Abstract
The cortical nucleus LMAN provides the output of a basal ganglia pathway that is necessary for vocal learning in juvenile songbirds. The shell subregion of LMAN gives rise to recurrent loops that may subserve specific learning-related functions. We report here that lesions within the LMANshell pathway cause no immediate disruption of vocal behavior, but prevent the development of stable vocal sequences as well as the ability to imitate vocal sounds.
In both songbirds and humans, vocal learning entails forming a memory of vocal sounds from adult “tutors” based on auditory experience during development, and then using feedback of self-produced vocalizations to adjust motor commands until vocal output matches the neural memory of those sounds1. LMAN is composed of separate core and shell subregions, which give rise to independent parallel pathways that traverse the basal ganglia and thalamus (Fig. S1A)2. LMANcore projects to vocal motor cortex (RA) and thence to hindbrain vocal motor and respiratory circuits. In contrast, LMANshell projects to Ad, an area of motor cortex adjacent to RA; Ad makes a prominent projection to a dorsal thalamic zone (DTZ) that sends projections back to LMAN as well as to the cortical motor-control nucleus HVC (Fig. S1 for abbreviations). Thus the LMANshell pathway forms recurrent loops that are likely to make feedback connections to LMAN as well as feed-forward connections to HVC, and thereby play an important role in vocal learning. Lesions targeted to LMANcore in juvenile birds, but not adults, cause immediate disruption of vocal behavior3. However, no functional role for LMANshell circuitry has been identified. As an initial test of whether the recurrent loop from LMANshell→Ad→DTZ→LMANshell contributes to acquisition of learned vocal behavior, we lesioned Ad during early stages of auditory-motor integration in juvenile zebra finches (~45 days of age) and examined resultant changes in vocal behavior.
Juvenile birds underwent surgery after being raised by their natural father and hearing his tutor song (all experimental procedures were approved by the USC Institutional Animal Care & Use Committee; Supplementary Methods online). Birds with significant lesion damage to Ad produced substantially disrupted song behavior as adults (Fig. S2; sample audio files in Supplementary Information). The morphology of individual syllables was largely normal with respect to basic phonological features. However, lesioned birds produced songs with little or no stereotypy in terms of temporal sequence of syllables. The increased variability of syllable ordering following Ad lesions was reflected in lowered scores of linearity and consistency (Supplementary Methods, Fig. S3, Supplementary Table S1). Linearity measures the degree to which syllables are produced in a specific order (maximum score = 1.0). Control birds produced stereotyped linear sequences by 75 days of age (0.84 ± 0.05, mean ± SEM), whereas birds with Ad lesions did not (0.49 ± 0.02; U = 1, p < 0.001). We also calculated a consistency score for each bird, which measures the frequency with which the most common syllable transitions occur (maximum score = 1.0). Control birds produced more consistent syllable sequences than did birds with Ad lesions (0.96 ± 0.01 versus 0.80 ± 0.03; U = 3.5, p <0.001). As an additional means of assessing sequence stereotypy, we calculated the proportion of song motifs that included the syllable sequence produced most often by each bird (Fig. S3). Control birds produced their highest-probability sequence 73% of the time on average, whereas lesioned birds produced their most frequent sequence only 32% of the time (U = 4, p < 0.001). In summary, adult vocal behavior of lesioned birds was abnormal in that they never developed stable syllable sequences.
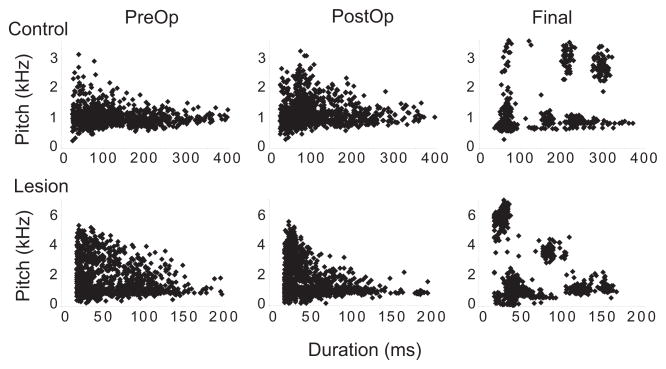
Examination of vocal behavior within one week following Ad lesions revealed no tendency for immediate disruption (Fig. S4). Lesioned birds continued to produce normal juvenile song behavior for at least 6 days post-lesion. We quantified spectral characteristics of song behavior by measuring six different phonological features for every syllable produced in all songs of adult birds (pitch, FM, AM, entropy, pitch goodness, and mean frequency4; Supplementary Methods). Settings used to measure syllables in adult songs (Final) were then applied to songs produced prior to surgery (PreOp; mean 2.5 days pre-surgery) and within the first week post-surgery (PostOp; mean 2.0 days post-surgery) in order to determine whether lesioned birds showed any immediate disruption for these six features. Mean values for each of the six phonological features analyzed were not different between control and lesioned birds, nor did they show substantial changes at any of the three time points analyzed (Fig. S5A). Representative scatter plots of pitch against duration for all syllables produced by one lesioned and one control bird were essentially the same pre-operatively and post-operatively (Fig. 1). By the Final (adult) song recording, both lesioned and control birds developed discrete clusters of syllables. Thus, adult birds that received lesions of Ad as juveniles generated stereotyped syllable types, despite a lack of stable sequencing of those syllables.
