Abstract
Supramaximal concentrations of cholecystokinin or its analogue caerulein have been shown to stimulate the proteolytic activation of zymogens within the pancreatic acinar cell and initiate acute pancreatitis. Previous studies suggest that a low pH compartment might be required for activation. To test this hypothesis, the effects of agents that modulate intracellular pH on caerulein-induced trypsin and chymotrypsin activation were studied. Pretreatment of pancreatic acini with the proto-ionophore monensin (10 µM) and the weak base chloroquine (40 µM) inhibited activation. Pre-incubation with the vacuolar ATPase (V-ATPase) inhibitors bafilomycin A1 and concanamycin A also decreased activation in a concentration-dependent manner with 50% inhibition at ~50 and 25 nM, respectively. Caerulein stimulation caused a time- and concentration-dependent translocation of soluble V-ATPase V1 subunits to a membrane fraction, a marker of V-ATPase activation. Carbachol also stimulated translocation at supramaximal concentrations. Elevation of cytosolic Ca2+ by thapsigargin was sufficient to induce translocation. Thus, stimulation of V-ATPase activity appears to be required for agonist-induced zymogen activation in the pancreatic acinar cell.
The premature activation of digestive zymogens in the pancreatic acinar cell plays a central role in the initiation of acute pancreatitis, but the mechanisms leading to activation remain unclear. Concentrations of cholecystokinin that are at least 10-fold greater than that generated by the physiologic response to a meal, a treatment known as hyperstimulation, cause zymogen activation and pancreatitis in vivo. Similarly, hyper-stimulation of isolated pancreatic acini by cholecystokinin or its analogue caerulein causes zymogen activation in vitro (1, 2). Such activation appears to take place in unidentified membrane-bound organelles. Previous findings suggest that the generation of a low pH compartment in acinar cells is a feature of acute pancreatitis and may be required for zymogen activation. First, in both the caerulein hyperstimulation and the choline-deficient, ethionine-supplemented diet models of acute pancreatitis, acidic vacuoles are generated within acinar cells (3). Second, chloroquine, a weak base that non-selectively increases pH in acidic organelles, reduces the severity of pancreatitis in the caerulein hyperstimulation and choline-deficient, ethionine-supplemented diet models of pancreatitis (4, 5). Third, both proposed mechanisms of zymogen activation, trypsinogen autoactivation and trypsinogen activation by the lysosomal protease cathepsin B, proceed optimally at an acidic pH (6). Finally, chloroquine and monensin, agents that non-selectively increase intracellular pH, block the proteolytic conversion of procarboxypeptidase A1 to its active form in isolated acini following caerulein hyperstimulation (1). Although these studies support a role for acidification in zymogen activation, they provide no information on the mechanism.
Because vacuolar ATPase (V-ATPase)1 acidifies many intracellular compartments, it is a candidate for generating the low pH compartment observed during pathologic zymogen activation in the acinar cell. Furthermore, the soluble A and E subunits have been localized to the apical region of the pancreatic acinar cell; at least some of this immunoreactivity is associated with zymogen granules (7). V-ATPase is a proton pump present in eukaryotic cells that establishes the acidic luminal pH (4.5–6.5) of organelles such as lysosomes, endosomes, and secretory granules (8, 9). In contrast to F-ATPases, which function as ATP synthases, and P-ATPases, which function as cation ex-changers, V-ATPases function exclusively as ATP-dependent proton pumps that transport protons from the cytosol into organelle lumina or the extracellular space. V-ATPases are multisubunit complexes composed of an integral membrane domain (Vo) and a soluble peripheral domain (V1). The soluble domain must assemble with the membrane-bound domain to activate the pump (10).
In the present study, we demonstrate that caerulein stimulation of pancreatic acinar cells causes an increase in trypsin and chymotrypsin activity that is inhibited by agents that non-selectively increase intracellular pH, chloroquine, and monensin. Similarly, the specific V-ATPase inhibitors bafilomycin A1 and concanamycin A significantly inhibit this caerulein-induced zymogen activation. Caerulein also causes a time- and concentration-dependent increase in the fraction of a soluble V-ATPase subunit associated with membrane-bound organelles in vitro. Furthermore, the muscarinic agonist carbachol and the elevation of cytosolic Ca2+ by thapsigargin also caused this V-ATPase translocation. The findings suggest that agonist stimulation results in the translocation of soluble V-ATPase subunits to membranes and the subsequent V-ATPase activation that is linked to the proteolytic activation of trypsin and chymotrypsin.
MATERIALS AND METHODS
Animals and Materials
Male Sprague-Dawley rats (80–120 g) were obtained from the Charles River Breeding Laboratories, Wilmington, MA. Caerulein was obtained from Amersham Biosciences; all other chemicals and supplies were obtained from Sigma unless otherwise noted.
