Abstract
We have shown previously that vaccination with recombinant chlamydial protease-like activity factor (rCPAF) plus interleukin-12 as an adjuvant induces robust protective immunity against primary genital Chlamydia muridarum challenge in mice. Since CPAF is a protease, we compared the effects of enzymatically active and inactive (heat-denatured) rCPAF to determine whether proteolytic activity is expendable for the induction of protective immunity against chlamydial challenge. Active, but not inactive, rCPAF immunization induced high levels of anti-active CPAF antibody, whereas both induced robust splenic CPAF-specific IFN-γ production. Vaccination with active or inactive rCPAF induced enhanced vaginal chlamydial clearance as early as day 6 with complete resolution of infection by day 18, compared to day 30 in mock-vaccinated and challenged animals. Importantly, significant and comparable reductions in oviduct pathology were observed in active and inactive rCPAF vaccinated mice compared to mock-vaccinated animals. Thus, rCPAF induced anti-chlamydial immunity is largely independent of enzymatic activity and secondary or higher order protein conformation.
INTRODUCTION
Chlamydia trachomatis, a Gram negative obligate intracellular bacterium, is the leading cause of bacterial sexually transmitted disease (STD) worldwide [1–3]. Genital chlamydial infections are observed predominantly in young adults [1]. In females, the majority of cases are left untreated due to lack of symptoms and despite availability of efficacious antimicrobial therapy [2]. Untreated infections ascend to the upper genital tract (UGT) and cause severe sequelae including pelvic inflammatory disease, ectopic pregnancy, and infertility [4]. The continued increase in the incidence of chlamydial STD worldwide [5] underscores the need for a safe and efficacious preventive vaccine. To this end, a recent report based on theoretical modeling suggests that prophylactic vaccines could have a tremendous impact towards reducing chlamydial infections and disease in the foreseeable future [6].
Chlamydial protease-like activity factor (CPAF) is a bacterial protease, secreted into the host cytosol during chlamydial infection [7, 8], that degrades several host proteins [9] including host transcription factors RFX-5 and USF-1 responsible for MHC expression [7], cytoskeletal proteins such as keratin-8 [10], and pro-apoptotic proteins [11]. Thus, CPAF has been suggested to be potentially important for the virulence of the bacterium [9]. However, upon rupture of infected cells, cytosolic CPAF is likely released into the extracellular environment, taken up by neighboring uninfected cells, processed via the exogenous MHC II pathway, and presented to CD4+ T cells [12]. In this regard, CPAF has been shown to be a dominant immunogen in Chlamydia sero-positive human individuals [13–15]. This suggested that vaccination strategies to induce CPAF-specific CD4+ T cells may mediate protective immunity against genital chlamydial infections.
We previously have demonstrated the efficacy of intranasal immunization with recombinant (r) CPAF, plus interleukin-12 (IL-12) as adjuvant, towards significantly enhancing chlamydial clearance and reducing the development of reproductive pathology following primary genital challenge [16]. We further demonstrated that the rCPAF-induced immunity was mediated by IFN-γ producing CPAF-specific CD4+ T cells [17, 18]. Additionally, neither the MHC I pathway (CD8+ T cells) [18] nor B cells and antibodies was required for the observed protective effects of the rCPAF vaccination [19]. Collectively, these findings suggested that linear antigenic epitopes on the rCPAF molecule, that elicit T cell responses, may be sufficient to induce protective immunity, raising the promising possibility that the protease-activity of CPAF can be eliminated to induce safe, in addition to highly effective, anti-chlamydial immunity.
In this study, we used rCPAF that was proteolytic (active) or rendered non-proteolytic by heat-denaturation (inactive), together with IL-12 as an adjuvant, and evaluated protective immunity against primary genital chlamydial challenge in female BALB/c mice. Inactive rCPAF induced comparable enhancement of genital chlamydial clearance and reduction of upper genital pathologies when compared to active rCPAF.
MATERIALS AND METHODS
Chlamydia muridarum
Chlamydial stocks were prepared as described previously [20, 21]. Confluent monolayers of HeLa cells were grown in Dulbecco’s modification of Eagle’s medium with 10% FBS and infected with Chlamydia muridarum. Cells were lysed using a sonicator and elementary bodies (EBs) were purified on Renograffin gradients as described previously. Stocks were stored at −80°C in sucrose–phosphate-glutamine (SPG) buffer and diluted appropriately for the challenge.
rCPAF and IL-12
The CPAF gene from C. muridarum genome was cloned into a PGEX vector, transformed into a BL21 Escherichia coli strain, and expressed as a fused protein with glutathione S-transferase (GST) as described previously [7]. Single colonies of bacteria were isolated and cultures were grown at 37°C and then induced for 1.5 hr at 25°C with 0.1mM isopropyl-beta-D-thiogalactopyranoside (IPTG). CPAF fused to GST was purified using glutathione Sepharose 4B beads (Bioplus Research Chemicals, Dublin, OH). rGST alone also was cloned into E. coli PGEX vector systems [7] and purified as above. The purified protein was subjected to electrophoresis on an SDS-polyacrylamide gel and subsequently stained with coomassie blue. The protein was transferred onto a PVDF membrane and probed with mouse anti-CPAF n-terminus monoclonal antibody (54b). Inactivation of the enzymatic activity of CPAF was carried out by heating the protein at 100°C for 5 min. Purified GST-CPAF was used for all experiments and intranasal (i.n.) immunizations were carried out with the addition of recombinant mouse IL-12 (R&D systems, Minneapolis, MN) as a mucosal adjuvant [22, 23].
