Abstract
The regulation of Ca2+ influx through the phosphorylation of the L-type Ca2+ channel, Cav1.2, is important for the modulation of excitation-contraction (E-C) coupling in the heart. Cav1.2 is thought to be the target of multiple kinases that mediate the signals of both the renin-angiotensin and sympathetic nervous systems. Detailed biochemical information regarding the protein phosphorylation reactions involved in the regulation of Cav1.2 is limited. The PKC family of kinases can modulate cardiac contractility in a complex manner, such that contractility is either enhanced or depressed, and relaxation is either accelerated or slowed. We have previously reported that Ser1928 in the C-terminus of α1c was a target for PKCα, ζ and ε phosphorylation. Here, we report the identification of seven PKC phosphorylation sites within the α1c subunit. Using phospho-epitope specific antibodies to Ser1674 and Ser1928, we demonstrate that both sites within C-terminus are phosphorylated in HEK cells in response to PMA. Phosphorylation was inhibited with a PKC inhibitor, bisindolylmaleimide. In Langendorff-perfused rat hearts, both Ser1674 and Ser1928 were phosphorylated in response to PMA. Phosphorylation of Ser1674, but not Ser1928, is PKC isoform-specific, as only PKC α, βI, βII, υ, δ and Θ, but not PKC ε, ζ and η, were able to phosphorylate this site. Our results identify a molecular mechanism by which PKC isoforms can have different effects on channel activity by phosphorylating different residues.
Ca2+ homeostasis in the heart is maintained through the actions of channels and pumps, tuned to increase cardiac contractility in response to neurohormonal stimulation. Treatment of several major cardiovascular diseases, including hypertension, heart failure and cardiac hypertrophy are dependent, in part, upon the modulation of neurohomonal pathways. Cav1.2, the L-type, voltage-gated calcium (Ca2+) channel present in the sarcolemma of cardiomyocytes, is required for excitation-contraction (E-C) coupling in the heart (1). It is well established that Cav1.2 plays a key role in modulating cardiac function in response to classic signaling pathways, such as the renin-angiotensin system (RAS) and sympathetic nervous system (SNS) (2). Typically these pathways alter cellular function by regulating kinases. Cav1.2 is thought to be the target of multiple kinases that mediate the signals of both the RAS and SNS.
The PKC family comprises 12 different isoforms, which are broadly classified according to their activation characteristics (3). In heart, PKC isoforms are activated by membrane receptors coupled to phospholipase C via Gq/G11 heterotrimeric G proteins (4, 5). Phospholipases activated via G-protein coupled receptors result in hydrolysis of inositol phospholipids and production of diacylglycerol (DAG). Tumor-promoting phorbol esters act as an analog of DAG. PKC isoforms are differentially responsive to neurohormones, suggesting that they play distinct and specific roles in cardiac function. Numerous agonists (phenylephrine, norepinephrine, ATP, carbachol, endothelin, angiotensin and thrombin) accelerate phosphoinositide turnover in cardiac muscle, thereby leading to PKC activation (6). Angiotensin II and endothelin-1 have been reported to increase (7-9), decrease (10) or have no effect (11) on basal ICa in the heart. The coupling of α1A-adrenoceptor with Gq/11-PLC-PKC-CaMKII pathway potentiates ICa, whereas α1B-adrenoceptor interacts with Go, of which the βη-complex might directly inhibit channel activity (12). Several direct activators of PKC have variable effects on Cav1.2 including activation, inhibition and activation followed by inhibition in cardiomyocytes (13-17). Techniques that preserve the cytoplasmic environment appear to preserve the up-regulation of ICa in response to agonists.