Acinar Experimental Protocol
Acini were prepared as described previously and incubated at 37 °C (15). Acini were pretreated for 30 min with the following agents that modulate intracellular pH: monensin (10µM in 2.47 mM MeOH), chloroquine (40 µM), bafilomycin A1, and concanamycin A (100 nM in 1% Me2SO). The concentration-dependent effects of bafilomycin (10–50 nM in 0.05% Me2SO) and concanamycin A (1–50 nM in 0.05% Me2SO) on activation were examined. Following pretreatments, acini were treated with caerulein (100 nM) or carbachol (1 mM) for 2–60 min unless stated otherwise. For investigation of the role of cytosolic Ca2+, acini were treated with thapsigargin (10 µM) in Me2SO.
Enzymatic Activity Assays
Samples (acini with medium) were stored at −80 °C prior to assay. Trypsin and chymotrypsin activities were assayed using fluorescent enzyme substrates and a microtiter plate reader as described previously (15). Enzyme activities were normalized to total amylase activity and expressed as relative fluorescence units per second per microgram of total amylase. Amylase activity in acinar homogenates was determined using the Phaebadas test kit (Pharmacia Diagnostic, Rochester, NY).
Other Assays
Amylase secretion was expressed as percentage of amylase release following 60 min of caerulein treatment. To examine cell injury, lactate dehydrogenase secretion was assayed (Promega, Madison, WI) and expressed as percentage of release.
Subcellular Fractionation and V-ATPase Detection
Assays for both trypsin and chymotrypsin activation were done prior to fractionation. If an increase in activation of >50% for trypsin or >200% for chymotrypsin was not seen for acini treated with caerulein (100 nM), the experiment was not used for studies examining V-ATPase translocation. For subcellular fractionation, acini were homogenized on ice in 300 µl of 0.3 M sucrose, 10 mM HEPES, pH 7.4, with protease inhibitors (5 mM benzamidine and a protease inhibitor mixture, used as directed by Roche Diagnostics) and subjected to low speed centrifugation (500 g for 10 min at 4 °C) to remove nuclei and unbroken cells. The resulting postnuclear supernatant was subjected to high speed centrifugation (288,000 × g for 10 min) at 4 °C in a Beckman Optima TLX Ultracentrifuge to form a cytosolic and particulate fraction. The fractions were diluted in SDS sample buffer, heated for 5 min at 70 °C, and then electrophoresed on 12% polyacrylamide gels at 125 V and transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Billerica, MA) for immunoblot analysis. Membranes were incubated with polyclonal rabbit antibodies to the E subunit of V-ATPase (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:200 (v/v) or with polyclonal rabbit antibodies to the E subunit or A subunit at a dilution of 1:1000 (v/v) (provided by Prof. Irene Schulz, Homburg, Germany and described in Ref. 7) in a Western blocking reagent (5% Carnation milk powder (Nestle, Glendale, CA) and 0.05% Tween 20 in phosphate-buffered saline) at room temperature for 2 h. The membranes were then washed in Western blocking reagent and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG at 1:10000 (v/v) dilution in Western blocking reagent for 1 h. After washing, the V-ATPase protein was visualized by enhanced chemiluminescence (Pierce).
Statistical Analysis
Data represent the means ± S.E. of independent experiments, with each experiment being performed in duplicate or triplicate. A paired Student’s t test was used to determine significance.
RESULTS
Effects of Subcellular pH Manipulation
Preincubation of isolated pancreatic acini with either monensin or chloroquine inhibited zymogen activation induced by caerulein hyperstimulation (Fig. 1). Lower levels of activation were also observed after physiologic caerulein stimulation (1 nM); this response was also inhibited by monensin and chloroquine (data not shown). To define the role of V-ATPase on zymogen activation, the effects of specific cell-permeant V-ATPase inhibitors were examined.
FIG. 1. Agents that raise intracellular pH decrease zymogen activation.
Isolated acini were pretreated with medium alone as control (Ctl), 40 µM chloroquine (Chlor), or 10 µM monensin (Mon) for 30 min and then exposed to 100 µM caerulein (Cer) for 1 h. Black bars indicate caerulein alone; gray bars indicate caerulein plus chloroquine or monensin. Chymotrypsin and trypsin activities were determined by fluorometric assay as described under “Materials and Methods.” Values represent mean ± S.E. *, p < 0.05 versus caerulein alone.
