Abstract
Gene-environment interaction may play a role in the etiology of schizophrenia. Transgenic mice expressing dominant-negative DISC1 (DN-DISC1 mice) show some histological and behavioral endophenotypes relevant to schizophrenia. Viral infection during neurodevelopment provides a major environmental risk for schizophrenia. Neonatal injection of polyriboinosinic-polyribocytidylic acid (polyI:C), which mimics innate immune responses elicited by viral infection, leads to schizophrenia-like behavioral alteration in mice after puberty. To study how gene-environmental interaction during neurodevelopment results in phenotypic changes in adulthood, we treated DN-DISC1 mice or wild-type littermates with injection of polyI:C during the neonatal stage, according to the published method, respectively, and the behavioral and histological phenotypes were examined in adulthood. We demonstrated that neonatal polyI:C treatment in DN-DISC1 mice resulted in the deficits of short-term, object recognition, and hippocampus-dependent fear memories after puberty, although polyI:C treatment by itself had smaller influences on wild-type mice. Furthermore, polyI:C-treated DN-DISC1 mice exhibited signs of impairment of social recognition and interaction, and augmented susceptibility to MK-801-induced hyperactivity as compared with vehicle-treated wild-type mice. Of most importance, additive effects of polyI:C and DN-DISC1 were observed by a marked decrease in parvalbumin-positive interneurons in the medial prefrontal cortex. These results suggest that combined effect of neonatal polyI:C treatment and DN-DISC1 affects some behavioral and histological phenotypes in adulthood.
Keywords: schizophrenia, gene-environment interaction, viral infection, polyI:C, susceptibility gene, DISC1, transgenic mice
Introduction
Genetic susceptibility factors for schizophrenia have recently become available; these include neuregulin-1, dysbindin, and disrupted-in-schizophrenia 1 (DISC1) [9]. Maternal viral infection in the first and second trimesters of pregnancy in humans increases the risk of schizophrenia in young adulthood [3,4,22]. Furthermore, the possible interaction between environmental and genetic susceptibility factors, especially during neurodevelopment, is proposed as a promising disease etiology of schizophrenia [5,14].
Here we study a possible interaction of genetic and environmental factors by injecting a synthetic double-stranded RNA, polyriboinosinic-polyribocytidylic acid (polyI:C) into transgenic mice that express a dominant-negative form of DISC1 (DN-DISC1). We chose DISC1 as a genetic factor on which to focus, because its role during neurodevelopment is well characterized [6,13]. DN-DISC1 mice show some behavioral (sensorimotor gating deficits, depression-like behavior and hyperactivity) and histological (enlarged lateral ventricles and reduction in the immunoreactivity of parvalbumin in the cortex) endophenotypes relevant to schizophrenia [10]. PolyI:C is a toll-like receptor 3 ligand that induces a strong innate immune response, and has been used to mimic viral infection during neurodevelopment [15,23]. Moreover, we recently reported that neonatal injection of polyI:C in mice results in schizophrenia-like behavioral alterations in adulthood [11]. In the publication, we discussed the rationale in choosing the timing of polyI:C injection during the mouse neonatal stage that corresponds to the human second trimester [11]. Accordingly, neonatal DN-DISC1 mice were repeatedly injected with polyI:C for 5 days from postnatal day 2 to 6, which correspond to post conception day 128 to 158 for cortical events and 93 to 115 for limbic events of brain development in humans (http://translatingtime.net; see also [7]).
In the present study, we demonstrate that combined effect of neonatal polyI:C treatment and DN-DISC1 affects some behavioral and anatomical phenotypes in adulthood. Of note, as far as we are aware, this is the first experimental demonstration that “neonatal” interaction of major genetic and environmental susceptibility factors for schizophrenia results in the dramatic change in the parvalbumin-positive interneurons in the medial prefrontal cortex (mPFC), one of the best hallmarks for schizophrenia [17,20].
