Abstract
The regulation of vitamin D receptor (VDR), a key mediator in the vitamin D pathway, in breast cancer etiology has long been of interest. We have shown here that the transcriptional repressor protein SLUG inhibits the expression of VDR in human breast cancer cells. To explore the possibility that SLUG regulates the VDR gene promoter, we cloned a 628 bp fragment (−613 to +15) of the human VDR gene promoter. This region contains three E2-box sequences (CAGGTG/CACCTG), the classical binding site of SLUG. SLUG specifically inhibited VDR gene promoter activity. Chromatin-immunoprecipitation (ChIP) assays revealed that SLUG is recruited on the native VDR gene promoter along with the co-repressor protein CtBP1 and the effector protein HDAC1. These data suggests that SLUG binds to the E2-box sequences of the VDR gene promoter and recruits CtBP1 and HDAC1, which results in the inhibition of VDR gene expression by chromatin remodeling.
Keywords: SLUG, VDR, E2-box, Transcriptional repression, CtBP1, HDAC1
The vitamin D receptor (VDR) [1–3] is a ligand-regulated transcription factor that mediates most biological effects of 1,25-dihydroxyvitamin D (VD). In vitro studies have shown that the VDR ligand, VD, modulates key proteins involved in signaling, proliferation, differentiation, and survival of normal mammary epithelial cells. However, many transformed breast cancer cells lose sensitivity to VD owing to down-regulation of VDR function [1–3].
SLUG is a member of the SNAI family of C2H2-zinc finger family of transcriptional repressors [4–6]. It is involved in the epithelial–mesenchymal transition during development [5], acts as an inhibitor of apoptosis [7], and causes tubulogenesis during breast and kidney developments [4,5]. The genes inhibited by SLUG include E-cadherin [8], claudins [9], BRCA2 [10], and cytokeratins [11]. Our ChIP-DSL analysis of 20,000 human gene promoter array revealed that more than 150 promoters bind to SLUG at their promoters (Mittal, M.K. and Chaudhuri, G., unpublished data). VDR gene is one of the candidate SLUG-regulated genes. Here, we report that SLUG indeed binds in vivo to the VDR gene promoter in human breast cell nucleus and inhibits VDR gene expression by chromatin remodeling.
Materials and methods
Cell culture and reagents
Human breast cancer cells MCF-7, MDA-MB-468, MDA-MB-231 and BT549 were obtained from ATCC (Manassas, VA) and were cultured in ATCC-recommended media [10,11]. FLAG M2 antibody was purchased from Sigma Chemical Co. (St. Louis, MO). CtBP1 and HDAC1 antibodies were purchased from UPSTATE Millipore (Burlington, MA). VDR and SLUG (G18) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Generation of stable clones
Human SLUG (hSLUG) gene ORF (NM_003068) was PCR amplified [10] from the cDNAs derived from MDA-MB-231 cells with ClaI and BamHI site-containing primers (5′-CAAGGTACCATGCCGCGCTCCTTCCTGC-3′, and 5′-CAAGGATCCG TGTGCTACACAGCAGCC-3′). The reverse primer did not have the endogenous stop codon. The PCR product was cloned at the ClaI/BamHI sites of p3xFLAG-CMV-14 vector (Sigma). The SLUG-3xFLAG sequences were then amplified with 5′-CACCATGCCGCGCTCCTTCC T-3′ and 5′-ATCACTACTTGTCATCGTCATCCTTGTAGTCG-3′ primers to clone directionally into the Gateway entry vector pENTR/D-TOPO (Invitrogen, Carlsbad, CA). The SLUG-3xFLAG ORF was then transferred to pLenti4/TO/V5-DEST vector (Invitrogen) by recombination using Gateway cloning reagents and protocols (Invitrogen). Transfection of 293 FT packaging cells with the plasmid constructs and human breast cells with the virus were done as described before [10]. The blasticidin (25 μg/ml)-resistant tetracycline repressor-expressing cells were further transduced by pLenti4/TO/V5-SLUG-3xFLAG-containing virus as described above, and the stable cell line was selected with 250 μg/ml zeocin. These double-resistant cell lines were maintained in blasticidin and zeocin. Expression of the SLUG protein was induced by tetracyclin (1 μg/ml) for 24–48 h.
