behavior Analyst Certification Board® “Guidelines for Responsible Conduct,” behavior analysts are “responsible for review and appraisal of likely effects of all alternative treatments, including those provided by other disciplines…” (p. 14). An appraisal based on review of the current literature is challenging when the intervention has not yet been subjected to adequate scientific scrutiny. In such cases, behavior analysts should inform caregivers and professionals that the science is lacking and caution them about using such unproven therapies.
Appraisal could be extended to an experimental analysis when caregivers are not dissuaded from employing unproven therapies. A behavior analyst could offer his or her expertise in the measurement and analysis of behavior to help evaluate therapeutic outcomes. Caregivers are most concerned about the effects of a therapy for their own children, and behavior analytic methods provide the ideal means for generating this type of information. For example, behavior analysts have evaluated such therapies as facilitated communication, a gluten-free and casein-free diet, and the drug secretin for individual children (Irvin, 2006; Montee, Miltenberger, & Witrrock, 1995; Richman, Reese, & Daniels, 1999).
One unproven intervention that has garnered attention among parents and autism professionals is hyperbaric oxygen therapy (Schechtman, 2007). Hyperbaric oxygen therapy (HBOT) involves the inhalation of oxygen (20% to 100% concentration) inside a pressurized chamber. The pressure provided by the HBOT chamber (typically 1.3 to 1.5 absolute atmospheres [ATA]) promotes the dissolution of oxygen into the blood. The oxygen is then circulated near dormant or injured tissue in the body. HBOT has been used to treat a variety of medical problems. Among those uses currently recognized by the Food and Drug Administration (FDA) are the treatment of burns, gas gangrene, carbon monoxide poisoning, decompression sickness, certain problem wounds, and exceptional blood loss (McDonagh et al., 2003). HBOT also has been used to treat a variety conditions for which there is little evidence of benefit, such as strokes, traumatic brain injury, and cerebral palsy. (Adamides, Winter, Lewis, Cooper, Kossmann, & Rosenfeld, 2006; Carson, McDonagh, Russman, & Helfand, 2005; Liptak, 2005; McDonagh et al., 2003).
Recently, several authors have suggested that HBOT can improve the symptoms of autism by reversing neurological abnormalities that might be associated with this disorder (Buckley, 2005; Rossignol & Rossignol, 2006). Although such neurological abnormalities have not been verified and no controlled studies have been conducted on the behavioral outcomes of HBOT with this population, some authors have reported benefits for children even with low pressures (1.3 ATA in the portable chambers approved by the FDA for home use) and less than 100% oxygen concentrations (i.e., 21% to 40% FiO2; see Buckley, 2005). These findings have led to speculations that HBOT might prove beneficial for children with autism by improving socialization, language, and attending (among other abilities) and by reducing problem behavior.
For this reason, some physicians are now prescribing HBOT therapy for children with autism. Typically, prescriptions are for 60-min sessions (i.e., “dives”) in the chamber to occur once or twice per day, five days per week. Parents are actively seeking facilities that can provide this service or purchasing portable hyperbaric chambers that are FDA-approved for in-home use. Parents and physicians are reporting improvements in language and cognition, as well as decreases in problem behavior, within 10 to 40 dives (Buckley, 2005; Rossignol & Rossignol, 2006). This unproven therapy is estimated to cost more than $15,000 per person (McDonagh et al., 2003). Potential side effects, although rarely reported with mild (low-pressure) HBOT of 40 dives, include seizure; oxygen toxicity; aspiration; and pain, rupture, or hemorrhage in the ear.
In light of the potential costs associated with this unproven therapy (in the form of time, expense, and potential physical side effects), behavior analysts should work closely with caregivers who have secured HBOT services for their children. Controlled evaluations using behavior analytic methods are ideal for determining if unproven therapies like HBOT offer any benefits beyond those afforded by ongoing behavioral services.
In this paper, we describe the methods and results of a behavior analytic evaluation of HBOT, along with the considerations and challenges that arose when conducting this type of study. The purpose of the study was to conduct a systematic evaluation of this unproven therapy with several children who were attending a day program for children with autism. After receiving a hyperbaric oxygen chamber from a private donor, the program director decided to provide this therapy to children whose parents requested it. However, the director wanted to systematically evaluate the outcomes for these children. Three parents who had requested HBOT services through the day program but whose children had not yet initiated the therapy were invited to participate in the controlled evaluation.