Materials and Methods
Animals
Transgenic mice expressing a dominant-negative mutant DISC1 under the expression control of CaMKII promoter (DN-DISC1; line 10) [10] were used in this study. Littermates (both males and females) generated by cross breeding of wild-type (WT, C57BL/6N) female and DN-DISC1 male mice were used for experiments. They were housed under a standard 12-h light/dark cycle (light phase 9:00-21:00) at a constant temperature of 23 ± 1°C, with free access to food and water throughout the experiments.
The animals were handled in accordance with the guidelines established by the Institutional Animal Care and Use Committee of Nagoya University, the Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Treatment
All litters were randomly divided into two groups: vehicle and polyI:C-treated mice. Mice were treated by subcutaneous injection with either pyrogen-free saline or 5 mg/kg polyI:C (Sigma-Aldrich, St. Louis, MO) daily between postnatal days 2 and 6. Animals were weaned at postnatal day 21, and divided along gender lines at postnatal day 28. Four groups [e.g., vehicle-treated WT (vehicle/WT), polyI:C-treated WT (polyI:C/WT), vehicle-treated DN-DISC1 (vehicle/DN-DISC1) and polyI:C-treated DN-DISC1 (polyI:C/DN-DISC1)] were derived from multiple (at least 3) litters to preclude possible differences in individual maternal behaviors as a mitigating factor in any subsequent long-lasting changes induced in the offspring. Behavioral analyses were started at 8 weeks old, and carried out in the following orders: Y-maze test, novel object recognition test, prepulse inhibition test, fear conditioning test, social interaction test and MK-801-induced hyperactivity.
Behavioral assays
Y-maze test
Y-maze test was carried out as described previously [10]. Each arm is 40 cm long, 12 cm high, 3 cm wide at the bottom, and 10 cm wide at the top. The arms converge in an equilateral triangular central area that is 4 cm at its longest axis. Each mouse is placed individually at the center of the apparatus and allowed to move freely through the maze during an 8-min session. The series of arm entries is recorded visually. Alternation is defined as successive entries into the three arms, on overlapping triplet sets. The percent alternation is calculated as the ratio of actual to possible alternations (defined as the total number of arm entries minus 2) multiplied by 100. Spontaneous alternation (%) defined as successive entries into the three arms on overlapping triplet sets is associated with the capacity of short-term memory.
Novel object recognition test
A novel object recognition test was carried out as described previously [11]. Mice were individually habituated to an open-box (30 × 30 × 35 high cm) for 3 days. During the training session, two novel objects were placed into the open field and the animals was allowed to explore for 10 min. The time spent exploring each object was recorded. During retention sessions, the animals were placed back into the same box 1 hr after the training session, in which one of the familiar objects used during training was replaced by a novel object, and allowed to explore freely for 5 min. A preference index in the retention session, a ratio of the amount of time spent exploring the novel object over the total time spent exploring both objects, was used to measure cognitive function. In the training session, the preference index was calculated as a ratio of the time spent exploring the object that was replaced by the novel object in the retention session, over the total exploring time.
Prepulse inhibition test
The prepulse inhibition (PPI) test was carried out as described previously [11]. After the animals were placed in the chamber under moderately bright light conditions (180 lux) (San Diego Instruments, San Diego, California), they were allowed to habituate for 10 min, during which 65 dB background white noise was present. The animals then received 10 startle trials, 10 no-stimulus trials and 40 PPI trials. The intertrial interval was between 10 and 20 sec and the total session lasted 17 min. The startle trial consisted of a single 120 dB white noise burst lasting 40 msec. PPI trials consisted of a prepulse (20 msec burst of white noise at 69, 73, 77 or 81 dB intensity) followed, 100 msec later, by the startle stimulus (120 dB, 40 msec white noise). Each of the four prepulse trials (69, 73, 77 or 81 dB) was presented 10 times. Sixty different trials were presented pseudo-randomly, ensuring that each trial was presented 10 times and that no two consecutive trials were identical. The resulting movement of the animal in the startle chamber was measured for 100 msec after startle stimulus onset (sampling frequency 1 kHz), rectified, amplified and fed into a computer, which calculated the maximal response over the 100-msec period. Basal startle amplitude was determined as the mean amplitude of the 10 startle trials. PPI was calculated according to the formula: 100 × [1-(PPx/P120)] %, in which PPx was the mean of the 10 PPI trials (PP69, PP73, PP75 or PP80) and P120 was the basal startle amplitude.