Figure 1. Distribution of pitch in lesioned versus control birds.
Scatter plots of Pitch against Duration for all syllables produced by one control bird (top panels; W688) and one lesioned bird (bottom panels; Lb772) at three different time points: immediately prior to surgery (PreOp), within one week post-surgery (PostOp), and in adulthood (Final). Each data point represents an individual syllable, such that discrete clusters signify repeated production of a specific syllable type.
We also calculated the coefficient of variation (CV) across syllables for each feature in order to indicate the overall scatter for each feature in a comparable metric. The average CV (across syllables for each bird) for all phonological features did not differ between groups (although CV for pitch increased over time and was marginally higher in adult lesioned birds; Figs. S5B, S6). These data show that Ad lesions do not produce disruption of song behavior within the first week post-surgery, nor do they produce gross disruption of spectral aspects of vocal motor output even in adult birds.
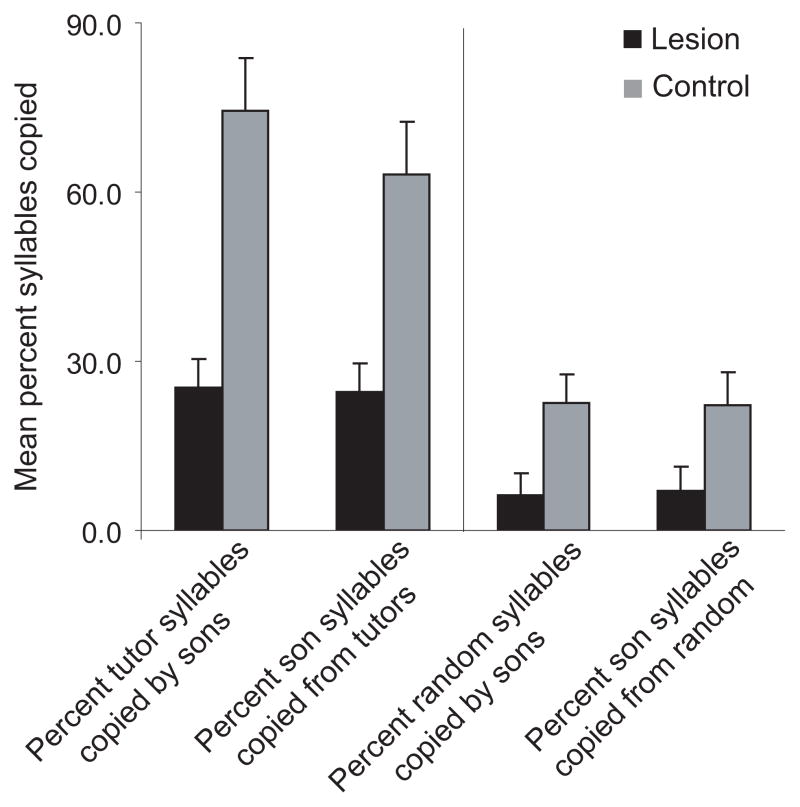
We compared the adult songs of Ad-lesioned birds to those of their tutors in order to judge whether lesioned birds could successfully imitate a tutor song. As a control, we also compared the songs of six birds randomly chosen from our breeding aviary that had received no experimental treatment to those of their tutors. Two measures were quantified: (1) the percent of syllables in the tutor song that were copied by each son, plus (2) the percent of syllables in the sons’ songs that were copied from the tutor (Supplementary Methods). Birds that received lesions of Ad as juveniles copied significantly fewer syllables from the tutor song than did untreated birds (Fig. 2; U = 2, p <0.02). In addition, songs of lesioned birds contained fewer syllables copied from the tutor relative to controls (U = 1, p <0.02; Fig. S7).
Figure 2. Imitation of tutor (father) songs by lesioned versus control birds.
Left set of bars show (a) the percent of syllables in the tutor songs copied by the sons, and (b) the percent of syllables in the sons’ songs copied from the tutors in lesioned versus control birds by the time they reached adulthood. Right set of bars depict a comparable “bootstrap” analysis showing (a) the percent of syllables in randomly chosen tutor songs copied by the sons and (b) the percent of syllables in the sons’ songs copied from random tutors in lesioned versus control birds as adults. An average of 78% of Ad was lesioned in these 5 birds. Scores are depicted as mean + SEM.