Cell-Free Keratin 8 Degradation Assay
CPAF activity was confirmed using a cell free assay as previously described [10] with keratin-8 from the crude extract of the cytosolic fraction of HeLa cells (CE) as the substrate. Active rCPAF, inactive rCPAF, Chlamydia-infected HeLa cell subfraction (S100 fraction-native CPAF), or GST were used as enzyme sources and incubated with the substrate (CE) for 3 hr at 37°C. Undegraded and degraded keratin-8 fragments were detected using a mouse monoclonal anti-keratin-8 antibody (M20; Sigma, St. Louis, Mo), followed by goat anti-mouse secondary antibody conjugated to horseradish peroxidase (Southern Biotechnology, Birmingham, Alabama), with analysis by enhanced chemiluminescence (ECL).
Mice
4–5 week old female BALB/c mice were obtained from the National Cancer Institute (Wilmington, MA) and housed at the University of Texas at San Antonio. Food and water was supplied ad libitum and all experimental procedures followed the guidelines of the Institutional Animal Care and Use Committee (IACUC).
Immunization
Mice (6 per group) were immunized i.n. with active rCPAF (15 μg), inactive rCPAF (15 μg), rGST alone (5 μg; based on GST protein content in 15 μg GST-rCPAF fusion) or PBS on days 0, 14, and 28 along with 0.5 μg of rIL-12. Mice were primed with 0.5 μg of IL-12 alone on days −1 and +1. Optimal doses for rCPAF and IL-12 administration were based on our previous findings [16, 20].
Cytokine Response
Antigen-specific cytokine production from splenocytes was measured as described previously [16, 20]. Twenty days after the last booster immunization with rCPAF+IL-12, mice (3 per group) were euthanized, spleens collected, and single cell suspensions made. Splenocytes (106 cells/well) were plated along with 0.1 μg of active rCPAF, inactive rCPAF, rGST, or the unrelated protein bovine serum albumin (BSA), and incubated for 72 hr. Supernatants were collected and assayed for IFN-γ and IL-4 production by ELISA using BD OptEIA™ kits (BD Pharmingen, San Diego, CA). The levels of respective cytokines were quantified by measuring the absorbance at 630 nm using a μQuant ELISA plate reader (BioTek Instruments, Winooski, VT).
Detection of Total Antibody and Isotype Levels
Ten days after the last booster immunization, mice were bled and serum was separated to measure antibody levels as described previously [16, 20]. Microtiter plates were coated with 1μg/well of active rCPAF, inactive rCPAF, rGST, or the unrelated antigen BSA, and incubated overnight at 4°C. Serum was serially diluted and incubated for 2 hr, followed by incubation with goat anti-mouse total Ig (H+L), IgM, IgG1, IgG2a, IgG2b, or IgG3 conjugated to horseradish peroxidase (HRP) as secondary antibody (Southern Biotechnology). Tetramethylbenzidine substrate was added and the absorbance quantified at 630 nm using a μQuant ELISA plate reader (BioTek Instruments, Winooski, VT). Reciprocal antibody titers were calculated for each group of mice using 50% maximal binding of serum.
Chlamydia muridarum Challenge and Monitoring of Bacterial Shedding
Mice were rested for one month after the final immunization and challenged intravaginally (i.vag.) with 5×104 inclusion forming units (IFU) of Chlamydia muridarum, as described previously [16]. Subcutaneous (s.c.) depo-progesterone injections were given at 10 and 3 days prior to challenge to synchronize the estrous cycles of the mice. Vaginal bacterial shedding was determined every third day for a period of 30 days following challenge, by swabbing the infected mouse vaginas and incubating the swabbed material on HeLa cell monolayers grown on glass cover slips in 24 well plates. Cells were fixed with 2% paraformaldehyde and stained using a genus-specific rabbit monoclonal antibody and FITC labeled goat anti-rabbit secondary antibody. Inclusions were counted in ten random mid-line fields for days 3 to 12, and over the entire coverslips for later time points. The mean ± SE of recovered chlamydial IFU per group of mice is reported.
Assessment of Pathology in the Upper Genital Tract
Based on our extensive analyses conducted previously [16], UGT pathology was evaluated on day 80 post-challenge. Mice were euthanized, genital tracts removed and the presence of gross hydrosalpinx was noted. The tissues were then photographed from a fixed distance using a 6 mega-pixel Fuji F10 camera. Images were stored at high resolution and printed on sheets (A4 size) of paper. The cross-sectional diameter of the oviducts was measured for the largest oviduct loop and the measurements for individual mice and mean ± SE for each group is reported as described previously [19]. As a baseline for our measurements, a normal mouse oviduct cross-sectional diameter (0.5 mm) was used.
Genital tracts were collected at day 80 post challenge, embedded into paraffin blocks, sectioned (5μm thickness), stained with hematoxylin and eosin and oviduct dilatation observed using a Zeiss Axioskop 2 research microscope. Micrographs were obtained at 10X magnification and printed on sheets (A4 size) of paper. As a measure of oviduct pathology, including luminal dilatation, loss of epithelial folds, and reduction in wall thickness, the largest luminal diameter of the oviduct and wall thickness was measured using a scale in millimeters and expressed as a ratio of diameter to wall thickness. As a baseline for our measurements, a normal mouse oviduct diameter/wall thickness ratio (20) was used. The ratio of individual oviduct diameter/wall thickness and the mean ± SE for each group of mice is reported [24].