Method
Participants
Three children whose parents had requested HBOT services for their children participated. Professionals who were not involved in the study previously had diagnosed all of the children with autism using standardized autism diagnostic instruments. Lillie was a 7-year-old girl who had been receiving intensive behavioral intervention for 8 months prior to the start of the study. She could imitate a variety of motor movements and vocal speech (words and 2- to 3-word phrases), follow 2-step directions, count to 10, match words to pictures, identify and write some letters of the alphabet, identify several body parts, and request several preferred items and activities using words and phrases. Lillie engaged in a variety of inappropriate behavior during instruction, including aggression and disruption. Carl was a 6-year-old boy who had been receiving intensive behavioral intervention for 3.5 years at the time of the study. He could imitate a variety of motor movements and some speech sounds, follow 2-step directions, count up to 4 from an array, spell words of objects shown in pictures, identify several body parts, and request several preferred items using signs. Carl engaged in aggression and disruption during instruction. Harvey was 6 years old and had been receiving intensive behavioral intervention for about 4.5 years when the study began. He could imitate 1-step motor movements, match pictures to objects, identify several body parts, and request several preferred items using signs. Carl engaged in aggression and stereotyped behavior during instruction.
The parents consented to include their children in the study after being told the following: (a) The treatment would begin at the discretion of the experimenter, (b) instructional sessions would be videotaped, (c) the videotapes would be observed and scored by trained research assistants, (d) the data would be used for publication and educational purposes, (e) the parent could withdraw the child from the study at any time, and (e) the child would never be identified by name in publications and presentations. To be eligible to participate, the parent had to obtain a physician's prescription for the HBOT dives, as well as a medical release for the child. The medical release had to be updated every 30 days during the course of the study. Permission to conduct the evaluation was obtained through the university's institutional review board.
Setting and Materials
The study was conducted at a private educational center providing behavior analysis services to children with developmental disabilities. The children attended the center each day from 8:30 am to 4 pm (Harvey) or from 9 am to 3 pm (Lillie and Carl), 5 days per week. Educational programming, provided in individual and small group formats, targeted a variety of skill areas (academic, communication, peer interaction, self-care, and play). All programming was supervised by a Board Certified Behavior Analyst®, who was employed by the center. Instructional sessions conducted for the purpose of this study were run in small treatment rooms with only the therapist present. The rooms contained small tables and chairs, instructional and leisure materials, and a video camera. The HBOT sessions took place in a medium size room that served as office space for center staff. The therapists who participated in the study had been working at the center for 2 to 3 years prior to the start of the study. The therapists were trained to implement the procedures described below by the first author. Training consisted of verbal and written instructions, practice, and feedback.
The HBOT sessions took place in a chamber that provided the participants with 88% (+/− 3%) oxygen at 1.3 ATA (according to the manufacturer) and is sold for in-home use (Vitaeris 320, sold by VitaO2). The chamber was 233 cm long and 111 cm wide, with an 86-cm diameter when fully inflated.
Response Measurement and Data Collection
As with any therapy, information about the outcomes of HBOT is limited to the specific responses measured. With this in mind, the current literature on HBOT and anecdotal reports posted on the Internet were examined to identify potential outcomes that could be readily measured within the context of services provided at the day center. Parents and professionals have reported increases in socialization, language, attending, and compliance, as well as decreases in stereotypic behavior, aggression, disruption, and self-injury, among children receiving HBOT (Buckley, 2005; Rossignol & Rossignol, 2006).
Specific responses related to task engagement, language, and problem behavior were defined for each participant, as shown in Table 1. Certain behaviors of the therapist also were measured. This included delivery of verbal instructions, defined as task-related instructions without gestural, model, or physical prompts; delivery of praise, defined as any positive statement (e.g., “good job,” “good try”) not including statements of feedback only (e.g., “That's correct.”); and delivery of tangibles, defined as providing access to food or non-task related objects. It was important to track possible changes in teacher behavior that could explain observed changes in child behavior. For example, an increase in the density of reinforcement for compliance could cause an increase in the child's compliance. If a change in the density of reinforcement corresponded with the introduction of HBOT, changes in child behavior could be attributable to either a change in the therapist behavior or to the HBOT.