Immunofluorescence analysis
Cells were cultured in 8-well chamber slides for 24 h in complete media, washed with PBS, fixed and permeabilized with ice-cold methanol for 10 min. After blocking in 5% goat serum in PBS, the cells were incubated with the primary antibody (Santa Cruz Biotechnology) followed by secondary antibody conjugated with the red fluorescent dye (ALexa Fluor R555-conjugated donkey anti mouse IgG, Invitrogen). The cells were subsequently stained with DAPI (Sigma). Finally, each slide was examined by fluorescence microscopy in a Nikon TE2000-E inverted wide-field microscope. Each representative image was examined and digitally recorded at the same cellular level and magnification.
RT–PCR analysis
RNA was extracted from cells using Trizol reagent (Invitrogen) and PCR amplifications were performed as described [10]. Primers used were: for SLUG, 5′-ATGCCGCGCTCCTTCCTGC-3′ and 5′-ATGGAGGAGGGGGACTCACTCG-3′, for VDR, 5′-CCAGTTCGT GTGAATGATGG-3′ and 5′-AGATTGGAGAAGCTGGACGA-3′, for β-actin, 5′-GCTCGTCGTCGACAACGGCTC-3′ and 5′-CAAACATGATCTG GGTCATCTTCTC-3′.
Luciferase reporter assay
We PCR amplified human VDR gene promoter (−613 to +15, NM_000376) from total DNA isolated from BT549 cells with 5′-GCTGCCAAGGTGATATCGGG-3′ and 5′-CGCTGCCGCCTTTTGACAAG-3′ primers. The amplified DNA was cloned into the pCR-II-TOPO vector (Invitrogen) and subsequently subcloned into the Eco RI site of pRL-Null vector (Promega). Cells were seeded on 24-well tissue culture plates and post 24 h, they were transfected with pGL3-Control and pRL-VDR promoter construct using Lipofectamine 2000 transfection reagent (Invitrogen). Luciferase activity was assayed 48 h later using a dual luciferase assay kit (Promega), as described before [10].
Chromatin-immunoprecipitation assay
Chromatin-immunoprecipitation assays were performed as described previously [10]. Immunoprecipitations were performed using FLAG (for SLUG), CtBP1 or HDAC1 antibodies. VDR gene promoter DNA was amplified from the ChIP DNA using the primers described above.
Immunoblot analysis
Cells from stable clones were grown in complete medium. Protein extracts were made and Western blotting was performed as described [10]. Cell lysates containing equal amounts of protein were resolved by 4–12% SDS–PAGE, transferred to nitrocellulose membranes, probed with the appropriate antibodies, and detected by means of enhanced chemiluminescence [10].
Results and discussion
Inducible expression of FLAG-tagged SLUG in the SLUG-negative MDA-MB-468 and MCF-7 cells
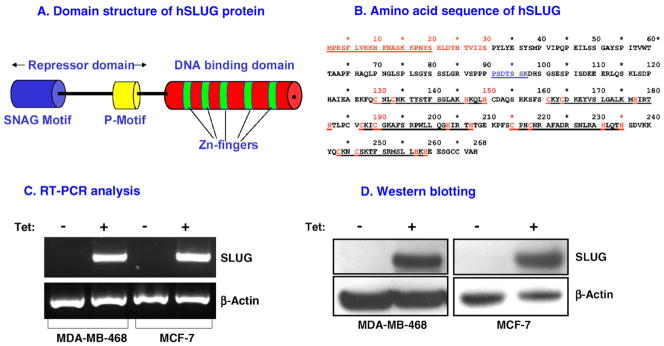
Human SLUG protein is 268 amino acids long with two functional domains: (i) the N-terminal repressor domain and (ii) the C-terminal DNA binding domain (Fig. 1A and B). The DNA binding domain has five C2H2 type zinc fingers, which are essential for the interaction of this repressor protein with the E2-box sequences (CAGGTG/CACCTG) at the promoters of SLUG-target genes [4–6]. The repressor domain consists of two distinct motifs: (i) the SNAG motif, which is conserved in many other proteins including other SNAI family members, Gfi1 and Gfi2, and ZEB1 and ZEB2 [4–6] and (ii) the P-motif, which we found through mutational analyses as essential for the repressor function of human SLUG (Bailey, C.K. and Chaudhuri, G., unpublished data). We replaced the seven amino acids of the P-motif of hSLUG (Fig. 1B) with alanine and used the resultant functionally inactive SLUG as a control in the studies described here.