Fear conditioning test
To examine contextual memory in polyI:C/DN-DISC1 mice, we used context-dependent conditioned fear test according to previous report [1]. In the conditioning phase, each mouse is placed in the training cage (30 × 30 × 40 cm) equipped with a metal floor, and a 15-sec tone (85 dB) is delivered (conditioned stimulus). During the last 5 sec of the tone stimulus, a foot shock of 0.8 mA is delivered as an unconditioned stimulus through a shock generator. This procedure is repeated four times with 15 sec intervals. Twenty-four hr after the conditioning, context-dependent test was carried out. For context-dependent test, mouse is placed in the training cage, and the freezing response is measured for 2 min in the absence of the conditioned stimulus. Two hr after context-dependent test, tone-dependent test was carried out. For tone-dependent test, the freezing response was measured in the neutral cage for 1 min in the presence of a continuous-tone stimulus identical to the conditioned stimulus using mice which had been subjected to context-dependent test.
Social interaction test
We used the experimental paradigm described by Ibi et al. [11] to measure social behavior (e.g., social interaction, aggression and escape behavior). Vehicle/WT and polyI:C/DN-DISC1 mice were individually housed in a home cage (29 × 18 × 12 cm) for 2 days before the trial. We used 10-15-week-old male C57BL mice as intruders which had not shown aggressive behavior. In the first trial (5 min duration), an intruder mouse was introduced into the resident’s home cage. The duration of social interaction (close following, inspection, anogenital sniffing, and other social body contacts except aggressive behavior), aggression (attacking/biting and tail rattling) and escape behavior were analyzed. Four trials, with an inter-trial interval of 30 min, were used to analyze social behavior using the same intruder mouse.
MK-801-induced hyperactivity
To investigate the possible changes in sensitivity of glutamate N-methyl D-aspartate (NMDA) receptor in polyI:C/DN-DISC1 mice, locomotor activity induced by MK-801 (Sigma-Aldrich) was measured as described previously [11]. Each mouse was placed in a standard transparent rectangular rodent cage (25 × 30 × 18 cm) and allowed a 120-min habituation period before MK-801 (0.3 mg/kg, i.p.) treatment. Locomotor activity was then measured for 180 min immediately after MK-801 treatment, using an infrared sensor (NS-AS01; Neuroscience, Tokyo, Japan) placed over the cage.
Histological analyses
In histological analysis, 8-week-old mice that had not previously subjected to behavioral analysis were deeply anesthetized with diethyl ether and perfused transcardially with saline, followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4). Their brains were removed, post-fixed in the same fixative, and then cryoprotected. Thirty μm-thick coronal brain sections were cut on a cryostat and mounted on slides. The section used for the analysis of the mPFC was collected between stereotaxic coordinates bregma 1.78 to 1.54 according to the brain atlas [21] and those in the hippocampus was collected according to Ibi et al. [12].
BrdU-staining
5-Bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich) at a dose of 75 mg/kg was injected i.p. 3 times at 2 hr intervals at 4 weeks old, and the number of BrdU-labeled cells in the hippocampus was counted at 8 weeks [12]. Sections were treated overnight with 0.1% Nonidet P-40/0.01 M PBS (pH 7.2) at 4°C and denatured by microwave oven in 0.01 M citrate buffer (pH 6.0). After blocking in 10% goat serum/PBS with 0.1% NP-40 for 30 min, BrdU-positive cells in the sections were detected using a BrdU labeling and detection kit 2 (Roche Diagnotics GmbH, Germany) according to the manufacture’s instructions.
Ki67 and parvalbumin staining
Sections were incubated with 10% goat serum/PBS with 0.1% Triton X-100, and then incubated with rabbit anti-Ki67 antibody (1:2000; Novocastra, Newcastle, UK) or rabbit anti-parvalbumin antibody (1:500; Calbiochem, San Diego, CA) overnight at 4°C. They were washed with 0.01 M PBS and incubated with biotinylated goat anti-rabbit antibody (1:200; BA-1000, Vector Laboratories, Burlingame) at room temperature for 1 hr. The sections were washed and processed with avidin-biotinylated horseradish peroxidase complex (Vector ABC kit, Vector Laboratories), and action was visualized using diaminobenzidine.