Statistical Analyses
For comparison of two groups, the Student t test (for normally distributed values) or the Mann-Whitney rank sum test (for values not distributed normally) was used to compare values of continuous variables. For experiments with more than two groups of animals, analysis of variance (ANOVA) followed by multiple comparison of means was used. To analyze differences in the time required for bacterial clearance, the Kaplan-Meier test was used. Differences between groups were considered statistically significant if P values were ≤ 0.05. All data shown are representative of two or three independent experiments conducted under the same conditions, and each experiment shown was analyzed independently.
RESULTS
Generation of Native and Denatured rCPAF
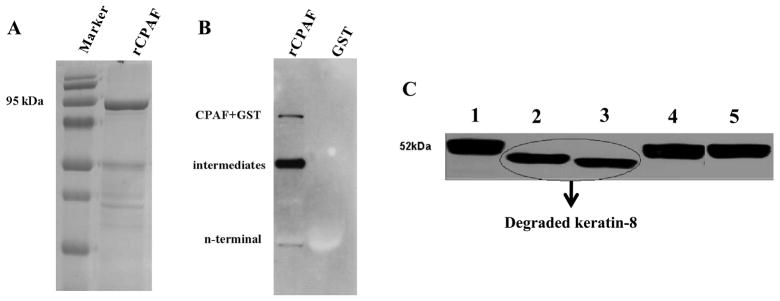
Recombinant CPAF tagged to GST (GST-CPAF) was expressed in the E. coli pGEX vector system and purified using Sepharose 4B beads, as described previously [7]. The purified protein was identified as ~95 kDa band that corresponded to the combined molecular weights of rCPAF (~69 kDa) and GST (~26 kDa) (Fig. 1A). Moreover, Western blot analysis using mouse anti-CPAF n-terminus monoclonal antibody (54b) [25; 26] confirmed the purified protein as rCPAF (Fig. 1B). The purified rCPAF was aliquoted, and some aliquots were denatured at 100°C for 5 min. The non-denatured and denatured proteins were incubated for 3 hr with cyotsolic extract from HeLa cells, and the mixture was subsequently subjected to SDS-PAGE electrophoresis. Western blot analysis using anti-human keratin-8 antibody demonstrated that non-denatured rCPAF degraded keratin-8, whereas the denatured rCPAF did not (Fig. 1C). These results indicate the generation of enzymatically-active (referred to as “active”), and denatured (referred to as “inactive”) rCPAF, which were used for all subsequent analyses.
Figure 1. Molecular size and enzymatic activity of rCPAF.
(A) GST-rCPAF was purified using glutathione Sepharose 4B and loaded in a 10% SDS-polyacrylamide gel and stained with coomassie blue. (B) GST-rCPAF and GST alone were probed with mouse anti-CPAF c-terminus monoclonal antibody. (C) Purified active or inactive rCPAF from C. muridarum, or S100 fraction from HeLa cells infected with C. trachomatis (L2S100), or GST were incubated with cytosolic extract (CE) from the HeLa cells at 37°C for 3 hr. The full length and degraded keratin-8 fragments were detected using mouse anti-human keratin-8 primary antibody followed by goat anti-mouse secondary antibody and developed using an ECL reagent. Lanes 1: CE only, 2: CE+native CPAF, 3: CE+active rCPAF, 4: CE+denatured rCPAF, and 5: CE+GST.
CPAF-Specific Immune Responses Following Immunization
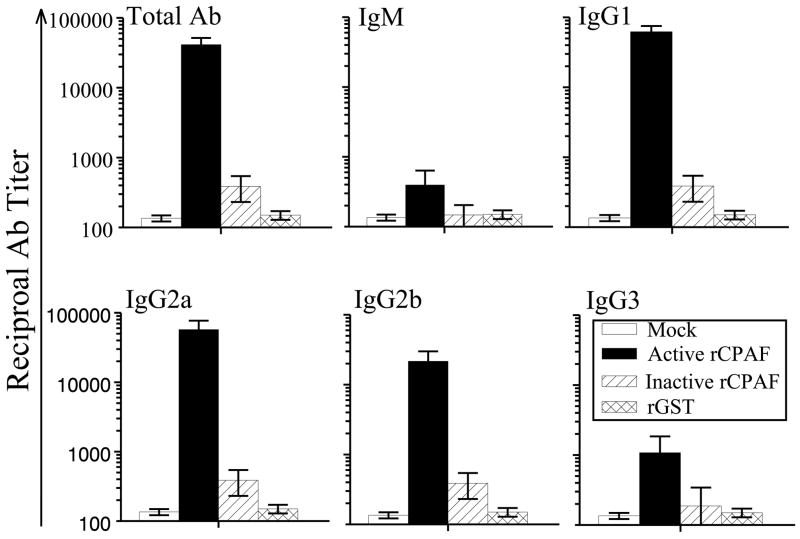
Groups of four-six week-old female BALB/c mice (n=9) were immunized i.n. with active rCPAF (15 μg per mouse), inactive rCPAF (15 μg per mouse), rGST, or PBS (mock) on day 0, and booster immunizations administered on days 14 and 28. IL-12 (0.5 μg per mouse) was used as an adjuvant. Additionally, IL-12 alone was given i.n. to the mice on days −1 and +1. On day 40 after the initial immunization, mice were bled and sera separated for antibody analyses. Since the CPAF produced during infection in vivo would be in native configuration and enzymatically active, we measured anti-active rCPAF antibody responses. As shown in Fig. 2, active CPAF vaccinated mice showed elevated levels of anti-CPAF total IgG (41429 ± 10271), IgG1 (62365 ± 12811), IgG2a (57435 ± 19603), and IgG2b (21618 ± 8311), but low levels of IgG3 (1081 ± 766) and IgM (395 ± 240). The titers of anti-GST antibodies were subtracted from each condition, and therefore the bars shown correspond only to anti-CPAF antibody levels. Interestingly, sera from mice immunized with inactive CPAF displayed minimal levels of anti-active CPAF antibodies. As expected, there were minimal levels of anti-active CPAF antibodies observed in GST and PBS vaccinated mice and in wells coated with the unrelated antigen BSA. Collectively, these results suggest that antibodies induced against CPAF are largely dependent upon the conformation of the protein.