Fig. 1.
Inducible expression of FLAG-tagged SLUG in MCF-7 and MDA-MB-468 cells. (A) Domain structure of hSLUG protein. (B) Amino acid sequences of hSLUG protein showing different functional domains. The SNAG motif (residues 1–20), the P-motif (residues 91–97) and the five zinc finger motifs (residues 130–150, 161–181, 187–207, 215–235, and 243–259) are underscored. RT-PCR (C) and Western blotting (D) analyses data showing tetracycline-inducible expression of hSLUG mRNA and protein, respectively. β-Actin mRNA and protein were used as loading controls in these studies.
To study the binding of SLUG to its target gene promoters, we expressed recombinant SLUG in SLUG-negative human breast cells, e.g., MCF-7 and MDA-MB-468 [10]. Attaching the 3xFLAG epitope at the C-terminus of hSLUG protein did not alter the repressor activity of this protein. We employed a lentiviral vector system to express recombinant SLUG from a tetracycline-inducible promoter. Fig. 1C and D shows that the SLUG mRNA and the 3xFLAG-tagged SLUG protein are abundantly expressed both in the recombinant MDA-MB-468 and MCF-7 cells in a tetracycline-inducible manner. These recombinant cells inducibly expressing functionally active 3xFLAG-tagged hSLUG were used for the studies described below.
Repression of VDR gene expression by hSLUG in MDA-MB-468 and MCF-7 cells
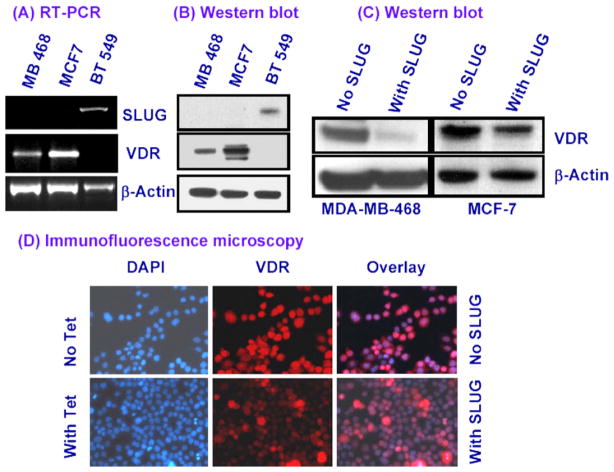
While studying SLUG-binding gene promoters in human breast cells using the 3xFLAG-SLUG-expressing MDA-MB-468 and MCF-7 cells employing the ChIP-DSL technique [12] with the reagents and human 20,000 gene promoter chip from Aviva Systems Biology (San Diego, CA), we discovered that VDR gene promoter binds strongly with the SLUG protein. Since VDR and SLUG proteins are relevant in human breast cancer etiology, we characterized further the interactions of SLUG and the VDR gene promoter in the human breast cells. With few cultured human breast cells, we found that there is an inverse relationship between SLUG and VDR gene expressions. The noninvasive MDA-MB-468 and MCF-7 cells do not express SLUG gene and they have significant levels of VDR mRNA and protein (Fig. 2A and B). Whereas, the highly invasive BT549 cells expresses SLUG but no VDR (Fig. 2A and B). When we induced the expression of SLUG in the recombinant MDA-MB-468 and MCF-7 cells, the levels of the VDR protein decreased significantly (Fig. 2C). Expression of non-functional SLUG protein did not cause any such effect on the VDR protein levels (data not shown). Our immunofluorescence microscopy data further verified the down-regulation of VDR gene expression in the presence of SLUG in the recombinant MDA-MB-468 cells (Fig. 2D). These data strongly suggests that SLUG inhibits the expression of VDR gene in human breast cells.