For double staining of parvalbumin/Nissl in the mPFC, sections were incubated with blocking solution (10% donkey serum/PBS with 0.1% Triton X-100) and then rabbit anti-parvalbumin antibody (1:500; Calbiochem) diluted in blocking solution was applied to sections, which were then incubated overnight at 4°C and for 30 min at room temperature. After washing in PBS, donkey anti-rabbit Alexa 488 (1:1000; Invitrogen, Eugene, OR) were added to sections for 2 hr at room temperature. NeuroTracer Fluorescent Nissl Stains (1:100; Invitrogen) diluted in PBS was applied to sections, which were then incubated for 20 min at room temperature.
Quantification for BrdU, Ki67 and parvalbmin-positive cells
All BrdU and Ki67-labeled cells in the subgranular zone (SGZ), hilus and granule cell layer (GCL) were assessed as described by Ibi et al. [12]. Parvalbumin-positive cells in the hippocampus and mPFC were counted under ×10 magnification using a light microscope (Axio Imager; Zeiss, Jene, Germany) and confocal-laser scanning microscope (LSM 510; Zeiss), respectively.
Statistical Analysis
One-way analysis of variance (ANOVA) followed by the Bonferroni test was used for multiple-group comparisons. Two-tailed Student′s t-test was used for two-group comparisons.
Results
Behavioral evidence of gene-environment interactions
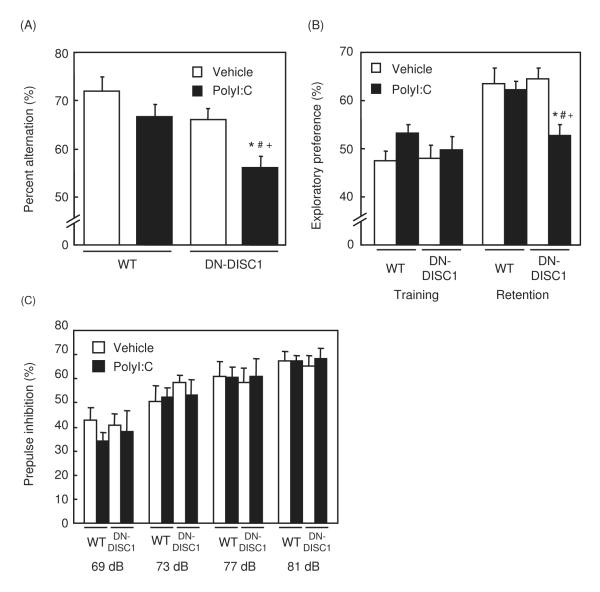
Neonatal WT and DN-DISC1 mice were treated with polyI:C or saline, and their behaviors were analyzed after being 8 weeks old. In the Y-maze test, neither genetic manipulation (vehicle/DN-DISC1) nor an environmental factor (polyI:C/WT) affected behavioral phenotypes compared with controls (vehicle/WT). Nonetheless, polyI:C/DN-DISC1 mice showed marked reduction in spontaneous alternation (Fig. 1A). There was no difference in the total number of arm entries among the four groups [vehicle/WT, 21.1 ± 1.0; polyI:C/WT, 24.7 ± 2.8; vehicle/DN-DISC1, 21.1 ± 0.9; polyI:C/DN-DISC1, 23.4 ± 1.7]. These results indicate a combined action of genetic (DN-DISC1) and environmental (polyI:C) factors, resulting in an impairment of short-term memory.
Fig. 1. Changes in short-term and recognition memories, and prepulse inhibition in polyI:C/DN-DISC1 mice.
(A) Spontaneous alternation behavior in Y-maze test. Vehicle/WT, n=13; polyI:C/WT, n=11; vehicle/DN-DISC1, n=17; polyI:C/DN-DISC1, n=13. Percent alternation (%): F(3,50)=6.529, p<0.001.