Figure 2. Humoral responses after vaccination.
Four to five week old female BALB/c mice (n=6) were immunized i.n. with active rCPAF, inactive rCPAF, rGST, or PBS (mock) plus IL-12 on day 0, and booster immunizations were given on days 14 and 28. Mice also were given IL-12 alone on days −1 and +1. Ten days after the final immunization, mice were bled, sera were obtained and analyzed for total antibody and various isotypes of antibodies against active rCPAF using an indirect ELISA. Results are represented as mean ± SE of reciprocal titers corresponding to the 50% maximum binding. Titers of rCPAF specific antibodies induced by immunization with active rCPAF immunization were significantly (P ≤ 0.05; Student’s t test) greater than those induced by immunization with inactive rCPAF or GST or PBS. Results are representative of two individual experiments.
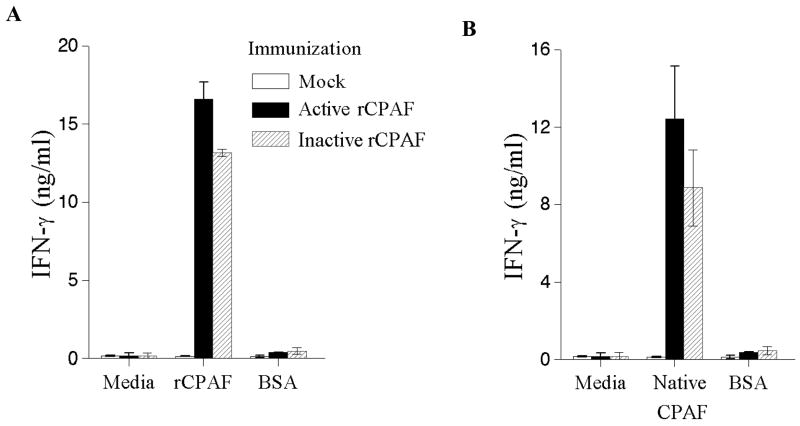
On day 50 following initial immunization, mice were euthanized, spleens collected and single cell suspensions made. The cells were stimulated with active rCPAF, rGST, or the unrelated antigen BSA. The supernatants were collected after 72 hr incubation and analyzed for IFN-γ and IL-4 levels using an indirect ELISA. As shown in Fig. 3A, splenocytes from both the active and inactive rCPAF vaccinated animals showed enhanced levels of IFN-γ upon stimulation with active rCPAF (16.6 ± 1 and 13.2 ± 0.2 ng, respectively) compared to mock- and GST-immunized mice. Some differences were noted in the levels of IFN-γ production between active rCPAF versus inactive rCPAF immunized mice, but there was no propensity for one group to consistently produce more cytokine than the other in repeat experiments. The responses against rGST were subtracted and the bars shown represent only anti-CPAF responses. Additionally, minimal cytokine production was observed upon stimulation with the unrelated antigen BSA. We also confirmed that elevated levels of cellular IFN-γ responses were induced against native CPAF C. trachomatis serovar L2 (S100 fraction of infected HeLa cells) in mice immunized with active or inactive C. muridarum rCPAF (Fig. 3B), with greater, albeit not statistically significant, differences in IFN-γ production from active rCPAF compared to inactive rCPAF immunized mice. There were minimal IL-4 responses in all the examined conditions. These results suggest that protein conformation is not a significant constraint for the induction of robust anti-CPAF IFN-γ responses.
Figure 3. Antigen-specific IFN-γ responses following vaccination.
Four to five week old female BALB/c mice (n=6) were immunized i.n. with active rCPAF, inactive rCPAF, rGST, or PBS (mock) plus IL-12 on day 0, and booster immunizations were given on days 14 and 28. Mice were also given IL-12 alone on days −1 and +1. Twenty days after the final booster immunization, the mice (n=3) were euthanized, spleens collected and single cell suspensions made and stimulated in vitro with the indicated antigens. (A) rCPAF-specific IFN-γ response. Splenic cells from each group were stimulated with active rCPAF, unrelated antigen BSA, or cultured in media alone, and analyzed for IFN- γ production using an indirect ELISA. (B) Native CPAF-specific IFN-γ response. Cells were stimulated with L2S100 (S100 fraction of Chlamydia infected HeLa cells), BSA, or cultured in media alone, and analyzed for IFN- γ production. Results are represented as CPAF-specific responses, after subtracting the responses against GST, and reported as mean ± SE of titers of mice for individual groups. Significant difference (P ≤ 0.05; Student’s t test) in cytokine secretion between mock-vaccinated mice versus active or inactive rCPAF groups.