Fig. 2.
Down-regulation of VDR in SLUG-expressing human breast cells. RT-PCR (A) and Western blotting (B) analyses data showing the expressions of SLUG and VDR mRNA and protein, respectively, in different human breast cancer cells. (C) Western blotting analysis data showing tetracycline-inducible repression of VDR protein levels in the recombinant MCF-7 and MDA-MB-468 cells. (D) Immunofluorescence analysis showing tetracycline-inducible repression of VDR protein levels in the recombinant MDA-MB-468 cells. (Left panel) The nuclei of the cells were stained with DAPI (blue); (middle panel) VDR protein was tagged with red Alexafluor dye; and (right panel) the superimposed photograph. β-Actin mRNA and protein were used as loading controls in these studies. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Inhibition of VDR gene promoter activity by hSLUG
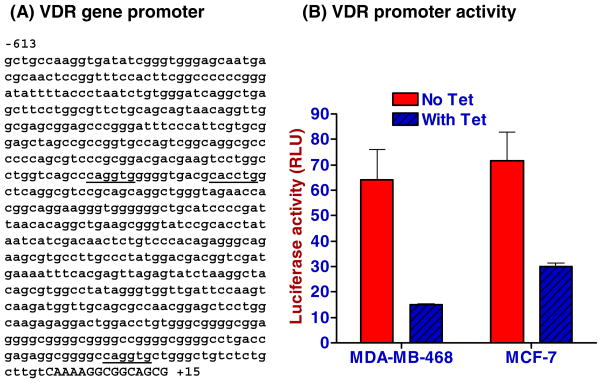
We then evaluated whether hSLUG can inhibit the cloned VDR gene promoter. We amplified a 628 bp (−613 to +15) promoter sequence (Fig. 3A) from human VDR gene and cloned that in front of Renilla luciferase gene in pRL-Null vector. This promoter sequence has three E2-boxes at the upstream of the transcription start site (Fig. 3A). Tetracycline induction of hSLUG expression in recombinant MDA-MB-468 and MCF-7 cells showed down-regulation of VDR gene promoter activities (Fig. 3B). These data suggest that hSLUG works through the E2-box containing minimal promoter sequence of human VDR gene to repress it.
Fig. 3.
Negative regulation of VDR gene promoter activity in SLUG-expressing human breast cells. (A) Nucleotide sequence of human VDR gene promoter showing (underscored) the SLUG-binding E2-box (CAGGTG/CACCTG) elements. The upstream sequences are shown in lower case letters. The 5′-end of the exon 1 sequences is in uppercase letters. (B) Dual luciferase assay data showing the repression of the function of VDR gene promoter in the recombinant MCF-7 and MDA-MB-468 cells. Results are means ± SE (n = 6). The differences in the luciferase activities between the control and the tetracycline-induced cells were statistically significant (p < 0.001).