V-ATPase Inhibitors Reduce Zymogen Activation in Vitro
In the nanomolar range, bafilomycin A1 and concanamycin A are high affinity inhibitors of V-ATPases. In the micromolar range they inhibit P-type ATPases and ATP-binding cassette transporters (11, 12). Pretreatment with bafilomycin A1 (100 nM) or concanamycin A (100 nM) completely blocked caerulein-induced trypsinogen and chymotrypsinogen activation in acini (Fig. 2). This inhibition by bafilomycin A1 and concanamycin A was concentration-dependent and half-maximal at ~50 and 25 nM, respectively.
FIG. 2. V-ATPase inhibitors reduce caerulein-induced chymotrypsinogen and trypsinogen activation.
Isolated acini were pretreated for 30 min with the specific V-ATPase inhibitors bafilomycin A1 or concanamycin A at the indicated concentrations and then exposed to 100 nM caerulein (CER) for 1 h. Chymotrypsinogen activation (left) and trypsinogen activation (right) were inhibited by both bafilomycin A1 and concanamycin A, respectively, in a concentration-dependent manner. Black bars indicate caerulein alone; gray bars indicate caerulein plus V-ATPase inhibitor. CTL, control. Values represent mean ± S.E. *, p < 0.05 versus caerulein alone.
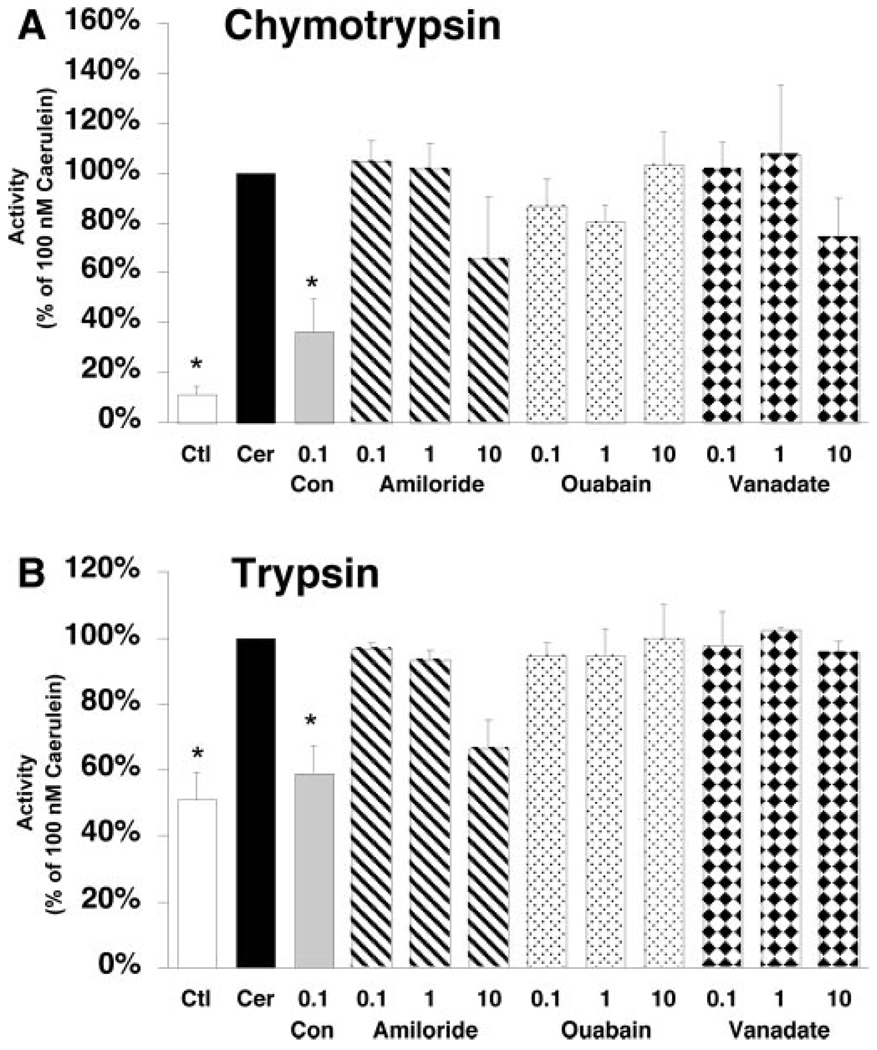
To exclude possible nonspecific or toxic effects of the inhibitors, the effects of these agents on acinar cell secretion and cell injury were examined. Bafilomycin A1 and concanamycin A had no effect on caerulein-stimulated amylase secretion (Table I) and did not lead to an increase in lactate dehydrogenase release from acinar cells, a marker of cell injury (data not shown). To confirm that bafilomycin A1 and concanamycin A were not inhibiting other classes of ATPase, the effects of P-ATPase inhibitors and ion exchangers on caerulein hyperstimulation-induced zymogen activation were examined. Isolated acini were pretreated with either the Na+-H+ exchange inhibitor amiloride, the Na+-K+ ATPase inhibitor ouabain, or the plasma membrane ATPase inhibitor vanadate. None of these compounds inhibited zymogen activation at 100 nM, the concentration at which V-ATPase inhibitors block hyperstimulatory caerulein-induced (100 nM) zymogen activation (Fig. 3). At high concentrations (10 µM), amiloride demonstrated a tendency to reduce zymogen activation.