Kinetics of Chlamydial Clearance in Mice Vaccinated with Active and Inactive rCPAF
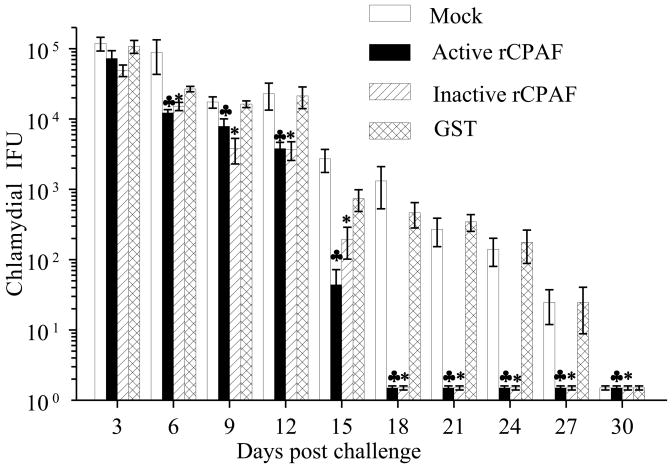
Mice were immunized with active rCPAF, inactive rCPAF, rGST or PBS on days 0, 14, 28 with IL-12 as adjuvant. Mice also were given IL-12 alone on days −1 and +1. After the final immunization, mice were rested for 30 days and challenged i.vag. with 5×104 IFU of C. muridarum. Vaginal chlamydial shedding was monitored every third day after the challenge, until day 30. As shown in Fig. 4, active and inactive rCPAF immunized mice exhibited significant reductions in vaginal chlamydial shedding as early as day 6 (12244 ± 1357 and 15200 ± 2033, respectively) compared to PBS and GST immunized groups (88385 ± 45228 and 26740 ± 2365, respectively), and the differences were apparent each time-point thereafter. Cessation of bacterial shedding was observed as early as day 9 in some inactive rCPAF immunized mice, or day 15 in active rCPAF immunized mice, with all animals in both groups exhibiting complete resolution of infection by day 18 after challenge (Table 1). In comparison, some PBS and GST immunized mice exhibited cessation of vaginal chlamydial shedding by days 21 and 24, respectively, with all mice in these groups completely clearing the infection by day 30 after challenge. These results indicate that immunization with either active or inactive rCPAF induces significant and comparable enhancements in bacterial clearance after primary genital chlamydial challenge.
Figure 4. Chlamydial shedding from vagina following bacterial challenge in vaccinated mice.
Four to five week old female BALB/c mice (n=6) were immunized i.n. with active rCPAF, inactive rCPAF, rGST, or PBS (mock) plus IL-12 on day 0, and booster immunizations were given on days 14 and 28. Mice were also given IL-12 alone on days −1 and +1. One month after the final immunization, mice were challenged i.vag. with 5×104 IFU of C. muridarum. Vaginal swabs were obtained and analyzed for numbers of chlamydial IFU recovered. The shedding of bacteria was monitored every third day for 30 days. Results are represented as mean ± SE. Significant differences between mock-vaccinated versus active rCPAF (♣) or inactive rCPAF groups (*) (P ≤ 0.05, Mann-Whitney Rank Sum test).
Table 1. Percentage of mice shedding bacteria.
Four to five week old female BALB/c mice (n=6) were immunized i.n. with active rCPAF, inactive rCPAF, rGST, or PBS (mock) plus IL-12 on day 0, and booster immunizations were given on days 14 and 28. Mice also were given IL-12 alone on days −1 and +1. One month after the final immunization, mice were challenged i.vag. with 5×104 IFU of C. muridarum. The percentage of mice shedding bacteria every third day after i.vag. challenge for 30 days is shown. Significant differences in the time required for complete clearance of bacteria between mock-vaccinated versus active rCPAF (♣) or inactive rCPAF (*) vaccinated animals (P ≤ 0.05, Kaplan-Meier analysis). Results are representative of two individual experiments.
| Immunization (n=6/group) | % of mice shedding Chlamydia from the vagina Days after i.vag. challenge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | |
| Mock | 100 | 100 | 100 | 100 | 100 | 100 | 83 | 67 | 50 | 0 |
| ♣ Active rCPAF | 100 | 100 | 100 | 100 | 33 | 0 | 0 | 0 | 0 | 0 |
| * Inactive rCPAF | 100 | 100 | 83 | 83 | 83 | 0 | 0 | 0 | 0 | 0 |
| rGST | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 67 | 33 | 0 |
Upper Genital Tract Pathology in Mice Vaccinated with Active or Inactive rCPAF
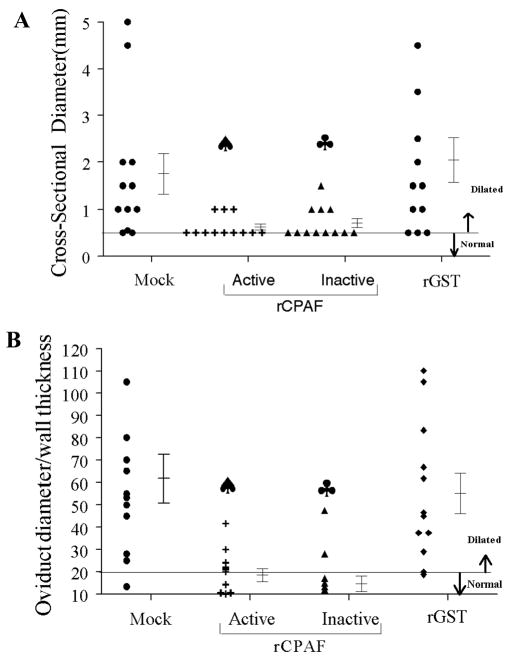
At day 80 after bacterial challenge, mice were euthanized and genital tracts were collected for examination of gross hydrosalpinx. As shown in Table 2, 83% each (66% bilateral, 17% unilateral) of mock- and rGST-immunized mice displayed hydrosalpinx. In comparison, hydrosalpinx was observed in significantly fewer (33% each) mice vaccinated with active rCPAF (17% bilateral, 17% unilateral), and inactive rCPAF (33% bilateral). The genital tracts also were photographed and the gross oviduct cross-sectional diameter was measured as a quantitative indicator of the degree of hydrosalpinx. As shown in Fig. 5A, mock-vaccinated mice displayed a high degree of oviduct dilatation (1.75 ± 0.43mm) when compared to age-matched naïve mice (0.50 mm). Active and inactive rCPAF vaccinated mice displayed significantly reduced oviduct dilatation (0.62 ± 0.06 and 0.71 ± 0.10, respectively) in comparison to mock-immunized animals. The oviduct dilatation in rGST-treated mice was comparable to that in mock-vaccinated animals.