In vivo binding of SLUG, CtBP1 and HDAC1 proteins to the VDR gene promoter in human breast cells
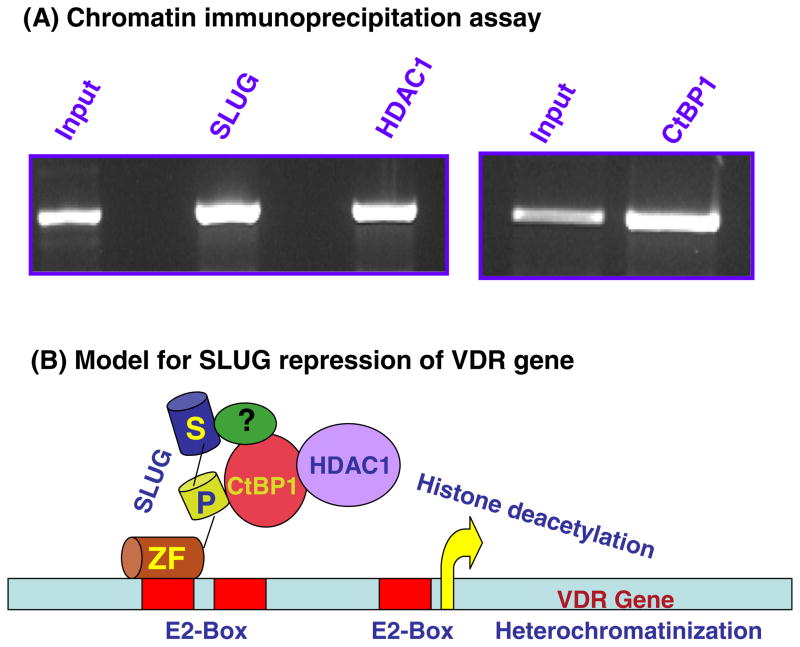
To test whether SLUG is indeed binds to VDR gene promoter in the breast cell nuclei and to validate the co-repressor and effector requirements in this repression process, we performed ChIP analysis with antibodies against FLAG, CtBP1 and HDAC1 in the recombinant MDA-MB-468 cells. We previously found that CtBP1 is the co-repressor for SLUG-repression of human BRCA2 gene [10]. We also detected HDAC1 as the effector for the heterochromatinization of human BRCA2 gene promoter [10]. Fig. 4A shows that VDR gene promoter indeed binds to SLUG protein in the nucleus of the recombinant MDA-MB-468 cells. CtBP1 and HDAC1 also bound to the VDR gene promoter when SLUG was expressed (Fig. 4A). These bindings were dependent on tetracycline induction of SLUG (data not shown), suggesting that SLUG binding to the VDR gene promoter is a prerequisite for CtBP1 and HDAC1 binding to this promoter. Non-functional P-motif mutated SLUG also could not recruit CtBP1 or HDAC1 to the VDR gene promoter (data not shown). Based on these observations, we propose a model for SLUG-mediated down-regulation of human VDR gene expression by chromatin remodeling (Fig. 4B). According to this model, SLUG is recruited to any or all three of the E2-box sequences at the VDR gene promoter through its DNA binding domain. We have shown previously that hSLUG cannot directly bind to CtBP1 [13]. Thus, assisted perhaps by other transcription regulator(s), CtBP1 is recruited at the P-motif of SLUG. CtBP1 then recruits HDAC1, and perhaps other effectors (e.g., HMT1), to catalyze histone modification and silencing of the VDR gene promoter. Although VDR gene promoter was shown to be regulated by other E2-box binding proteins in non-breast cells [14,15], ours is the first report of the direct involvement of SLUG in the modulation of the expression of VDR gene in human breast cells.
Fig. 4.
In vivo binding of SLUG, CtBP1 and HDAC1 to the VDR gene promoter in human breast cells. (A) ChIP analysis data showing the in vivo binding of hSLUG, CtBP1, and HDAC1 at the native VDR gene promoter in the lentivirus-transformed MDA-MB-468 cells. The expression of hSLUG was induced with tetracycline (1 μg/ml) for 48 h before the ChIP analysis. Anti-FLAG antibody was used to pull down the SLUG complex. (B) Model showing the possible mode of action of hSLUG to repress VDR gene expression in human breast cells. For simplicity, hSLUG binding to only one of the three E2-boxes is shown. The hypothetical protein, denoted with ‘?’, is proposed to help recruit CtBP1 at the P-motif of hSLUG. ZF, zinc finger of hSLUG; P, the P-motif; and S, the SNAG motif of hSLUG.