Table 1.
Definitions for the Child Responses Measured

Participants were videotaped three times each week during structured, 10-min instructional sessions at the center. Trained observers using hand-held computers or desktop PCs and Instant Data software later scored responses of the child and the therapist from the videotapes of the sessions. The observers were unaware of the purpose of the study, the intervention evaluated, or the point at which the intervention was introduced. For the children, observers scored the frequency of unprompted trials with task engagement, frequency of problem behavior, and frequency of spontaneous communication (words or signs, depending on the child). Problem behavior was measured using frequency recording because all target responses were brief and discrete. Data on the frequency of task engagement were converted to a percentage by dividing the number of unprompted instructional trials with task engagement by the total number of unprompted instructional trials delivered during the session. Data on the frequency of problem behavior and spontaneous communication were converted to responses per minute by dividing the total number of responses by session time. Frequency of unprompted instructional trials, praise, and tangibles delivered by the therapist also was scored. Data on the frequency of praise and tangible delivery were converted to a percentage by dividing the number of deliveries by the total number of trials with task engagement.
Interobserver agreement was measured during 25% of the sessions for each child by having a second observer independently score a random sample of the videotaped sessions. Each session was divided into consecutive 10-s intervals and the data records then compared. The number of intervals for which both observers recorded the same number of responses was divided by the total number of intervals in the session. The result was multiplied by 100 to obtain a percentage of agreement. Across children and sessions, mean percentage of agreement was 92.5% (range, 77% to 98%) for task engagement, 97.6% (range, 65% to 100%) for problem behavior, 91.3% (range, 78% to 100%) for spontaneous communication, 93.5% (range, 81% to 95%) for instruction delivery, 91.8% (range, 85% to 98%) for praise, and 96% (range, 74% to 99%) for tangible delivery.
The therapists also monitored the implementation of HBOT. For each scheduled dive, the therapist recorded the date; the times at which (a) the session started, (b) pressurization began, (c) full pressurization was reached, and (d) depressurization began; the total time at full pressurization; and the total time that the mask was worn (if not for the complete time). If the scheduled dive was canceled, this was noted on the data sheet, along with the reason for the cancelation. A scheduled HBOT session was canceled if the child was experiencing any cold, allergy, or flu symptoms, such as congestion, headache, fever, and cough. Lillie missed three scheduled HBOT dives due to congestion or absences, Carl missed four scheduled dives due to illness or congestion, and Harvey missed five scheduled dives due to cough and congestion. However, both Carl and Harvey still received a total of 40 dives. Lillie's parents decided to discontinue HBOT after 27 dives because she developed an eye infection. Although her physician determined that the infection was unrelated to HBOT, her parents did not want to resume the therapy after the infection was treated.
Procedures
As noted above, the children were videotaped three times each week during structured, 10-min instructional sessions at the center. These sessions were conducted before the HBOT intervention began and continued throughout the intervention period. For all children, sessions continued for an additional month after the HBOT sessions ended. The videotaped sessions occurred at the same time each day (e.g., always at 10 am) and either preceded (Harvey and Carl) or followed (Lillie) the HBOT dive by a minimum of 2 hours during the intervention phase.
Instructional sessions.
Evaluating the benefits of HBOT beyond those obtained through intensive behavioral intervention required careful control over the types of instructional targets presented during the measurement sessions. A representative sample of targets from the child's current curriculum (as provided by case supervisor of the child's educational program) was selected for use during the instructional sessions. Sessions included only those acquisition targets that were currently completed with 50% accuracy or less, on average, during the child's regular therapy sessions at the center. An acquisition target was removed from the sessions when the child's accuracy on the target during regular educational therapy sessions exceeded 50% correct for three consecutive days. Although these targets were removed from the 10-min sessions, the children continued to receive instruction on these targets during their regular therapy sessions at the center. A new target from the same skill domain but for which current performance did not exceed 50% accuracy replaced the previous target. This was done so that improvements in task engagement and problem behavior observed after the introduction of HBOT could not be attributed to increases in accuracy as a result of the continued behavior therapy. That is, the acquisition of the targeted skills as a result of ongoing behavior therapy might necessarily be associated with increases in task engagement and decreases in problem behavior during the observation sessions. Communication targets also were excluded so that changes in spontaneous communication could be measured during these sessions. A minimum of 12 and a maximum of 15 different acquisition targets were included in each session. Instruction on these targets was interspersed with instruction on previously mastered targets (those exceeding 80% correct; called “maintenance targets”) throughout each session. A maintenance target was presented after three consecutive presentations of acquisition targets. Four to five different maintenance targets were presented during each session.