Table 2. Percentage of mice developing hydrosalpinx.
Four to five week old female BALB/c mice (n=6) were immunized i.n. with active rCPAF, inactive rCPAF, rGST, or PBS (mock) plus IL-12 on day 0, and booster immunizations were given on days 14 and 28. Mice were also given IL-12 alone on day −1 and +1. One month after the final immunization mice were challenged i.vag. with 5×104 IFU of C. muridarum. At day 80 after challenge, mice were euthanized, dissected, and the upper genital tracts were observed in situ. The percentage of mice displaying hydrosalpinx, and those with bilateral and unilateral hydrosalpinx are reported. Significant difference between mock-vaccinated versus active rCPAF (♠) or inactive rCPAF (♣) vaccinated groups (P ≤ 0.05, Student’s t test). Results are representative of two individual experiments.
| Immunization (n=6/group) | % of mice with hydrosalpinx | Bilateral | Unilateral |
|---|---|---|---|
| Mock | 83 | 66 | 17 |
| Active rCPAF ♠ | 33 | 17 | 17 |
| Inactive rCPAF ♣ | 33 | 33 | 0 |
| rGST | 83 | 66 | 17 |
Figure 5. Oviduct pathology following chlamydial challenge in vaccinated mice.
Four to five week old female BALB/c mice (n=6) were immunized i.n. with active rCPAF, inactive rCPAF, rGST, or PBS (mock) plus IL-12 on day 0, and booster immunizations were given on days 14 and 28. One month after the final immunization, mice were challenged with 5×104 IFU of C. muridarum and pathology was observed on day 80 post challenge. (A) Macroscopic oviduct dilatation. At 80 days post-challenge, animals were euthanized and genital tracts were collected and photographed. The cross sectional diameter for dilated oviducts was measured and the highest one was reported, with mean ± SE per group of mice. Significant reduction in oviduct dilatation with active and inactive rCPAF vaccination compared to PBS or GST (P < 0.05, Student t- test). (B) Microscopic dilatation. Genital tracts were collected, embedded in paraffin blocks, and sectioned (5μm thickness). These sections were stained with haematoxylin and eosin and the extent of dilatation reported as largest oviduct diameter/wall thickness ratio with mean ± SE. Significant difference between mock-vaccinated versus active rCPAF (♠) or inactive rCPAF (♣) vaccinated animals (P ≤ 0.05, Student t test). Results are representative of two individual experiments.
If oviducts are dilated following genital chlamydial infection, the normal epithelial folds are flattened and the walls of the oviducts are stretched. Microscopic cross-sections, but not gross photographs, can be used to accurately quantify these changes. Micrographs of the stained tissue sections were obtained and the ratio of oviduct diameter to wall thickness was measured. As shown in Fig. 5B, mock-vaccinated mice displayed significantly greater microscopic oviduct dilatation (61.63 ± 10.82) when compared to naïve age-matched mice (20). In comparison, mice vaccinated with active or in rCPAF displayed significantly reduced oviduct dilatation (18.50 ± 2.91 and 14.67 ± 3.50, respectively) compared to mock-vaccinated animals, and these ratios were comparable to that found in naïve mice. As expected, rGST-treated mice displayed significantly dilated oviducts (55.08 ± 8.92) that were comparable to those found in mock-immunized animals. Additionally, C. muridarum challenged mice that were previously vaccinated with active or inactive rCPAF displayed significant and comparable reductions in mononuclear and plasma cells on day 80 after challenge (data not shown), when compared to challenged mock and rGST-immunized animals. Collectively, these results demonstrate that vaccination with both active and inactive rCPAF induce significant and comparable reductions in UGT pathology.
DISCUSSION
Previously, we showed that vaccination with proteolytically active rCPAF plus adjuvant induces enhanced vaginal chlamydial clearance and reduction of UGT pathology following primary genital chlamydial infection [16, 27]. To determine if the proteolytic activity of the CPAF is expendable in the induction of anti-chlamydial immunity, we evaluated the protective efficacy of rCPAF that was rendered proteolytically inactive by heat denaturation. We found that inactive rCPAF induces robust CPAF-specific IFN-γ production, but not antibody responses, in immunized mice. Importantly, inactive rCPAF vaccination induced significant enhancement of chlamydial clearance and reduction in oviduct pathology after primary genital challenge, which was comparable to the effects of active rCPAF.