(B) Exploratory preference in novel object recognition test. Vehicle/WT, n=12; polyI:C/WT, n=11; vehicle/DN-DISC1, n=10; polyI:C/DN-DISC1, n=14. Exploratory preference (%): F(3,43)=5.427, p<0.01.
(C) PPI (%) at four different prepulse intensities (69, 73, 77 and 81 dB) in PPI test. Vehicle/WT, n=9; polyI:C/WT, n=14; vehicle/DN-DISC1, n=11; polyI:C/DN-DISC1, n=9. Prepulse inhibition (%):F(3,42)=0.0562, p=0.982.
Values indicate the mean ± SE. *p<0.05 vs. vehicle/WT, #p<0.05 vs. polyI:C/WT, +p<0.05 vs. vehicle/DN-DISC1.
Similar effects were also observed as a marked decrease in exploratory preference to a novel object in the retention session of novel object recognition test, in which only polyI:C/DN-DISC1 mice displayed the deficit, whereas the other three groups showed no difference (Fig. 1B). There was no difference in total time exploring two objects among the four groups in the training [vehicle/WT, 30.0 ± 2.4 sec; polyI:C/WT, 33.6 ± 3.2 sec; vehicle/DN-DISC1, 33.6 ± 3.8 sec; polyI:C/DN-DISC1, 33.0 ± 2.8 sec]. Thus, polyI:C/DN-DISC1 mice have an impairment of object recognition memory.
On the contrary, in the PPI test of the startle reflex, no changes were observed in either polyI:C/WT, vehicle/DN-DISC1 or polyI:C/DN-DISC1 mice compared to vehicle/WT mice, indicating no impairment of sensorimotor gating in polyI:C/DN-DISC1 mice (Fig. 1C). There was also no difference in the acoustic startle amplitude [vehicle/WT, 215 ± 36; polyI:C/WT, 232 ± 36; vehicle/DN-DISC1, 162 ± 30; polyI:C/DN-DISC1, 184 ± 28].
We then focused on the characterization of schizophrenia-like behavioral abnormality in polyI:C/DN-DISC1 mice. Thus, in the following behavioral assays, behavioral changes in polyI:C/DN-DISC1 mice were compared with those in vehicle/WT mice without testing behaviors in polyI:C/WT or vehicle/DN-DISC1 mice.
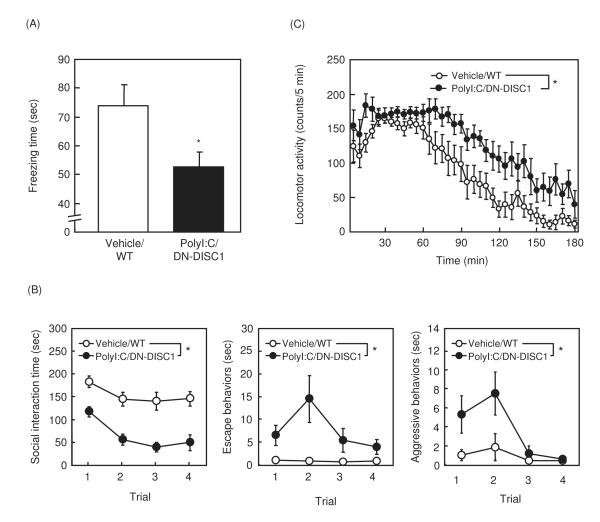
In the fear conditioning memory test, polyI:C/DN-DISC1 mice showed a significant decrease in context-dependent freezing time compared to vehicle/WT mice (Fig. 2A). However, there was no difference in tone-dependent freezing time (35.5 ± 2.1 sec in vehicle/WT mice, 33.0 ± 2.3 sec in polyI:C/DN-DISC1 mice) or sensitivity to electric footshock (0.14 ± 0.02 mA in vehicle/WT mice, 0.15 ± 0.02 mA in polyI:C/DN-DISC1mice), suggesting an impairment of hippocampus-dependent fear memory in polyI:C/DN-DISC1mice.
Fig. 2. Changes in fear memory, social behaviors and MK801-induced hyperactivity in polyI:C/DN-DISC1 mice.
(A) Context-dependent memory in fear conditioning test. Vehicle/WT, n=11; polyI:C/DN-DISC1, n=13.