TABLE I. Vacuolar ATPase inhibitors do not affect amylase secretion from the acinar cell.
Values represent mean ± S.E. Amylase activity was assayed in acinar cell media and homogenates after 60 minutes of stimulation as described under “Materials and Methods” and expressed as a percentage of amylase secretion.
| Treatment | Percent amylase secretion |
|---|---|
| Control | 5 ± 1 |
| Caerulein (100 nM) | 18 ± 3 |
| Caerulein (100 nM) + bafilomycin A1 (100 nM) | 16 ± 2 |
| Caerulein (100 nM) + concanamycin A (100 nM) | 18 ± 1 |
FIG. 3. P-ATPase inhibitors and ion exchange inhibitors do not reduce caerulein-induced zymogen activation.
Isolated acini were pretreated with the V-ATPase inhibitor concanamycin A (Con), the Na+-H+ exchange inhibitor amiloride, the Na+-K+ ATPase inhibitor ouabain, or the plasma membrane ATPase inhibitor vanadate (ranging from 0.1 to 10 µM) before stimulation with 100 nM caerulein (Cer). Ctl, control. Chymotrypsin (A) and trypsin (B) activities were quantitated as described under “Materials and Methods.” Values represent mean + S.E. *, p < 0.05 versus caerulein alone.
Agonist Stimulation Causes Redistribution of Soluble V-ATPase Subunits
V-ATPase activity requires assembly of a soluble complex (V1) composed of six tightly associated cytosolic subunits with the membrane-bound domain (Vo). As an indirect measure of V-ATPase activation, we assayed the redistribution of V1 from the soluble fraction to membranes after caerulein stimulation. To detect the redistribution of the 31-kDa E protein in soluble V1 subunits to organelles, postnuclear supernatants from acini were separated by centrifugation (288,000 × g) into soluble and particular fractions and subjected to immunoblot analysis. Caerulein hyperstimulation (100 nM) causes a dramatic increase in the level of the membrane-associated V-ATPase E subunit (Fig. 4A). Similar results were obtained with two different antibodies to the E subunit. Antibodies to the soluble 71-kDa A subunit detected a faintly labeled band that also redistributed to the particulate fraction after caerulein hyperstimulation (data not shown). Treatment of acini with supraphysiologic concentrations (1 mM) of the muscarinic agonist, carbachol, which causes the same zymogen activation as observed with caerulein (13), resulted in a similar ~4-fold increase in the level of the E subunit associated with membranes (Fig. 4A). Agonist-dependent zymogen activation requires an increase in intracellular Ca2+ within the acinar cell. Treatment of acini with thapsigargin, an agent that causes an increase in cytosolic Ca2+ by effecting its release from stores in the endoplasmic reticulum, also resulted in translocation of the E subunit. The magnitude of this effect was similar to that observed with caerulein (Fig. 4B). Because glucose has been reported to modulate the translocation of the V1 subunits to membranes in yeast, its effects were in examined in acini (14). However, E subunit translocation to membranes in response to caerulein hyperstimulation was the same in the presence or absence of glucose in the medium (data not shown).
FIG. 4. Translocation of the soluble E-subunit to membranes is agonist-dependent.
Acini were treated with medium as control (Ctl) or supraphysiologic concentrations of 0.1 µM caerulein (Cer), 1 mM carbachol (Carb) (A), or 10 µM thapsigargin (Thapsi) (B) for 15 min and then homogenized. A membrane fraction was formed from a post-nuclear supernatant, and the E subunit was detected using immunoblot analysis (see “Materials and Methods”). Results are representative of three separate studies.