Treatment of rCPAF at 100° C for 5 min resulted in reduction of the proteolytic activity of rCPAF to undetectable levels, as evidenced by the inability of the heat-treated preparation to degrade keratin-8. High temperature treatment denatures most proteins, via the breakage of weak interactions, primarily hydrogen bonds, leading to loss of the native secondary and higher order structure [28]. The function of a protein is dependent on the structure, and heat treatment expectedly resulted in loss of enzymatic activity of rCPAF. Native protein conformation also may play an important role in antibody recognition [2], as evidenced by the observation that sera from mice immunized with inactive rCPAF contained very low levels of antibody that recognized active rCPAF. As expected based on our previous findings [16, 20], mice immunized with active rCPAF displayed high levels of anti-CPAF antibody. Although active and inactive rCPAF may differ in secondary and tertiary conformation, the primary amino acid sequence would remain unaltered by heat denaturation since covalent peptide bonds are not broken by normal heat treatment [28]. T-cells predominantly recognize linear antigenic epitopes presented on the surface of major histocompatibility molecules (MHC) [29] and thus the primary amino acid sequence would be the critical determinant for T-cell recognition. This corroborates our finding that splenocytes from mice vaccinated with either active or inactive rCPAF recognized active rCPAF or native CPAF, and responded by producing elevated and comparable levels of IFN-γ.
Despite low levels of antibody responses, the induction of robust CPAF-specific IFN-γ production correlated with the enhanced chlamydial clearance and significant reduction of oviduct pathology in mice vaccinated with inactive rCPAF. Importantly, both inactive and active rCPAF immunization induced comparable enhancements in chlamydial clearance and reductions in UGT pathology. This corroborates our previous demonstration that rCPAF-induced protective anti-chlamydial immunity is dependent upon IFN-γ producing CPAF-specific CD4+ T cells [16–18], and does not require antibody production [19]. This also supports our initial hypothesis that anti-CPAF antibody would not be expected to neutralize chlamydial infectivity since CPAF is a reticulate body-specific protein that is secreted into the host cytosol, and not present on the infectious chlamydial elementary body [12]. In contrast, antibodies against proteins on the surface of the infectious elementary body would be capable of neutralizing chlamydial infectivity [30]. In the context of rCPAF vaccination, the predominant contribution of cellular IFN-γ production, and the lack of neutralizing effects of antibody, are evident from the fact that rCPAF-vaccinated mice display similar vaginal chlamydial shedding on day 3, but enhanced clearance by day 6 after challenge, when compared to mock-vaccinated animals. In support of suggestions from our previous reports [19], the results of this study directly demonstrate that anti-chlamydial protective immunity induced by rCPAF vaccination is not dependent upon conformational antigenic epitopes. Additional corroborative results from Lu and Zhong (unpublished observations) have shown that enzymatically inactive recombinant CPAF (due to E445A mutation in the catalytic site) induces enhanced chlamydial clearance comparable to that induced by enzymatically active CPAF.
The finding that conformational epitopes of rCPAF are largely dispensable for induction of anti-chlamydial protective immunity and reduction of UGT pathological sequelae has important implications for design of a chlamydial vaccine for human use. First, the ability to use a recombinant form of protein allows the rapid, easy, and abundant production of rCPAF for vaccination. Second, the fact that CPAF does not have to be in the native conformation to induce protective immunity indicates that a non-proteolytic, safe rCPAF preparation can be used for vaccination. Third, rCPAF denatured by heat treatment induces robust protective anti-chlamydial immunity and hence the vaccine preparation is likely to be highly stable for transport and would withstand temperature fluctuations. This would be of particular benefit to communities with low economic resources where the inability to maintain a “cold chain” would reduce the efficacy of most vaccines, and where incidence of genital chlamydial infections is high [3]. Fourth, rCPAF cloned from the C. muridarum genome provided robust protective immunity against UGT sequelae produced by C. muridarum challenge, in this study, that was comparable to that induced by rCPAF cloned from C. trachomatis serovar L2 genome in our previous studies [16, 20, 27]. This indicates that the protective epitopes in rCPAF are highly conserved across different species of Chlamydia, suggesting the possibility of inducing efficacious cross-serovar protection against genital chlamydial infections. Finally, the presence of conserved protective antigenic epitopes across different serovars of CPAF also justifies the mapping of such conserved epitopes for preparation of a subunit vaccine with the minimal fragment of rCPAF required to induce optimal protective immunity.
Given that a primary chlamydial infection does not induce complete resistance to infection in 100% of mice [2], significant shortening in the period of chlamydial shedding and prevention of upper genital pathological sequelae would be a more realistic goal for a subunit vaccine than the induction of sterilizing immunity. The results from this study, in addition to those from our previous reports, demonstrate several distinct advantages of inclusion of rCPAF as a component of a prospective anti-chlamydial vaccine. In the context of a need for a preventive vaccine against genital chlamydial infections, our results underscore the tangible appeal of rCPAF as a potential vaccine candidate for human use.
Acknowledgments
This work was supported by National Institutes of Health Grant 1RO1AI074860.