(B) Social behaviors in social interaction test. Vehicle/WT, n=8; polyI:C/DN-DISC1, n=8. Social interaction time: F(1,14)=41.172; p<0.0001, escape behaviors: F(1,14)=7.012; p<0.05, aggressive behaviors: F(1,14)=7.316; p<0.05.
(C) MK-801-induced hyperactivity. Vehicle/WT, n=9; polyI:C/DN-DISC1, n=10. F(1,17)=11.232, p<0.01.
Values indicate the mean ± SE. *p<0.05 vs. vehicle/WT.
In the social interaction test, time of interaction was markedly decreased, while escape and aggressive behaviors were increased, in polyI:C/DN-DISC1 mice, compared with vehicle/WT mice (Fig. 2B). MK-801-induced hyperactivity was significantly augmented in polyI:C/DN-DISC1 mice, compared to vehicle/WT mice (Fig. 2C).
Histological analyses in polyI:C/DN-DISC1 mice
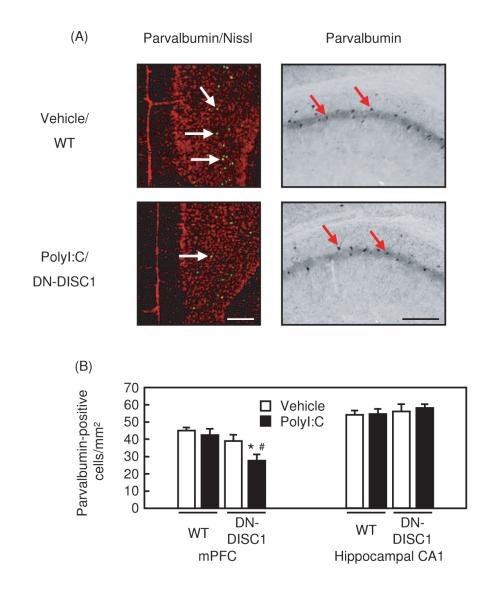
Selective reduction in the immunoreactivity of parvalbumin, an indicator of a set of interneurons in the cerebral cortex, has frequently been reported in autopsied brains from patients with schizophrenia [17]. Very interestingly, polyI:C treatment in the neonatal stage developed the selective reduction in the immunoreactivity of parvalbumin, specific to the mPFC in DN-DISC1 mice (Fig. 3A, B).
Fig. 3. Changes in parvalbumin-positive interneurons in polyI:C/DN-DISC1 mice.
(A) Representative photographs showing parvalbumin-positive cells in the mPFC [left (parvalbumin-positive cell, green; Nissl-positive cell, red)] and hippocampal CA1 region (right). Upper panels, vehicle/WT mice; lower panels, polyI:C/DN-DISC1 mice. Scale bar: 200 μm.
(B) Changes in the number of parvalbumin-positive interneurons. mPFC: vehicle/WT, n=10; polyI:C/WT, n=12; vehicle/DN-DISC1, n=8; polyI:C/DN-DISC1, n=8. F(3,35)=4.996, p<0.001.
Hippocampal CA1 region: vehicle/WT, n=3; polyI:C/WT, n=4; vehicle/DN-DISC1, n=3; polyI:C/DN-DISC1, n=4. F(3,10)=0.386, p=0.766.
Values indicate the mean ± SE. *p<0.05 vs. vehicle/WT, #p<0.05 vs. polyI:C/WT.