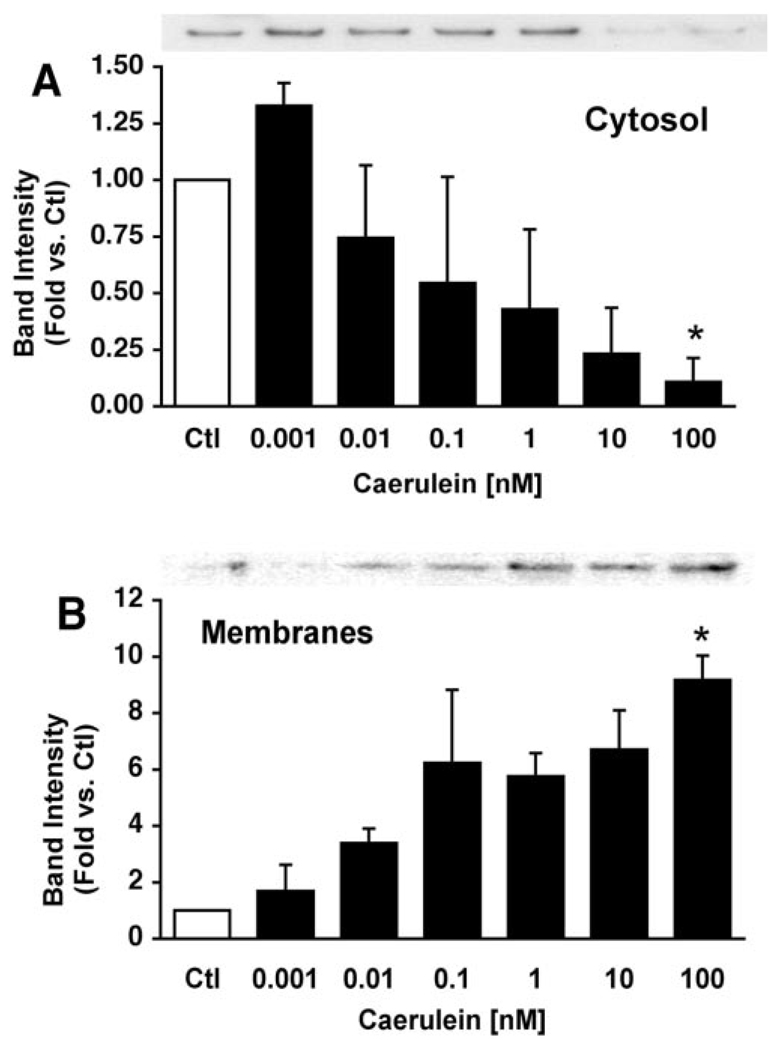
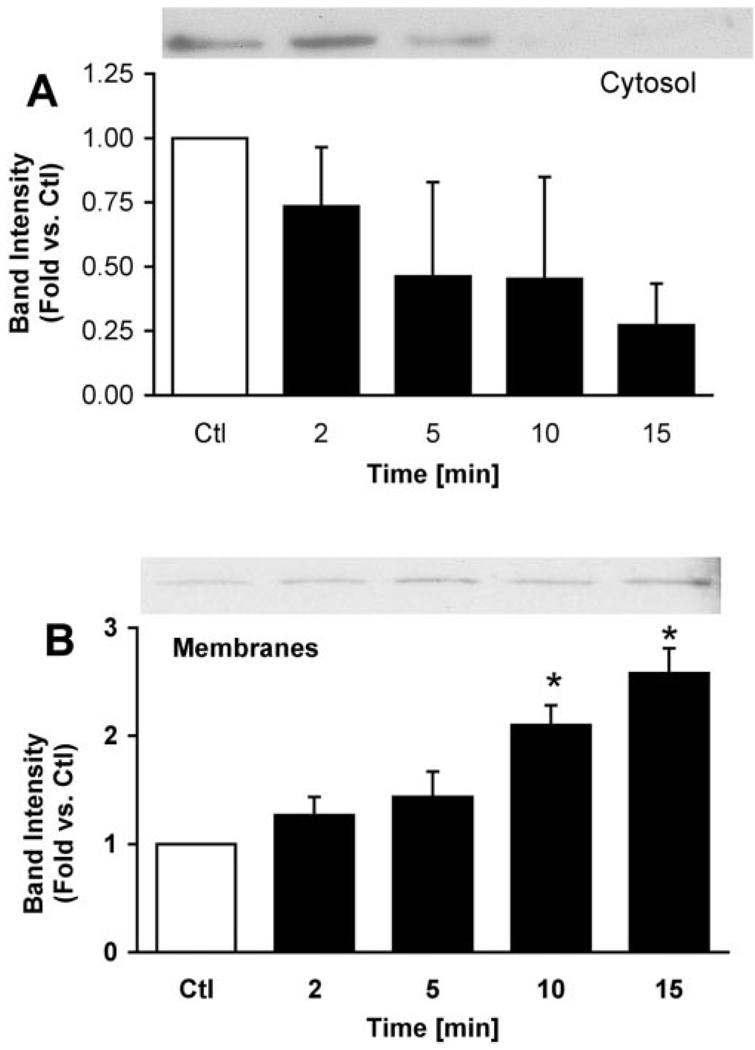
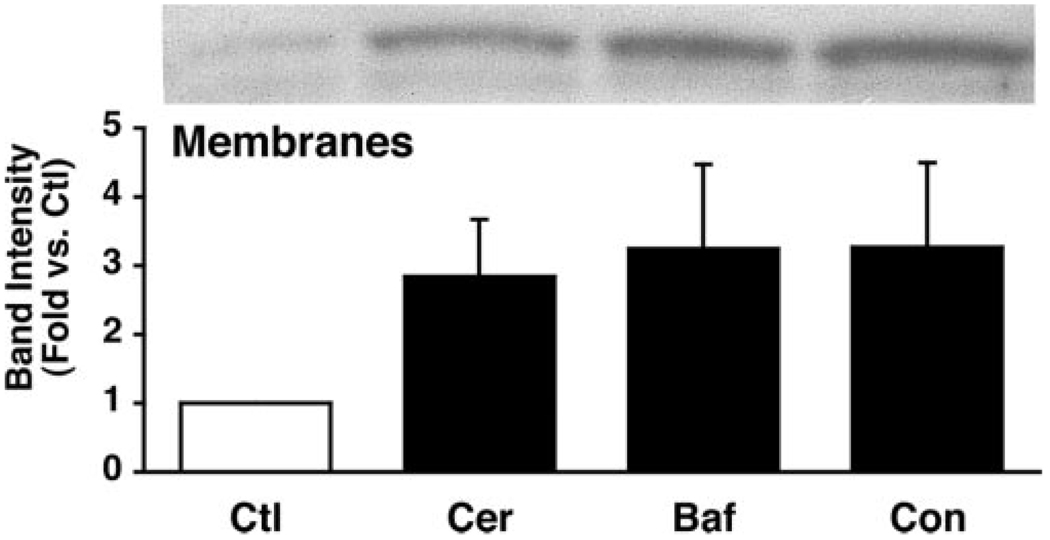
Translocation of the E subunit to membranes was concentration-and time-dependent (Fig. 5 and Fig. 6). Increasing concentrations of caerulein resulted in increased levels of the membrane-associated E subunit from ~5 to10-fold after 15 min (Fig. 5B). This concentration-dependent response corresponds to that reported for caerulein-induced zymogen activation (1, 15). The E subunit rapidly translocated to membranes after caerulein hyperstimulation. Within 2 min of stimulation, the levels of the E subunit associated with membranes had increased slightly. After 10 min of caerulein hyperstimulation the levels of E subunit had doubled in the membrane fraction. Decreases in E subunit levels in the soluble fraction were often observed, but these changes exhibited much greater variability than those observed for its association with membranes (Fig. 6A). The levels of the E subunit associated with the membrane appeared to be maximal by 15 min and did not increase further after 1 h (not shown). Notably, translocation of the E-subunit (detected after 2 min) to membranes appears to precede zymogen activation (first detected at ~10 min in our system) (1, 15). Although pretreatment with the V-ATPase inhibitors bafilomycin A1 and concanamycin A decreased zymogen activation (Fig. 2), they had no effect on caerulein-induced E subunit translocation to membranes (Fig. 7).
FIG. 5. Caerulein stimulation causes a concentration-dependent translocation of soluble V-ATPase subunits to membranes in pancreatic acini.