We thank Dr. Anand Ramasubramanian at the Department of Biomedical Engineering, UTSA, for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005 Feb;5(2):149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 2.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002 Jun;70(6):2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates. 2001:11–15. [Google Scholar]
- 4.Kelly KA. Cellular immunity and Chlamydia genital infection: induction, recruitment, and effector mechanisms. Int Rev Immunol. 2003 Jan;22(1):3–41. doi: 10.1080/08830180305229. [DOI] [PubMed] [Google Scholar]
- 5.Rekart ML, Brunham RC. Epidemiology of chlamydial infection: are we losing ground? Sex Transm Infect. 2008 Apr;84(2):87–91. doi: 10.1136/sti.2007.027938. [DOI] [PubMed] [Google Scholar]
- 6.Gray RT, Beagley KW, Timms P, Wilson DP. Modeling the impact of potential vaccines on epidemics of sexually transmitted Chlamydia trachomatis infection. J Infect Dis. 2009 Jun 1;199(11):1680–1688. doi: 10.1086/598983. [DOI] [PubMed] [Google Scholar]
- 7.Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med. 2001 Apr 16;193(8):935–942. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong F, Zhong Y, Arulanandam B, Zhong G. Production of a proteolytically active protein, chlamydial protease/proteasome-like activity factor, by five different Chlamydia species. Infect Immun. 2005 Mar;73(3):1868–1872. doi: 10.1128/IAI.73.3.1868-1872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong G. Killing me softly: chlamydial use of proteolysis for evading host defenses. Trends Microbiol. 2009 Oct;17(10):467–474. doi: 10.1016/j.tim.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong F, Su H, Huang Y, Zhong Y, Zhong G. Cleavage of host keratin 8 by a Chlamydia-secreted protease. Infect Immun. 2004 Jul;72(7):3863–3868. doi: 10.1128/IAI.72.7.3863-3868.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong F, Pirbhai M, Xiao Y, Zhong Y, Wu Y, Zhong G. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect Immun. 2005 Mar;73(3):1861–1864. doi: 10.1128/IAI.73.3.1861-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy AK, Guentzel MN, Zhong G, Arulanandam BP. Chlamydial Protease-Like Activity Factor- Insights into immunity and vaccine development. J Reprod Immunol. 2009 Dec;83(1–2):179–184. doi: 10.1016/j.jri.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma J, Bosnic AM, Piper JM, Zhong G. Human antibody responses to a Chlamydia-secreted protease factor. Infect Immun. 2004 Dec;72(12):7164–7171. doi: 10.1128/IAI.72.12.7164-7171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma J, Dong F, Pirbhai M, Zhong G. Inhibition of proteolytic activity of a chlamydial proteasome/protease-like activity factor by antibodies from humans infected with Chlamydia trachomatis. Infect Immun. 2005 Jul;73(7):4414–4419. doi: 10.1128/IAI.73.7.4414-4419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma J, Zhong Y, Dong F, Piper JM, Wang G, Zhong G. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect Immun. 2006 Mar;74(3):1490–1499. doi: 10.1128/IAI.74.3.1490-1499.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect Immun. 2007 Feb;75(2):666–676. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphey C, Murthy AK, Meier PA, Guentzel MN, Zhong G, Arulanandam BP. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4+ T cells. Cell Immunol. 2006 Aug;242(2):110–117. doi: 10.1016/j.cellimm.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Murthy AK, Guentzel MN, et al. Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008 Mar 1;180(5):3375–3382. doi: 10.4049/jimmunol.180.5.3375. [DOI] [PubMed] [Google Scholar]
- 19.Murthy AK, Chaganty BK, Li W, et al. A limited role for antibody in protective immunity induced by rCPAF and CpG vaccination against primary genital Chlamydia muridarum challenge. FEMS Immunol Med Microbiol. 2009 Mar;55(2):271–279. doi: 10.1111/j.1574-695X.2008.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy AK, Cong Y, Murphey C, et al. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect Immun. 2006 Dec;74(12):6722–6729. doi: 10.1128/IAI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jupelli M, Guentzel MN, Meier PA, Zhong G, Murthy AK, Arulanandam BP. Endogenous IFN-gamma production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice. J Immunol. 2008 Mar 15;180(6):4148–4155. doi: 10.4049/jimmunol.180.6.4148. [DOI] [PubMed] [Google Scholar]
- 22.Arulanandam BP, O’Toole M, Metzger DW. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J Infect Dis. 1999 Oct;180(4):940–949. doi: 10.1086/314996. [DOI] [PubMed] [Google Scholar]
- 23.Arulanandam BP, Metzger DW. Modulation of mucosal and systemic immunity by intranasal interleukin 12 delivery. Vaccine. 1999 Jan 21;17(3):252–260. doi: 10.1016/s0264-410x(98)00157-1. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Guentzel MN, Seshu J, Zhong G, Murthy AK, Arulanandam BP. Induction of cross-serovar protection against genital chlamydial infection by a targeted multisubunit vaccination approach. Clin Vaccine Immunol. 2007 Dec;14(12):1537–1544. doi: 10.1128/CVI.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong F, Sharma J, Xiao Y, Zhong Y, Zhong G. Intramolecular dimerization is required for the Chlamydia-secreted protease CPAF to degrade host transcriptional factors. Infect Immun. 2004 Jul;72(7):3869–3875. doi: 10.1128/IAI.72.7.3869-3875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong F, Pirbhai M, Zhong Y, Zhong G. Cleavage-dependent activation of a Chlamydia-secreted protease. Mol Microbiol. 2004 Jun;52(5):1487–1494. doi: 10.1111/j.1365-2958.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 27.Cong Y, Jupelli M, Guentzel MN, Zhong G, Murthy AK, Arulanandam BP. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine. 2007 May 10;25(19):3773–3780. doi: 10.1016/j.vaccine.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 5. New York: W.H. Freeman and Company; 2008. The Three-Dimensional Structure of Proteins; pp. 113–152. [Google Scholar]
- 29.Murphy K, Travers P, Walport M. Janeway’s Immunobiology. 7. New York and London: Garland Science; 2008. Antigen Recognition by B-cell and T-cell Receptors; pp. 110–217. [Google Scholar]
- 30.Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997 Apr;15(5):575–582. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]