Instructional trials were delivered every 30 s, so that an equal number of instructional trials were delivered during each session. It was important to keep this variable consistent across sessions because the number of trials in a session could influence the child's level of task engagement and problem behavior. A four-step “least-to-most” prompting procedure consisting of verbal, model, and physical prompts was used for all targets. First, the therapist delivered a verbal instruction (e.g., “Touch red.”). If the child failed to respond within 5 s or responded incorrectly, the experimenter removed the materials for 5 s without saying anything and then represented the materials and instruction. If the child again failed to respond within 5 s or responded incorrectly, the therapist delivered a model prompt along with the verbal instruction. If the child failed to respond within 5 s or responded incorrectly following the model prompt, the experimenter used the least amount of physical guidance needed to get the child to engage in the correct response. A second model prompt replaced the physical prompt in the prompt sequence if the target required a vocal response from the child (i.e., the response couldn't be physically guided). Each trial terminated when the child exhibited the correct response or failed to respond correctly following the second model (for verbal responses only).
Reinforcement in the form of praise and access to a tangible item for the remainder of the 30-s interval (or a small food treat) was delivered for each correct, unprompted response. Praise was delivered for responses that followed a model prompt. Correct responses on maintenance tasks were reinforced on a fixed ratio-2 schedule. Prior to each instructional session, tangible and food items to be delivered following correct responses were selected by conducting a brief multiple stimulus preference assessment (DeLeon & Iwata, 1996). Up to five items identified by the child's parent or regular therapist as highly preferred were presented to the child, who was instructed to “pick one.” The child was given access to the item selected, and then the remaining items were presented with the instruction “pick one.” This continued until the child had selected three items. The therapist rotated among these items during the instructional session and occasionally provided choices between instructional trials. Problem behavior generally did not produce any differential consequences; however, brief blocking, reprimands, or redirection were used if needed (e.g., to prevent injury to the child or others; to retrieve the experimenter's materials)
HBOT sessions.
Therapy consisted of 40 60-min sessions in a hyperbaric oxygen chamber. The HBOT was restricted to 40 dives (a) to minimize exposure to an unproven therapy, (b) because of previous reports of improvements following 40 dives (e.g., Rossignol & Rossignol, 2006), and (c) because of the increased risk of side effects associated with additional dives. Prior to initiating the first formal dive of the therapy, the child was gradually acclimated to the oxygen mask and the chamber using the following procedures (with reinforcement provided for tolerance to each step). First, the child was encouraged to sit in the HBOT chamber and to remain inside for 3 min to 5 min while it was zipped closed but not turned on. While sitting in the chamber, the child was permitted to play with the oxygen mask to become familiar with it. The therapist then prompted the child to place the oxygen mask over his or her face and to wear the mask during the 3 min to 5 min acclimation periods. Each afternoon, the child was prompted to remain near the HBOT while it was activated so that the participant could acclimate to the loud noise produced by the chamber. Both of these procedures were implemented several times each week, over a period of approximately two weeks.
At this point, the regular 60-min HBOT sessions commenced. Sessions in which the child did not wear the oxygen mask for the entire session were not included in the total 40 dives. During the HBOT session, the therapist helped the child remove his shoes and place his or her preferred items into the HBOT before getting in. Once in the HBOT, the child placed the oxygen mask on, either independently or with a prompt from the therapist. The therapist remained in the HBOT chamber with the child and completed paperwork. The child had continuous access to preferred items (e.g., portable DVD players, puzzles, books) while in the HBOT. The items were chosen based on the results of ongoing preference assessments and parental report. Food and drinks, such as fruit loops, gum, and juice or water, were also available. The gum and drinks were used to help the child's ears pop when the pressure in the HBOT was changing quickly, at the beginning and end of the session. It should be noted that, relative to baseline, the introduction of HBOT was necessarily associated with a 60-min daily reduction in instruction at the center, along with an increase in access to preferred activities. Although these changes could have potentially influenced the results, controlling for their effects would have required us to remove 60 min of the children's regular instructional time each day during baseline. We decided that the clinical costs associated with this approach outweighed the potential benefits.