Acini were treated with increasing concentrations of caerulein for 15 min and fractionated to obtain cytosolic (A) and particulate (B) fractions. Proteins were separated by SDS-PAGE and immunoblotted with anti-E subunit antibodies. Representative immunoblots are shown (n = 3). Band intensities were quantified by laser densitometry and expressed as mean ± S.E. *, p < 0.05 versus control (Ctl) for panels A and B.
FIG. 6. Caerulein stimulation causes a time-dependent translocation of soluble V-ATPase subunits to membranes in pancreatic acini.
Acini were treated with 100 nM caerulein for the indicated periods and fractionated to obtain cytosolic (A) and particulate (B) fractions. Proteins were separated by SDS-PAGE and immunoblotted with anti-E subunit antibodies. Representative immunoblots are shown (n = 3). Band intensities were quantified by laser densitometry and expressed as mean ± S.E. *, p < 0.02 versus control (Ctl) for panels A and B.
FIG. 7. V-ATPase inhibitors do not affect caerulein-induced redistribution of soluble V-ATPase subunits.
Acini were pretreated with 100 nM bafilomycin A1 (Baf) or 100 nM concanamycin A (Con) for 15 min and then treated with 100 nM caerulein (Cer) for an additional 15 min. Ctl, control. Following fractionation to obtain cytosolic and particulate fractions, proteins in the membrane fraction were separated by SDS-PAGE and immunoblotted with anti-E subunit antibodies. A representative immunoblot is shown. Band intensities from independent experiments were quantified by laser densitometry and expressed as mean ± S.E.