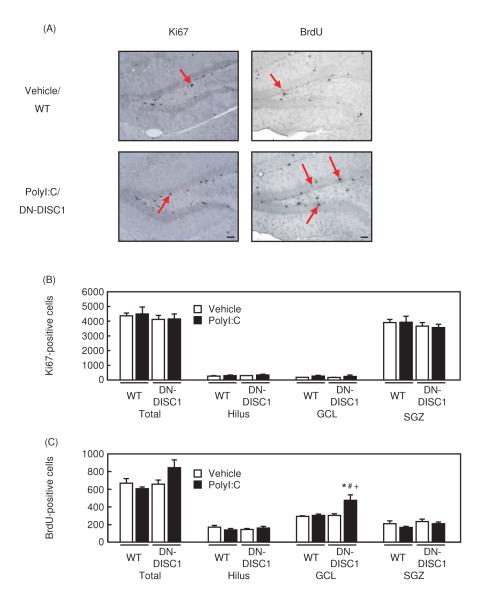
Because polyI:C/DN-DISC1 mice showed a disturbance of the hippocampus-dependent fear memory, we examined whether there were histological alterations in the hippocampus of polyI:C/DN-DISC1 mice. We conducted Nissl-staining, BrdU staining, and immunohistochemistry for Ki67 (a marker of cell proliferation) [2,8]. Although there were no changes in Nissl-staining and immunostaining of Ki67 (Fig. 4A, B), a significant increase in the number of BrdU-positive cells in the GCL of the hippocampus was observed (Fig. 4A, C).
Fig. 4. Changes in Ki67 and BrdU-positive cells in polyI:C/DN-DISC1 mice.
(A) Representative photographs showing Ki67-positive, and BrdU-positive cells in the DG of hippocampus. Upper panels, vehicle/WT mice; lower panels, polyI:C/DN-DISC1 mice. Scale bar: 200 μm.
(B) Changes in the number of Ki67-positive cells in the hippocampus. Vehicle/WT, n=3; polyI:C/WT, n=4; vehicle/DN-DISC1, n=3; polyI:C/DN-DISC1, n=4. Total: F(3,10)=0.231, p=0.873, Hilus: F(3,10)=0.318, p=0.812, GCL: F(3,10)=0.926, p=0.463, SGZ: F(3,10)=0.358, p=0.785.
(C) Changes in the number of BrdU-positive cells in the hippocampus. Vehicle/WT, n=5; polyI:C/WT, n=5; vehicle/DN-DISC1, n=5; polyI:C/DN-DISC1, n=5.
F(3,16)=6.406, p<0.01. There were no significant differences in other brain areas. Values indicate the mean ± SE. *p<0.05 vs. vehicle/WT, #p<0.05 vs. polyI:C/WT, +p<0.05 vs. vehicle/DN-DISC1.
Discussion
In agreement with the previous report by Hikida et al. [10], behavioral and histological abnormalities in DN-DISC1 mice were mild and subtle. In the present study, we demonstrated that combination of neonatal polyI:C treatment with DN-DISC1 resulted in the deficits of short-term, object recognition, and hippocampus-dependent fear memories in adulthood, although polyI:C treatment by itself had little influence on WT mice. Furthermore, polyI:C/DN-DISC1 mice exhibited signs of impairment of social recognition and interaction and augmented susceptibility to MK-801-induced hyperactivity. DN-DISC1 mice is reported to display a small, but significant, deficit in PPI of the startle reflex in the previous study [10], whereas there was no significant difference between vehicle/WT and vehicle/DN-DISC1 mice in the present study. The discrepancy in the PPI deficit of DN-DISC1 mice may be explained by the difference in experimental schedule. Behavioral analysis was carried out at the age of 12-32 weeks in the previous report [10] while we started the analysis from 8-weeks old mice. Accordingly, it is possible that the changes in PPI response in DN-DISC1 mice may be undetectable at younger ages as we selected [24].
The present study demonstrated a significant impact of DN-DISC1 expression on parvalbumin-positive interneurons in the mPFC (Fig. 3A, B), which is consistent with the previous findings [10]. Importantly, the post-hoc analysis revealed the additive effect of neonatal immune activation induced by polyI:C treatment and genetic impact of DN-DISC1, leading to a marked decrease in parvalbumin-positive interneurons in the mPFC of polyI:C/DN-DISC1 mice in adulthood. This pathological change is currently regarded as the best hallmark for the pathophysiology of schizophrenia, which is likely to underlie the cognitive dysfunction in patients with schizophrenia [17,20].