Each participant's parents and therapists filled out a daily reporting form designed to monitor potential side effects of the HBOT. The therapy would have been discontinued if any undesirable symptoms were reported.
Experimental Design
A controlled evaluation was necessary to rule out the possibility that changes in behavior were due to a confounding variable rather than to the HBOT. Special consideration had to be given to the experimental design, however, because (a) the effects of HBOT are considered to be irreversible, and (b) the children were receiving intensive behavioral intervention prior to and simultaneously with HBOT. A reversal design was not a viable option because removal of HBOT after receipt of the prescribed “dosage” was not expected to reverse the potential behavioral improvements produced by HBOT. The multiple-baseline design, in which an intervention is sequentially introduced across different participants, behaviors, or settings, is a commonly used design when a reversal is not possible or desirable (Baer, Wolf, & Risley, 1968). A concurrent multiple-baseline across participants design seemed like the best option, given the hypothesized neurological effects of HBOT.
With the multiple-baseline design, the effects of an intervention are demonstrated if responding on each baseline changes when and only when the independent variable is introduced. For each baseline, changes in responding must correspond with the introduction of the intervention on that baseline and should not occur before the intervention has been implemented. However, we anticipated that some improvements in behavior might be observed across the participants' baselines as a result of ongoing behavior-analytic therapy. If so, the independent variable would have to be introduced on trending baselines, making it difficult to determine (a) the ideal point in time to introduce the HBOT across successive baselines, and (b) the extent to which the HBOT was associated with improvements in behavior. To complicate matters, it was not clear how quickly HBOT should produce observable improvements in behavior after the child started to receive the therapy. These complications, resulting from the type of independent variable evaluated (HBOT) and the nature of the evaluation (superimposition of HBOT on ongoing treatment), were addressed in several ways.
First, the baseline phase for each successive participant lasted a minimum of 20 days longer than that for the previous participant because improvements resulting from HBOT have been reported to occur within 20 dives. Baseline was conducted across at least 20 days for the first participant, 40 days for the second participant, and 60 days for the third participant. This strategy ensured that each participant would be exposed to HBOT for an ample length of time before introducing the intervention with the next participant. Second, if baseline trends were apparent, HBOT was introduced after a consistent pattern of responding appeared to emerge, based on visual inspection of the graphed data. Finally, the slopes of the trends prior to and following HBOT were compared in the final data analysis. Fitting a line to the data points using a feature of Excel 2007 created these slopes.
Results
The percentage of unprompted instructional trials with task engagement for the three participants is shown in Figure 1. Calendar dates are inserted below session numbers. It should be noted that Lillie was initially unwilling to wear the oxygen mask for the full duration of the dives. (The therapist held the oxygen tube under her nose whenever she took the mask off.) Thus, these HBOT sessions were not considered part of the 40 total prescribed dives. The data collected during this period are shown for analysis purposes. The arrow on the graph indicates the first instructional session that was conducted after Lillie began consistently wearing the mask for the full dive.
Figure 1.
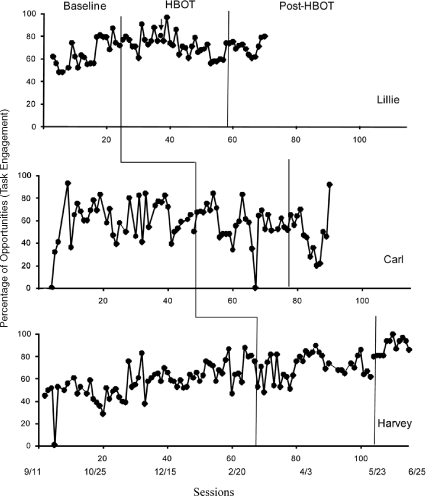
Percentage of task engagement across baseline, HBOT, and post-HBOT conditions for Lillie, Carl, and Harvey. Calendar dates appear below the session numbers.