Although all PKC isoforms preferentially phosphorylate peptides with hydrophobic amino acids at position +1 C-terminal of the phosphorylated serine and basic residues at position −3, individual PKC isoforms have distinct optimal substrates (18). PKC, purified from avian brain, has been shown to phosphorylate the α1c and β2a subunits in vitro (19). A systematic study of the phosphorylation of α1c by different PKC isoforms has not been completed. Several studies have suggested that the N-terminus of α1c is important for PKC up-regulation of channel function (20, 21). Phosphorylation of α1c Thr27 and Thr31 was proposed, based upon electrophysiological studies utilizing heterologous expression of mutant channels, to mediate PKC-induced inhibition of channel activity (22). No biochemical evidence exists for the phosphorylation of these residues in cells or in the heart. Recently, we reported that the α1c Ser1928 was phosphorylated by PKCα, PKCε and PKCζ (23). Here, we demonstrate the PKC phosphorylation of several targets within α1c protein, in an isoform-specific manner. We demonstrate that the phosphorylation occurs in response to a PKC activator in a heterologous expression system and in cardiac myocytes. The results suggest that the α1c subunit can be differentially regulated by the different PKC isoforms, based upon phosphorylation of specific residues.
EXPERIMENTAL PROCEDURES
cDNA clones and site-directed mutagenesis
The rabbit α1c subunit (NCBI accession number X15539) and β2a subunit (NCBI X64297) in pcDNA3 (Invitrogen) were used for HEK cell expression. The preparation of the rabbit α1c GST fusion proteins was described previously (23). Site-directed mutagenesis was performed using QuikChange XL kit (Stratagene). All clones were sequenced on both strands prior to use. Transfections into HEK293 cells were performed with Lipofectamine 2000 (Invitrogen).
Preparation of phospho-epitope specific antibodies
The general α1c, α1c phospho-Ser1928, phospho-Ser528 and phospho-Ser533 antibodies have been previously described (23, 24). The phospho-Ser1674 (pS1674) antibody was prepared at Zymed utilizing the peptide: NH2-CEQGLVGKPpSQRN-COOH. The phospho-PKC substrate antibody was purchased from Cell Signaling Technology (#2261).
PKC kinase assay
For PKC kinase reactions, samples were washed twice with PKC washing buffer (conventional PKCs: 20mM HEPES, pH7.4, 10mM MgCl2 100μM CaCl2; novel and atypical PKCs: 20mM HEPES, pH 7.4, 10mM MgCl2 100μM EGTA). Conventional PKC kinase assays were performed in 15μl phosphorylation buffer containing 20mM HEPES pH7.4, 10mM MgCl2, 100μM CaCl2, 1mg/ml phosphatidylserine (PS), 200μg/ml DAG, 100 μM ATP. Novel and atypical PKC isoform-phosphorylation assays were performed with an identical buffer except the Ca2+ was replaced with 100 μM EGTA. 5μCi 32PγATP was added to the assay buffer to radiolabel the substrates of the kinase assay as indicated. Phosphorylation reactions, which were optimized for individual PKC isoforms (Panvera, Invitrogen), were performed for 10-30 min at 30°C. Samples were size-fractionated on SDS-PAGE, extensively washed, stained with Coomassie, fixed and dried. 32P-γATP was detected using autoradiography. All in vitro kinase assays were repeated at least three times.
PKD kinase assay
Samples were washed twice with PKD washing buffer containing 12.5 mM Tris (pH7.5), 10 mM MgCl2, 1 mM EGTA, 0.5 mM Na3VO4, 5 mM β-glycerophosphate and 0.01% Triton X-100. PKD assay was carried out in phosphorylation buffer by adding 2.5 mM DTT, 100 μM ATP and 100 ng PKD1 in washing buffer at 30°C for 10-30 min.
Cardiac perfusion
All animal care and procedures were approved by Columbia University College of Physician and Surgeons Institutional Animal Care and Use Committee and was in accordance with the NIH and institutional guidelines. Rats were injected with heparin and then anesthetized with pentobarbitol. The hearts were rapidly excised and placed in ice-cold Tyrode solution containing (mM): 134 NaCl, 5.4 KCl, 1.0 MgCl2, 10 HEPES, 10 glucose, 2 CaCl2 (pH adjusted to 7.4 with NaOH). The aorta was cannulated and mounted on a Langendorff perfusion apparatus. The hearts were perfused for 5 min with Tyrode solution, followed by 15 min perfusion with Tyrode solution containing calyculin 50nM, PMA 0.5 μM and calyculin 50nM, 4 α-phorbol (0.5 μM) and calyculin 50nM or control solution (without PMA and calyculin). Perfusions were done at 36° C.