For the past a couple of years, many lines of genetic mouse models based on susceptibility genes for schizophrenia have become available and characterized mainly by behavioral alterations [10,13,18,19,25]. Nonetheless, only a few studies have addressed for possible gene-environmental interactions in the context of schizophrenia [16]. We believe that the present study has two major strengths: first, a most promising pair of genetic and environmental factors for schizophrenia is tested together for the phenotypic assessment; second, of most importance, the present study address a specific interneuron deficit, well-accepted histological hallmark for schizophrenia, and examine how gene-environmental interactions during the “critical neurodevelopmental period” can result in this objective phenotype in “adulthood”. Furthermore, we also demonstrated that such gene-environmental interactions specifically affect some, but not all, types of behaviors elicited by the genetic factor. The concept of neonatal “critical neurodevelopmental period” proposed in the present study is consistent with the observation by the Cannon and Silva’s group [19], in which their group demonstrated the critical requirement of DISC1 function in neonatal days by using inducible transgenic mice for mutant DISC1. On the other hand, from the clinical point of view, “critical development period” of schizophrenia is postulated not only in early (pre- and perinatal) stage but also in late (pubertal) stage in human [26]. Further studies are required to clarify the effect of pubertal exposure to psychosocial stresses or polyI:C in DN-DISC1 mice on phenotypic changes.
To elucidate a genuine gene-based effect, it is important to use more than two independent lines of transgenic mice. The present study investigated the gene-environmental interaction by using only one line of DN-DISC1 mice (line 10) because basic characterization of these behavioral deficits has already conducted in two independent lines (line 10 and 37) which showed that the behavioral and histological phenotypes in DN-DISC1 mice are due to the disruption of DISC1 gene [10]. However, to completely exclude a possibility that another, unknown gene may participate in the interaction, we need to use another line of DN-DISC1 (line 37).
In summary, taking effects in several behavioral and pathophysiological deficits into consideration, we propose that neonatal polyI:C treatment in DN-DISC1 mice may provide a model for schizophrenia that reflects gene-environmental interactions.
Acknowledgements
We thank Drs. Pamela Talalay and Akiko Takagi-Hayashi for critical reading of this manuscript. This study was supported in part by a Grant-in-Aid for Scientific Research (No. 19390062) from the Japan Society for the Promotion of Science, Research on Risk of Chemical Substances, Health and Labor Science Grants supported by Ministry of Health, Labour and Welfare, Academic Frontier Project for Private Universities; matching fund subsidy from MEXT, 2007-2011, Takeda Science Foundation, AstraZeneca Research Grant 2008, and by JST, CREST. NIH grants of MH-084018, MH-069853, as well as grants from Stanley, CHDI, HighQ, S-R, and NARSAD support AS.
Footnotes
Disclosure/Conflict of Interest The authors declare that there is no conflict of interest in the publication of the present work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Braun N, Papadopoulos T, Müller-Hermelink HK. Cell cycle dependent distribution of the proliferation-associated Ki-67 antigen in human embryonic lung cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56:25–33. doi: 10.1007/BF02889998. [DOI] [PubMed] [Google Scholar]
- [3].Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cannon M, Clarke MC. Risk for schizophrenia-broadening the concepts, pushing back the boundaries. Schizophr Res. 2005;79:5–13. doi: 10.1016/j.schres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- [5].Caspi A, Moffitt TE. Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- [6].Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- [7].Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- [8].Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- [9].Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- [10].Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, et al. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64:297–305. doi: 10.1016/j.neures.2009.03.015. [DOI] [PubMed] [Google Scholar]
- [12].Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- [13].Ishizuka K, Paek M, Kamiya A, Sawa A. A review of disrupted-in-schizophrenia-1 (disc1): Neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- [14].Jaffee SR, Price TS. Gene–environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Laviola G, Ognibene E, Romano E, Adriani W, Keller F. Gene-environment interaction during early development in the heterozygous reeler mouse: Clues for modelling of major neurobehavioral syndromes. Neurosci Biobehav Rev. 2009;33:560–572. doi: 10.1016/j.neubiorev.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [17].Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- [18].Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’Brien EP, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li W, Zhou Y, Jentsch JD, Brown RAM, Tian X, Ehninger D, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci USA. 2008;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San diego: 2004. [Google Scholar]
- [22].Patterson PH. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- [23].Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fatemi SH, Folsom TD. The Neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stefansson H, Petursson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]