DISCUSSION
Activation of digestive zymogens within the pancreatic acinar cell is a characteristic of acute pancreatitis. Preliminary studies have suggested that this activation requires a low pH compartment (1, 3, 4). Other reports have suggested that this activation may occur in a compartment related to lysosomes and/or within the secretory pathway (16–18). Because V-ATPases mediate the acidification of lysosomes, related organelles, and compartments within the secretory pathway, we examined their role in acinar cell zymogen activation. In this study, we show that agonist-induced zymogen activation is coupled to activation of a V-ATPase. Furthermore, we find that translocation of soluble V-ATPase subunits to membranes, an event that is essential for V-ATPase activation, can also be regulated by agonists.
Several of our observations support the conclusion that V-ATPase has a causative role in zymogen activation. First, caerulein-induced activation of trypsinogen and chymotrypsinogen in vitro is decreased by the specific, cell-permeant V-ATPase inhibitors bafilomycin A1 and concanamycin A. Second, the concentration dependence of caerulein-induced zymogen activation corresponds to that observed for translocation of the V1 subunit to membranes. Finally, translocation of the V1 subunit to the membrane appears to precede zymogen activation. Together, these findings support the hypothesis that a low pH compartment generated by V-ATPase regulates zymogen activation.
The assembly of the V1 complex on the membrane-bound V0 complex is one mechanism by which V-ATPase activity is regulated.Previous studies have found that free V1 and Vo domains may be in dynamic equilibrium with the fully assembled, enzymatically active V1Vo holoenzyme. The presence of both fully assembled and free inactive V1 domains in a bovine kidney cell line has been demonstrated (19). A similar equilibrium was observed in the pancreas in which a small fraction of the V1 domain is associated with membranes in the basal state. Although we found a direct relationship between V1 translocation to membranes and the concentration dependence of zymogen activation with cholecystokinin, additional studies are required to provide direct evidence of V1 and Vo interactions. Our studies suggest that elevations in cytosolic Ca2+ are sufficient to cause translocation of the V1 domain. However, whether these increases result in activation of the V-ATPase needs to be examined. In yeast, V-ATPase assembly has been linked to glucose metabolism. Glucose deprivation for 5 min results in reversible detachment of V1 domains from Vo domains (20). Glucose regulation of the assembly state of V-ATPase in yeast has been shown to be mediated by the RAVE complex, a heterotrimer of Rav1, Rav2, and Skp1 (10, 14). In preliminary studies we could not find a role for glucose in V1 translocation in the acinar cell (data not shown).
There are a number of mechanisms that might couple zymogen activation to H+ conductance by the V-ATPase activity. First, V-ATPase-dependent acidification of vesicles containing digestive zymogens may provide optimal pH conditions for proteolytic zymogen activation. In that context, cathepsin B appears to have a role in mediating trypsinogen activation (21, 22). Furthermore, in vitro studies have shown that cathepsin B-mediated activation of human cationic trypsinogen exhibits a pH optimum of 4 and does not occur above pH 5.2 (6). Trypsinogen autoactivation is also favored in an acidic milieu. Second, activation of the V-ATPase might couple proton movement to other cell signals that regulate zymogen processing. For example, V-ATPase has been linked to the regulation of cytosolic calcium signaling, an essential mechanism of zymogen activation. Thus, V-ATPase might modulate levels of cytosolic calcium by modulating the refilling of intracellular calcium pools or release from lysosomes (23, 24). Alternately, V-ATPase might drive a H+-Ca2+ exchanger that causes the accumulation of Ca2+ in vacuoles containing zymogens (25). Finally, V-ATPase might mediate a trafficking event that is critical for zymogen activation. In that context, V-ATPase has been shown to play an essential role in trans-SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex formation and vesicular fusion (26). This occurs through an interaction of the V0 subunits and is independent of V1 assembly with V0. However, the sensitivity of the zymogen activation to V-ATPase inhibitors and its correlation with V1 translocation makes it unlikely that V0-mediated vesicular fusion is mediating activation.
In summary, we demonstrate that at supramaximal concentrations, agonist-induced zymogen activation requires V-ATPase activity. Translocation of a V1 subunit to membrane-bound organelles, a marker of V-ATPase activation, was observed in an in vitro model of acute pancreatitis. We propose that V-ATPase-mediated acidification of zymogen-containing organelles stimulates pathologic zymogen activation in the pancreatic acinar cell.
Footnotes
This work was supported by a Goldwater Student Research Award (to S. D. W.), a Veterans Administration Senior Career Development Award (to F. S. G.), and National Institutes of Health Grant RO1 DK54021 (to F. S. G.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviation used is: V-ATPase, vacuolar ATPase.