Preparation of heart lysates
PKCα-overexpressing transgenic (C57), PKCα knockout mice (FVB), and corresponding littermate control hearts were obtained from 12 month old animals (25). Hearts were homogenized in 1% Triton-100/RIPA buffer containing 1%(v/v) Triton X-100, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10 mM EDTA, 10 mM EGTA, phosphatase inhibitor cocktail and protease inhibitors (complete mini-tablet, calpain I and II inhibitors, Roche).
Immunoblots
Proteins were transferred to nitrocellulose membrane, and probed with the phospho-specific and general antibodies, followed by anti-rabbit HRP-conjugated secondary antibody and ECL (Pierce). Detection was performed with a CCD camera (Carestream). Image quantification was performed using ImageQuant
Immunoprecipitations
Immunoprecipitations were performed overnight in a modified RIPA buffer as previously described (23).
Statistical analysis
Bar graphs with error bar data show mean ± standard deviations. Sample size ≥ 3 in all cases. Statistical analysis was performed by Student’s unpaired t-test.
RESULTS
The brain and liver contain virtually all PKCs, but most other tissues express only certain PKC isoforms. It is known that different isoforms mediate diverse cellular responses, defined by different resting and stimulus-induced subcellular localization and different target substrates, based upon optimal phosphorylation consensus sequences (26). Cardiomyocytes co-express conventional (PKCα), novel (PKCδ and PKCε) and atypical (PKCλ) isoforms; conventional PKCβ has also been variably detected by some investigators. Our prior work established that α1c Ser1928 was phosphorylated by PKCα, PKCε and PKCζ. We also showed that PKCα, but not PKCε and PKCζ phosphorylated unidentified residue(s) within a GST fusion protein containing rabbit α1c subunit amino acid residues 1509-1905 (23).
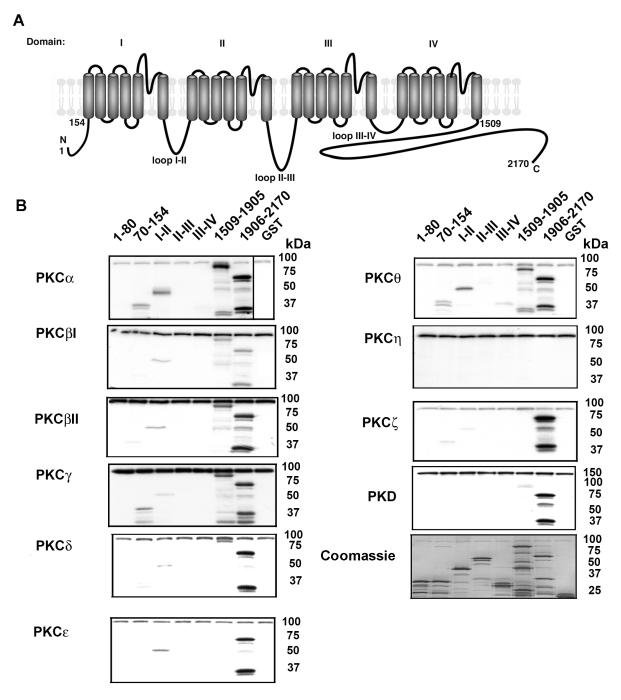
We utilized a panel of GST fusion proteins that encompassed all major intracellular regions, as substrates for in vitro PKC phosphorylation. The small intracellular loops between transmembrane segments (S2-S3; S4-S5) do not contain consensus PKC phosphorylation sites, and were thus not included in the panel. The α1c containing GST fusion proteins were differentially phosphorylated by PKC isoforms (Fig. 1). Many of the conventional PKC isoforms, namely PKCα, PKCβI, PKCβII and PKCγ, all phosphorylated GST fusion proteins I-II loop, 1509-1905 and 1906-2170. PKCα and PKCγ also phosphorylated GST fusion protein 70-154. The novel PKC isoforms phosphorylated the GST fusion proteins to different extents—PKCΘ phosphorylated 1906-2170, 1509-1905, the I-II loop and 70-154; PKCδ phosphorylated 1906-2170 and 1509-1905, and to a modest extent the I-II loop; PKCε phosphorylated 1905-2170 and I-II loop, but not 1509-1905; PKCη did not phosphorylate any GST fusion protein. The atypical PKC isoform, PKCζ phosphorylated GST fusion protein 1905-2170, but not 1509-1905 and only weakly phosphorylated 70-154 and the I-II loop. Protein kinase D (PKD), which is activated by PMA and is downstream of PKC, primarily phosphorylated the 1906-2170 fusion protein. These results suggest that the individual PKC isoforms can phosphorylate distinct regions within the α1c subunit. The differential phosphorylation of the α1c subunit by PKC isoforms may represent an important regulatory control mechanism to fine-tune the L-type Ca2+ channel response to distinct neurohormonal stimulation.