Both Lillie and Harvey showed increasing trends in task engagement across the baseline phases. HBOT was not associated with any obvious changes in the level, stability, or trend of task engagement compared to baseline for the three children. Lillie's task engagement remained relatively stable initially and then showed a downward trend after she consistently wore the oxygen mask. Her level of task engagement after the termination of HBOT (prematurely due to an eye infection) was similar to that observed during the initial exposure to HBOT. For Carl, levels of task engagement were variable but similar across the baseline, HBOT, and post-HBOT phases. The gradual increase in task engagement across Harvey's lengthy baseline appeared to continue with the introduction of HBOT, suggesting that HBOT did not provide any additional benefits to the ongoing behavior therapy. A somewhat abrupt increase in task engagement coincided with the removal of HBOT. The observer anecdotally noticed that the therapist began to deliver more prompts to attend to the task materials during this phase. Thus, the last 12 sessions in the HBOT phase and the 12 sessions in the post-HBOT phase were re-scored to collect additional data on the frequency of therapist-delivered prompts to attend. Results showed a notable increase in the rate of these prompts (M = .37 rpm during HBOT; M = .74 rpm following HBOT), providing one possible explanation for the abrupt increase in task engagement.
Responses per minute of problem behavior for each participant are shown in Figure 2. Baseline data for all of the participants show a gradual decrease in problem behavior, with the exception of the last two points for Lillie. These descending trends continue following the introduction of HBOT, and the overall results do not suggest that HBOT enhanced the effects of ongoing behavioral intervention. Most interesting, however, was the slight increase in problem behavior for Carl with the removal of HBOT. The increase was rather immediate, suggesting that a confounding component of the HBOT sessions (e.g., spending an hour each day with preferred activities) was responsible for the reduced levels of problem behavior during the HBOT phase. Such an abrupt increase in problem behavior would be unlikely to occur if the improvement in behavior was due to HBOT.
Figure 2.
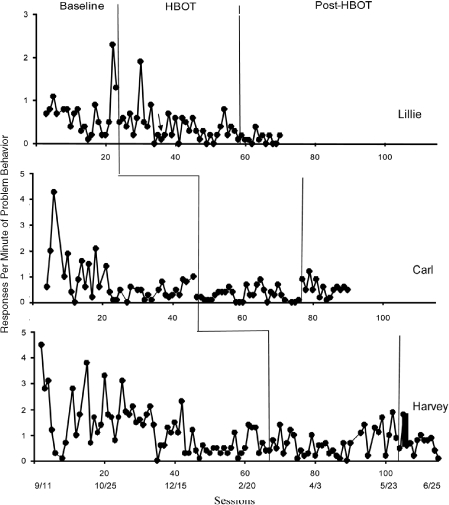
Responses per minute of problem behavior across baseline, HBOT, and post-HBOT conditions for Lillie, Carl, and Harvey. Calendar dates appear below the session numbers.
Finally, data on spontaneous communication for each participant are shown in Figure 3. For Lillie and Carl, levels of responding were similar prior to, during, and following HBOT. The introduction of HBOT for Harvey appeared to be associated with an increase in spontaneous communication. Similar levels persisted for up to a month after HBOT was terminated.
Figure 3.
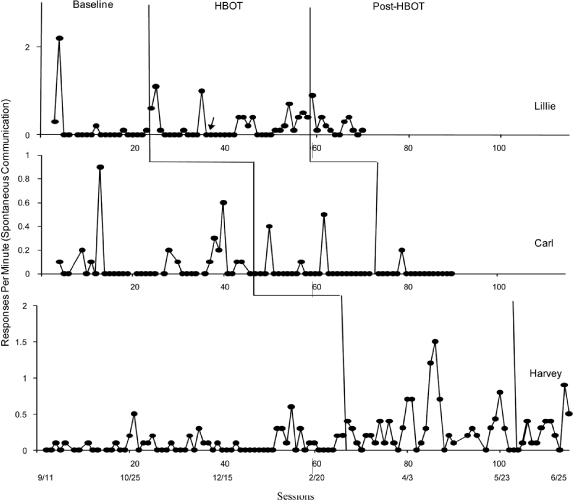
Responses per minute of spontaneous communication across baseline, HBOT, and post-HBOT conditions for Lillie, Carl, and Harvey. Calendar dates appear below the session numbers.