REFERENCES
- 1.Leach SD, Modlin IM, Scheele GA, Gorelick FS. J. Clin. Investig. 1991;87:362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saluja A, Saito I, Saluja M, Houlihan MJ, Powers RE, Meldolesi J, Steer M. Am. J. Physiol. 1985;249:G702–G710. doi: 10.1152/ajpgi.1985.249.6.G702. [DOI] [PubMed] [Google Scholar]
- 3.Niederau C, Grendell JH. J. Clin. Investig. 1988;81:229–236. doi: 10.1172/JCI113300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach SD, Bilchik AJ, Karapetian O, Gorelick FS, Modlin IM. Pancreas. 1993;8:64–69. doi: 10.1097/00006676-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Guillaumes S, Blanco I, Villanueva A, Sans MD, Clave P, Chabas A, Farre A, Lluis F. Pancreas. 1997;14:262–266. doi: 10.1097/00006676-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Kukor Z, Mayerle J, Kruger B, Toth M, Steed PM, Halangk W, Lerch MM, Sahin-Toth M. J. Biol. Chem. 2002;277:21389–21396. doi: 10.1074/jbc.M200878200. [DOI] [PubMed] [Google Scholar]
- 7.Roussa E, Alper S, Thevenod F. J. Histochem. Cytochem. 2001;49:463–474. doi: 10.1177/002215540104900406. [DOI] [PubMed] [Google Scholar]
- 8.Nishi T, Forgac M. Nat. Rev. Mol. Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 9.Merzendorfer H, Graf R, Huss M, Harvey WR, Wieczorek H. J. Exp. Biol. 1997;200:225–235. doi: 10.1242/jeb.200.2.225. [DOI] [PubMed] [Google Scholar]
- 10.Smardon AM, Tarsio M, Kane PM. J. Biol. Chem. 2002;277:13831–13839. doi: 10.1074/jbc.M200682200. [DOI] [PubMed] [Google Scholar]
- 11.Crider BP, Xie XS, Stone DK. J. Biol. Chem. 1994;269:17379–17381. [PubMed] [Google Scholar]
- 12.Drose S, Altendorf K. J. Exp. Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Schmid S, Modlin IM, Stoch A, Tang L, Rhee S, Nathanson M, Scheele GA, Gorelick FS. Am. J. Physiol. 1998;274:G734–G741. doi: 10.1152/ajpgi.1998.274.4.G734. [DOI] [PubMed] [Google Scholar]
- 14.Seol JH, Shevchenko A, Shevchenko A, Deshaies RJ. Nat. Cell Biol. 2001;3:384–391. doi: 10.1038/35070067. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Karne S, Kolodecik T, Gorelick FS. Am. J. Physiol. 2002;282:G501–G507. doi: 10.1152/ajpgi.00388.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, Steer ML. Am. J. Physiol. 1998;275:G352–G362. doi: 10.1152/ajpgi.1998.275.2.G352. [DOI] [PubMed] [Google Scholar]
- 17.Otani T, Chepilko SM, Grendell JH, Gorelick FS. Am. J. Physiol. 1998;275:G999–G1009. doi: 10.1152/ajpgi.1998.275.5.G999. [DOI] [PubMed] [Google Scholar]
- 18.Grady T, Mah’moud M, Otani T, Rhee S, Lerch MM, Gorelick FS. Am. J. Physiol. 1998;275:G1010–G1017. doi: 10.1152/ajpgi.1998.275.5.G1010. [DOI] [PubMed] [Google Scholar]
- 19.Myers M, Forgac M. J. Cell. Physiol. 1993;156:35–42. doi: 10.1002/jcp.1041560106. [DOI] [PubMed] [Google Scholar]
- 20.Kane PM, Parra KJ. J. Exp. Biol. 2000;203:81–87. doi: 10.1242/jeb.203.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. J. Clin. Investig. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Gastroenterology. 1997;113:304–311. doi: 10.1016/s0016-5085(97)70108-2. [DOI] [PubMed] [Google Scholar]
- 23.Camello C, Pariente JA, Salido GM, Camello PJ. Curr. Biol. 2000;10:161–164. doi: 10.1016/s0960-9822(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki M, Masgrau R, Morgan AJ, Churchill GC, Patel S, Ashcroft SJ, Galione A. J. Biol. Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 25.Okorokov LA, Silva FE, Okorokova Facanha AL. FEBS Lett. 2001;505:321–324. doi: 10.1016/s0014-5793(01)02852-6. [DOI] [PubMed] [Google Scholar]
- 26.Muller O, Bayer MJ, Peters C, Andersen JS, Mann M, Mayer A. EMBO J. 2002;21:259–269. doi: 10.1093/emboj/21.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]