Figure 1. PKC isoforms phosphorylate the α1c subunit.
A, Schematic is shown of the α1c subunit with the intracellular segments used to construct GST fusion proteins. B, α1c subunit-GST fusion proteins bound to glutathione-sepharose were subjected to PKC kinase reaction with [γ-32P] ATP. The amount of PKC isoform used for each kinase assay was normalized based upon moles of phosphate transferred to test substrate. The amount of GST fusion protein utilized is shown in the Coomassie-stained gel. The bands at ~ 80-100 kDa are autophosphorylated PKC. All blots are representative of 3 or more similar experiments.
Identifying phosphorylated residues in the N-terminus and I-II loop
Several studies have suggested that the N-terminus of α1c is important for PKC up-regulation of channel function (20, 21). Phosphorylation of α1c Thr27 and Thr31 was proposed, based upon electrophysiological studies utilizing heterologous expression of mutant channels, to mediate PKC-induced inhibition of channel activity (22). Both of these residues are within GST fusion protein 1-80, which was not phosphorylated by any of the tested PKC isoforms in the in vitro kinase assays (Fig. 1), although it is conceivable that the folding of the GST fusion protein is different in the full-length channel. GST fusion protein 70-154 was phosphorylated by several PKC isoforms, although in comparison to GST fusion protein 1905-2170, which is predominantly phosphorylated on a single residue (Ser1928) (23), the amount of 32P-γATP incorporation was significantly less. This suggests that the equivalent of less than 1 site is phosphorylated within 70-154 fusion protein. To test which site(s) were phosphorylated by PKCα, we made Ala-substitution mutants at amino acid residues 107-109 (SST to AAA), 124-126 (STT to AAA) and 138 (T to A), and expressed the fusion proteins. For all three mutant GST fusion proteins, no significant change in modest amount of phosphorylation was detected (data not shown). This suggests that multiple sites are weakly phosphorylated in this region.