Conclusions and Implications for Practice
To our knowledge, this is the first controlled evaluation of HBOT, an unproven therapy for autism that has grown more popular in recent years. The purpose of the study was to evaluate the potential benefits of HBOT for three children whose parents had requested this intervention. Results suggest that the form of HBOT provided to these participants did not improve task engagement or decrease problem behavior beyond that provided by ongoing behavior analytic services. HBOT also was not associated with changes in spontaneous communication for two of the three participants. Conclusions about the effects of HBOT on spontaneous communication are somewhat difficult to draw. It is possible that HBOT produced idiosyncratic outcomes across the participants, with positive effects obtained for Harvey only. Alternatively, the increase in Harvey's spontaneous communication may have been the result of (a) ongoing behavior analytic intervention, or (b) changes in an unknown variable that occurred simultaneously with the introduction of HBOT. Unfortunately, the viability of these alternative interpretations can't be determined without additional experimental analysis. However, these results failed to show any robust changes in behavior as a result of HBOT, indicating that the therapy was not worth the associated costs for these three children.
Further research is needed to determine if HBOT is a viable treatment for autism. A more intensive form of this therapy (e.g., higher oxygen concentrations, larger numbers or durations of dives) may produce beneficial outcomes. HBOT also may have behavioral effects that were not captured by the measurement system used in this study. The generality of the results also are limited because only three children participated. Furthermore, it should be noted that our approach to the study of this new biomedical treatment (i.e., use of a single-subject design; superimposition of HBOT on intensive behavioral intervention) raised a number of difficulties, as discussed in more detail below. In future work, the effects of HBOT might be evaluated with children who have not yet begun to receive behavioral intervention.
Several confounding features of HBOT also were not controlled during the study. Specifically, the introduction of HBOT was necessarily associated with a reduction in instruction (by 60 min per day), along with an increase in access to preferred activities (while in the HBOT). It is possible that the reduced instruction counteracted gains that were produced by HBOT in combination with intensive behavioral intervention. These confounds also could have reduced the participants' motivation to engage in problem behavior during the HBOT phase. Carl exhibited an immediate increase in problem behavior when HBOT was discontinued, an outcome that was consistent with this latter possibility. These confounds could have been controlled in baseline by permitting the children to engage with preferred activities for 60 min each day during their regular instructional time. We decided, however, that the clinical costs associated with this approach outweighed the potential benefits.
Although somewhat challenging, practitioners should consider conducting this type of evaluation when clients choose to implement unproven therapies while still receiving behavior analytic services. The study illustrates some of the considerations that enter into this type of evaluation. Issues related to response measurement, experimental design, procedural integrity, control over extraneous variables, identification and elimination of possible confounds, and data interpretation are at the forefront of any experimental evaluation. Among other things, practitioners must select the most appropriate responses to measure and the context(s) under which to measure them; establish a reliable measurement system; and design the procedures to best examine the intervention of interest. Ultimately, the experimental analysis must be arranged so that reasonable conclusions can be drawn about the effects of the independent variable on the dependent variable(s). Additional guidelines and considerations for conducting this type of experimental analysis are provided in Table 2.
Table 2.
Primary Areas of Consideration with Guidelines for Experimental Evaluation of Unproven Therapies

In the current evaluation, we addressed the challenges inherent in this type of investigation with varying degrees of success. For example, the responses selected were convenient to measure but somewhat circumscribed; certain controls were omitted due to practical concerns (e.g., requiring stable baselines before implementing HBOT, holding the amount of instructional time constant in baseline and treatment). A post-hoc analysis also showed that one important variable (prompts to attend for Harvey) was not held constant due to procedural drift by the therapist. On the other hand, a number of potentially important extraneous variables were controlled (e.g., the rate of demands and type of instructional targets during the observation sessions), and the introduction of HBOT was staggered across relatively lengthy baseline periods.
As parents and professionals continue to try new unproven therapies for autism, practitioners with expertise in behavior analysis can assist by obtaining objective, quantifiable data on the outcomes for individual children. The results may provide valuable information not just for individual consumers, but for the field of autism, as well. It is our hope that the evaluation described in this paper will serve as a useful exemplar for additional experimental analyses of unproven interventions.
Footnotes
For further information, contact Dorothea C. Lerman, Ph.D., 2700 Bay Area Blvd., Box 245, Houston, TX 77058; lerman@uhcl.edu.