Acknowledgments
This work was supported by the DOD-CDMRP IDEA Grant# W81XWH-06-1-0466 to G.C. Immunofluorescence analysis was performed with the help of Dr. S. Goodwin, Director, MMC Morphology Core (supported by NIH Grants U54NS041071-06, G12RR03032-19, U54CA91408, and U54RR019192-04).
Abbreviations
- VDR
vitamin D receptor
- VD
vitamin D
- CtBP1
C-terminal binding protein 1
- HDAC1
histone deacetylase 1
- HMT1
histone methyl transferase 1
References
- 1.Narvaez CJ, Zinser G, Welsh J. Functions of 1 alpha,25-dihydroxyvitamin D(3) in mammary gland: from normal development to breast cancer. Steroids. 2001;66:301–308. doi: 10.1016/s0039-128x(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 2.Welsh J, Wietzke JA, Zinser GM, Smyczek S, Romu S, Tribble E, Welsh JC, Byrne B, Narvaez CJ. Impact of the Vitamin D3 receptor on growth-regulatory pathways in mammary gland and breast cancer. J Steroid Biochem Mol Biol. 2002;83:85–92. doi: 10.1016/s0960-0760(02)00277-7. [DOI] [PubMed] [Google Scholar]
- 3.Slattery ML. Vitamin D receptor gene (VDR) associations with cancer. Nutr Rev. 2007;65:102–104. doi: 10.1111/j.1753-4887.2007.tb00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 5.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 6.Hemavathy K, Guru SC, Harris J, Chen JD, Ip YT. Human Slug is a repressor that localizes to sites of active transcription. Mol Cell Biol. 2000;20:5087–5095. doi: 10.1128/mcb.20.14.5087-5095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 9.Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, Reina M, Cano A, Fabre M, Vilaro S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2005;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripathi MK, Misra S, Khedkar SV, Hamilton N, Irvin-Wilson C, Sharan C, Sealy L, Chaudhuri G. Regulation of BRCA2 gene expression by the SLUG repressor protein in human breast cells. J Biol Chem. 2005;280:17163–17171. doi: 10.1074/jbc.M501375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripathi MK, Misra S, Chaudhuri G. Negative regulation of the expressions of cytokeratins 8 and 19 by SLUG repressor protein in human breast cells. Biochem Biophys Res Commun. 2005;329:508–515. doi: 10.1016/j.bbrc.2005.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon YS, Garcia-Bassets I, Hutt KR, Cheng CS, Jin M, Liu D, Benner C, Wang D, Ye Z, Bibikova M, Fan JB, Duan L, Glass CK, Rosenfeld MG, Fu XD. Sensitive ChIP-DSL technology reveals an extensive estrogen receptor alpha-binding program on human gene promoters. Proc Natl Acad Sci USA. 2007;104:4852–4857. doi: 10.1073/pnas.0700715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey CK, Misra S, Mittal MK, Chaudhuri G. Human SLUG does not directly bind to CtBP1. Biochem Biophys Res Commun. 2007;353:661–664. doi: 10.1016/j.bbrc.2006.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pálmer HG, Larriba MJ, García JM, Ordóñez-Morán P, Peña C, Peiró S, Puig I, Rodríguez R, de la Fuente R, Bernad A, Pollán M, Bonilla F, Gamallo C, de Herreros AG, Muñoz A. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 15.Peña C, García JM, Silva J, García V, Rodríguez R, Alonso I, Millán I, Salas C, de Herreros AG, Muñoz A, Bonilla F. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: clinicopathological correlations. Hum Mol Genet. 2005;14:3361–3370. doi: 10.1093/hmg/ddi366. [DOI] [PubMed] [Google Scholar]