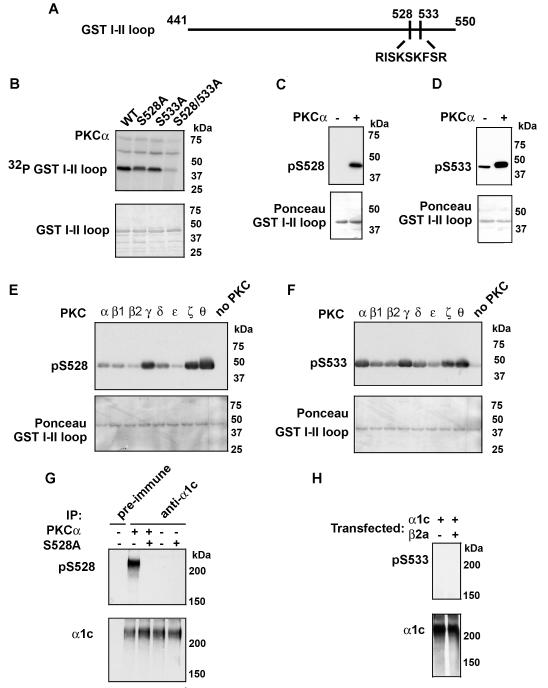
An examination of the amino acid sequence within the I-II loop revealed several potential phosphorylation sites. We created, by site-directed mutagenesis, single and double Ala-substitution mutants of the potential PKC phosphorylation residues within the I-II loop (Fig. 2A). Radiolabeling of the GST I-II loop fusion protein was reduced with each of the single mutants and was nearly completely abrogated by the double Ala-substition of Ser528 and Ser533 (Fig. 2B). We previously developed phospho-epitope specific antibodies for Ser528 and Ser533 (24), which were designed to report the phosphorylation of either Ser528 (pS528) or Ser533 (pS533) by protein kinase G (PKG). The antibodies detected the appropriate phosphorylated residue (Fig. 2C,D). The pS533 antibody weakly recognized the I-II loop under non-phosphorylated conditions, as previously reported (24). Having validated the specificity of the pS528 and pS533 antibodies, as reagents to track PKC phosphorylation, we used them to examine the phosphorylation of the GST I-II loop fusion protein by the different PKC isoforms. Consistent with our results using 32P-γATP incorporation (Fig.1B), we found that both Ser528 and Ser533 were phosphorylated by multiple PKC isoforms (Fig 2E, F). The specificity of the antibodies is demonstrated by the lack of significant signal in the non-phosphorylated lanes (right-most lanes, Fig 2E, F). These results suggest that Ser528 and Ser533 account for the PKCα phosphorylation of the GST fusion protein I-II loop (Fig. 1B) and are potential PKC phosphorylation sites within the full-length α1c subunit.
Figure 2. PKC phosphorylates α1c Ser528 and Ser533.
A, The schematic demonstrates the PKC phosphorylation sites in the α1c I-II loop. B, upper panel, Shown is autoradiogram of PKCα in vitro kinase reaction performed with [γ-32P] ATP and GST-fused WT, S528A, S533A and S528/S533A I-II loop. PKC phosphorylated Ser528 and Ser533. Lower panel, Coomassie-staining of autoradiogram demonstrating amount of fusion protein used. C-D, upper panels, WT GST fusion protein was phosphorylated with PKCα, size-fractionated on SDS-polyacrylamide gel, transferred to nitrocellulose and immunoblotted using anti-phospho-Ser528 and Ser533 antibodies (pS528 and pS533 respectively). PKC phosphorylates Ser528 and Ser533 in the GST fusion protein I-II loop. Lower panels, Ponceau staining indicates equivalent loading of GST fusion proteins. E-F, Shown are pS528 and pS533 immunoblots of in vitro kinase reactions of eight PKC isoforms. Lower panels, Ponceau staining indicates equivalent loading of GST fusion proteins. G, Extracts from HEK cells transfected with WT or S528A were prepared, followed by pre-immune or α1c immunoprecipitation and PKCα kinase reaction as indicated. Samples were size-fractionated on SDS-polyacrylamide gel, transferred to nitrocellulose membrane and probed with anti-phospho-Ser528 antibody (upper panel) or α1c antibody (lower panel). H, Extracts from HEK cells transfected with WT α1c, in the presence or absence of β2a subunit, were prepared, followed by α1c immunoprecipitation and PKCα kinase reaction as indicated. Samples were size-fractionated on SDS-polyacrylamide gel, transferred to nitrocellulose membrane and probed with anti-phospho-Ser533 antibody (upper panel) or α1c antibody (lower panel). All blots are representative of 3 or more similar experiments.