References
- Adamides A.A, Winter C.D, Lewis P.M, Cooper D.J, Kossmann T, Rosenfeld J.V. Current controversies in the management of patients with severe traumatic brain injury. ANZ Journal of Surgery. 2006;76:163–174. doi: 10.1111/j.1445-2197.2006.03674.x. [DOI] [PubMed] [Google Scholar]
- Baer D. M, Wolf M. M, Risley T. R. Some current dimensions of applied behavior analysis. Journal of Applied Behavior Analysis. 1968;1:91–97. doi: 10.1901/jaba.1968.1-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behavior Analyst Certification Board®. Guidelines for Responsible Conduct for Behavior Analysts. 2004. (available for download at www.bacb.com)
- Buckley J. A. How mild hyperbaric oxygen therapy works and why it is good for our children. Medical Veritas. 2005;2:67. [Google Scholar]
- Carson S, McDonagh M, Russman B, Helfand M. Hyperbaric oxygen therapy for stroke: A systematic review of the literature. Clinical Rehabilitation. 2005;19:819–833. doi: 10.1191/0269215505cr907oa. [DOI] [PubMed] [Google Scholar]
- Goin-Kochel R.P, Myers B. J, Mackintosh V.H. Parental reports on the use of treatments and therapies for children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2007;1:195–209. [Google Scholar]
- Hanson E, Kalish L.A, Bunce C.C, McDaniel S, Ware J, Petry J. Use of complementary and alternative medicine among children diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2007;37:628–636. doi: 10.1007/s10803-006-0192-0. [DOI] [PubMed] [Google Scholar]
- Harch P.G, Neubauer R.A. Hyperbaric oxygen therapy in global cerebral ischemia/anoxia and coma. In: Jain K.K, editor. Textbook of hyperbaric medicine. 3rd ed. Seattle, WA: Hogrefre and Huber Publishers; 1999. (Ed.) [Google Scholar]
- Irvin D.S. Using analogue assessment procedures for determining the effects of a gluten-free and casein-free diet on rate of problem behaviors for an adolescent with autism. Behavioral Interventions. 2006;21:281–286. [Google Scholar]
- Jacobson J.W, Foxx R.M, Mulick J.A. Controversial therapies for developmental disabilities. Mahwah, NJ: Erlbaum; 2005. [Google Scholar]
- Liptak G.S. Complementary and alternative therapies for cerebral palsy. Mental Retardation and Developmental Disabilities. 2005;11:156–163. doi: 10.1002/mrdd.20066. [DOI] [PubMed] [Google Scholar]
- McDonough M, Carson S, Ash J, Russman B.S, Krages K. P, Helfand M. Evidence report/technology assessment No 85. AHRQ Publication No. 04-E003. Rockville, MD: Agency for Healthcare Research and Quality; 2003. Hyperbaric oxygen therapy for brain injury, cerebral palsy, and stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montee B. B, Miltenberger R. G, Wittrock D. An experimental analysis of facilitated communication. Journal of Applied Behavior Analysis. 1995;28:189–200. doi: 10.1901/jaba.1995.28-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D.M, Reese R.M, Daniels D. Use of evidence-based practice as a method for evaluating the effects of secretin on a child with autism. Focus on Autism and Other Developmental Disabilities. 1999;14:204–211. [Google Scholar]
- Rossignol D.A, Rossignol L.W. Hyperbaric oxygen therapy may improve symptoms of autistic children. Medical Hypotheses. 2006;67:216–228. doi: 10.1016/j.mehy.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Schechtman M.A. Scientifically unsupported therapies in the treatment of young children with Autism Spectrum Disorders. Psychiatric Annals. 2007;37:639–645. doi: 10.3928/0090-4481-20070801-12. [DOI] [PubMed] [Google Scholar]
- Simpson R. L. Autism spectrum disorders: Interventions and treatments for children and youth. Thousand Oaks, CA: Corwin Press; 2005. [Google Scholar]
- Smith T, Antolovich M. Parental perceptions of supplemental interventions received by young children with autism in intensive behavior analytic treatment. Behavioral Interventions. 2000;15:83–97. [Google Scholar]
- Wong H.H.L, Smith R.G. Patterns of complementary and alternative medical therapy use in children diagnosed with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36:901–909. doi: 10.1007/s10803-006-0131-0. [DOI] [PubMed] [Google Scholar]