To further test whether lesioned birds were able to produce an imitation of their tutor’s song, songs of both control and lesioned birds were compared to randomly chosen tutors (Fig. 2). Both control and lesioned birds showed a lower incidence of imitation of random tutors compared to their actual tutors, although this difference was significant only for the control birds (Wilcoxon matched pairs test: T = 0, p < 0.05; lesioned birds, T = 1, p > 0.05). Interestingly, lesioned birds showed a lower incidence of matching to a random tutor than did control birds. Post-hoc inspection of the data revealed that the lesioned birds with the lowest rate of matching to random tutors tended to produce slightly more abnormal syllables, and thus may have been less likely to produce “generic” zebra finch syllables that might match. However, these birds did not show a lower rate of matching to their own tutor relative to other lesioned birds, suggesting that abnormal motor behavior was not responsible for the inability to copy a tutor. Arguably the most appropriate comparison for lesioned birds is to the level of imitation for normal control birds to random tutors: this comparison suggests that lesioned birds were unable to imitate tutors, since their incidence of matching to their tutors’ songs was equivalent to that for control birds to random tutors. In summary, these results indicate that birds with lesions of Ad either fail to preserve an auditory memory of the tutor’s song, or are unable to use that memory to guide the development of their own vocalizations in order to match the tutor song.
These results identify the LMANshell→Ad→DTZ→LMANshell recurrent loop as a novel functional circuit that is essential for vocal learning. In contrast to lesions targeting LMANcore circuitry, Ad lesions did not induce immediate or substantive disruption of vocal motor output, an outcome consistent with the lack of known projections from Ad onto descending motor circuitry2. Ad-lesioned birds showed no impairment of basic phonological aspects of vocal production, but had striking disruptions in temporal sequencing and were unable to imitate tutor vocal patterns. The basal ganglia are important for sequence learning across taxa in diverse motor-related tasks, and humans with basal ganglia damage are impaired in language sequencing5–7.
Closed-loop recurrent architecture of parallel circuits characterizes neural organization in both birds and mammals, suggesting that simultaneous processing of different types of information represents one mechanism by which such pathways contribute to motor control and cognition2, 8–10. For example, target location, direction of limb movement, and movement force are distributed across parallel circuits within brain regions for execution of limb movements in primates8. Anatomically distinct LMAN circuits (core versus shell) may subserve different learning-related functions. In contrast to LMANshell, the core region of LMAN serves functions more directly related to vocal motor output3, 11. Thus, core circuitry may be more involved with modulation of motor commands (performance), whereas shell circuitry may be more involved with evaluation of motor performance.
The defects in sequence learning of Ad-lesioned birds accord with recent data indicating that HVC is essential for timing of vocal sequences12, and suggest that the connections between the LMANshell pathway and HVC via MMAN may be key for this function. Lesions of MMAN in juvenile birds cause striking disruption of song, including the temporal sequence of syllables13, suggesting that the effects of Ad lesions are mediated at least partly through the Ad-DTZ-MMAN-HVC connection (Fig. S1E). The feed-back and feed-forward connections made by LMANshell circuitry are well suited in general to integrating information and making iterative comparisons of song-related feedback to other neural representations. Thus, the shell circuit may be an essential component of sensori-motor integration for matching vocal output to the auditory memory of vocal sounds. Of course, the effects of Ad lesions may not be song-specific. One interesting possibility is that the LMANshell circuit may perform generic functions on which normal vocal development depends. The cortical region dNCL is part of LMANshell circuitry, and is similar to an area of polymodal association cortex important for imprinting in chicks14. Thus it is possible that LMANshell circuitry could contribute to integration of different modalities, such as visual cues that are important for vocal learning15.
Supplementary Material
Acknowledgments
We are grateful to Dr. Vanessa Miller-Sims and Dr. Frank Johnson for assistance with data analysis. Supported by NIH grant NS037547.
Footnotes
Author Contributions. B.A. conducted the experiments; S.W.B. and B.A. designed the experiments; S.W.B analyzed the data and wrote the manuscript.
References
- 1.Doupe AJ, Kuhl PK. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 2.Bottjer SW. Ann N Y Acad Sci. 2004;1016:395–415. doi: 10.1196/annals.1298.037. [DOI] [PubMed] [Google Scholar]
- 3.Bottjer SW, Miesner EA, Arnold AP. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 4.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 5.Doyon J, et al. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Seger CA. Neuroscientist. 2006;12:285–290. doi: 10.1177/1073858405285632. [DOI] [PubMed] [Google Scholar]
- 7.Hikosaka O, et al. Trends Neurosci. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- 8.Alexander GE, Crutcher MD. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 9.Person AL, Gale SD, Farries MA, Perkel DJ. J Comp Neurol. 2008;508:840–866. doi: 10.1002/cne.21699. [DOI] [PubMed] [Google Scholar]
- 10.Graybiel AM. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 11.Aronov D, Andalman AS, Fee MS. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- 12.Long MA, Fee MS. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster EF, Bottjer SW. J Neurobiol. 2001;46:142–165. doi: 10.1002/1097-4695(20010205)46:2<142::aid-neu60>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Braun K, Bock J, Metzger M, Jiang S, Schnabel R. Behav Brain Res. 1999;98:211–218. doi: 10.1016/s0166-4328(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 15.Morrison RG, Nottebohm F. J Neurobiol. 1993;24:1045–1064. doi: 10.1002/neu.480240805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.