Having validated the specificity of the antibodies to track PKC phosphorylation of the I-II loop, we used them to examine phosphorylation of full-length recombinant α1c, co-expressed with β2a subunit in HEK293 cells. Recombinant channels were immunoprecipitated by an anti-α1c antibody from HEK cell extracts and subjected to PKCα in vitro kinase assay. In contrast to the GST fusion proteins, Ser528 (Fig 2G), but not Ser533 (Fig. 2H), demonstrated significant PKC phosphorylation. The specificity of Ser528 phosphorylation was demonstrated by the lack of a pS528 signal in the pre-immune serum lane, in the Ala-substituted α1c lane and in the lanes in which PKCα was not added (Fig. 2G). The lack of Ser533 phosphorylation in full-length recombinant channel compared to the GST I-II loop fusion protein may be due to the co-expression of the β2a subunit, which may sterically block access to PKC and/or change the I-II loop conformation. The α-interaction domain (AID) does not overlap with the phosphorylation sites on the I-II loop. We found that Ser533 was not phosphorylated even in the absence of the β2a subunit (Fig. 2H), suggesting that the I-II loop adopts a different, more inaccessible conformation in full-length channel compared to the GST fusion protein.
Identifying phosphorylated residues in the GST 1509-1905 fusion protein
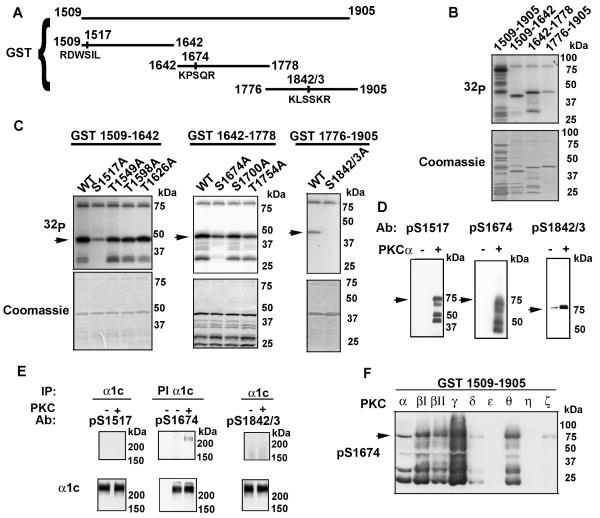
We divided the GST fusion protein 1509-1905 into three fragments; 1509-1642; 1642-1778 and 1776-1905 (Fig. 3A), to facilitate identification of the phosphorylation site(s), expressed each as GST fusion proteins in E. coli and purified the protein on glutathione sepharose. The purified fusion proteins were subjected to in vitro kinase assays with [γ-32P] ATP. All three fusion proteins were labeled in the in vitro kinase assay by PKCα, albeit the 1776-1905 protein demonstrated less 32P incorporation compared to 1509-1642 and 1642-1778 (Fig. 3B). An examination of the amino acid sequence within each of these GST fusion proteins revealed several potential phosphorylation sites. We created, by site-directed mutagenesis, single Ala-substitution mutants of the potential PKC phosphorylation residues. Radiolabeling of the GST 1509-1642 fusion protein was reduced by Ala-substitution of Ser1517, as demonstrated by the lack of phosphorylation of the truncated GST fusion products and significant reduction of phosphorylation of the full-length product (Fig. 3C). In a similar fashion, we found that a single Ala-substitution of Ser1674 substantially reduced phosphorylation of GST 1642-1778 and that a double Ala-substitution of Ser1842 and Ser1843 abrogated phosphorylation of GST 1776-1905. Ala-substitution of Ser1700 and Thr1754 did not substantially effect phosphate incorporation into the GST fusion protein. Therefore, our results suggest that Ser1517, Ser1674, Ser1842 and Ser1843 are phosphorylated by PKCα within the GST1509-1905 fragment. Mutation of these sites within GST 1509-1905 did not completely abrogate phosphorylation, but reduced >80% of the 32P incorporation (data not shown), suggesting that other, potentially minor, sites within this fragment are unidentified.
Figure 3. PKC phosphorylates α1c Ser1517, Ser1674 and Ser1842/1843.
A, The schematic demonstrates the PKC phosphorylation sites within the GST fusion protein 1509-1905. B, upper panel, Shown is autoradiogram of PKCα in vitro kinase reaction performed with [γ-32P] ATP and GST-fused 1509-1905, 1509-1642, 1642-1778, 1776-1905. Lower panel, Coomassie-staining of autoradiogram demonstrating amount of fusion protein used. C, upper panels, Shown are autoradiograms of PKCα in vitro kinase reactions performed with [γ-32P] ATP and WT and Ala-substituted GST-fused 1509-1642, 1642-1778, 1776-1905 proteins. Arrowheads indicate full-length GST fusion protein; lower bands are truncated GST proteins. Lower panel, Coomassie-staining of autoradiogram demonstrating amount of fusion protein used. D, WT GST-fused 1509-1905 fusion proteins were phosphorylated with PKCα, size-fractionated on SDS-polyacrylamide gel, transferred to nitrocellulose and immunoblotted using anti-phospho-Ser1517, Ser1674 and Ser1842/1843 antibodies (pS1517, pS1674 and pS1842/43 respectively). PKCα phosphorylates Ser1517, Ser1674 and Ser1842/1843. E, Extracts from HEK cells transfected with WT α1c + β2a subunits were prepared, followed by pre-immune (PI) or α1c immunoprecipitation and PKCα kinase reaction as indicated. Samples were size-fractionated on SDS-polyacrylamide gel, transferred to nitrocellulose membrane and probed with anti-phospho-Ser1517, Ser1674 and Ser1842/1843 antibodies (upper panel) or α1c antibody (lower panel). F, Shown is pS1674 immunoblot of in vitro kinase reactions of eight PKC isoforms. All blots are representative of 3 or more similar experiments.
We developed three phospho-epitope specific antibodies, designed to report the phosphorylation of Ser1517 (pS1517), Ser1674 (pS1674) and Ser1842/Ser1843 (pS1842/3). The antibodies detected the appropriate phosphorylated residue (Fig 3D). The pS1842/3 antibody weakly recognized the full-length GST 1509-1905 fusion protein under non-phosphorylated conditions.
Having validated the specificity of these antibodies, we used them to examine phosphorylation of full-length recombinant α1c, co-expressed with β2a subunit, in HEK293 cells. Recombinant channels were immunoprecipitated by an anti-α1c antibody from HEK cell extracts and subjected to PKCα in vitro kinase assay. In contrast to the GST fusion proteins, Ser1674, but not Ser1517 or Ser1842/Ser1843 demonstrated significant PKCα phosphorylation. These results suggest that PKCα phosphorylates Ser1674 in full-length recombinant α1c. The lack of phosphorylation of Ser1517 by PKCα in full-length channel may be due to lack of accessibility of PKCα to Ser1517 in full-length channel (Fig. 3E).
PKC isoforms can differentially phosphorylate GST 1509-1905; specifically, we showed that PKCα, PKCβI, PKCβII, PKCγ, PKCδ and PKCΘ, but not other PKC isoforms, can substantially phosphorylate GST1509-1905 (Fig. 1B) in an in vitro kinase assay. To test whether phosphorylation of Ser1674 was PKC isoform specific, we performed an in vitro kinase assay for each PKC isoform (equivalent specific activity 1500 nmole of phosphate transferred to substrate/min/mg protein), and detected phosphorylation using the pS1674 antibody. We found that PKCα, βI, βII, γ and Θ phosphorylated Ser1674; PKCδ and PKCζ very weakly phosphorylated Ser1674 and PKCε and PKCη did not phosphorylate Ser1674. These results demonstrate that α1c Ser1674 is differentially phosphorylated by PKC isoforms (Fig. 3F).
Ser1928 is phosphorylated by conventional, novel, atypical PKC isoforms and PKD
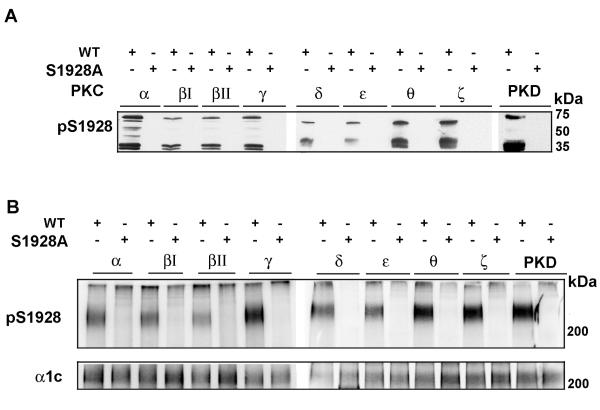
We have previously reported that PKCα, PKCε and PKCζ phosphorylated the α1c C-terminus at residue Ser1928 (23). In Fig. 1, we showed that PKD and all tested PKC isoforms except PKCη phosphorylated GST 1906-2170. In order to demonstrate whether Ser1928 was phosphorylated by the other PKC isoforms, we utilized a phospho-epitope specific antibody developed to specifically detect Ser1928 phosphorylation (23). Prominent immunoreactive bands were detected (with a range of mobilities corresponding to GST-fused full-length protein as well as truncated/proteolytic fragments) using eight PKC isoforms and PKD (Fig. 4A). No anti-phospho-Ser1928 antibody immunoreactivity was detected when a single Ala-substitution of Ser1928 was introduced into the GST fusion protein.
Figure 4. PKC isoforms phosphorylate α1c Ser1928.
A, GST fusion proteins (WT and S1928A 1906-2170 fragment) were phosphorylated by PKC isoforms and PKD, size- fractionated, transferred to nitrocellulose and immunoblotted with a phospho-specific antibody recognizing phosphorylated Ser1928 (pS1928). B, Extracts from HEK cells transfected with WT or S1928A were prepared, followed by α1c immunoprecipitation and kinase reaction by the different PKC isoforms and PKD. Samples were size-fractionated on SDS-polyacrylamide gel, transferred to nitrocellulose membrane and probed with anti-phospho-Ser1928 antibody (upper panel) or α1c antibody (lower panel).
Having determined that PKD and these PKC isoforms can phosphorylate Ser1928 in GST fusion proteins, we next asked whether these kinases can phosphorylate Ser1928 in full-length α1c. We co-expressed β2a and WT or Ala-substituted Ser1928 α1c in HEK cells. α1c immunoprecipitates were subjected to immune complex kinase assays with PKD and PKC isoforms. PKD and PKC isoforms α, β1, βII, γ, δ, ε, Θ and ζ phosphorylated Ser1928 in the full-length α1c (Fig. 4B), thus indicating that Ser1928 is a target for conventional, novel and atypical PKC isoforms.
α1c Ser1674 and Ser1928 are phosphorylated by PKC in HEK cells
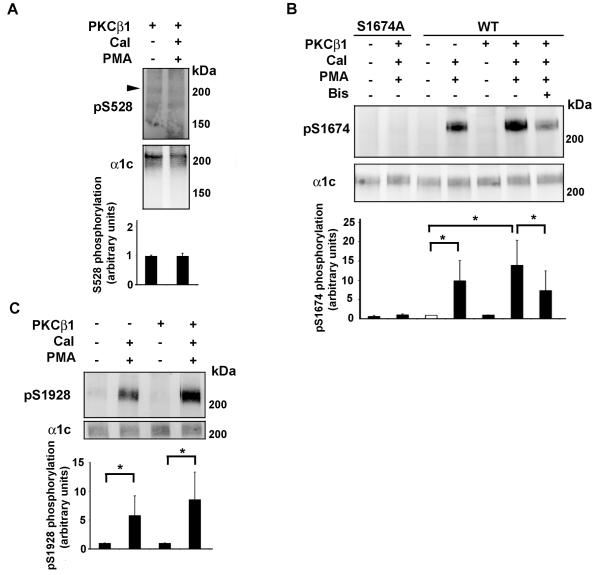
We determined α1c phosphorylation in HEK cells transfected with WT or Ala-substituted Ser528, Ser1674, or Ser1928, in the absence or presence of over-expressed PKCβI. Incubation of HEK cells transfected with α1c and β2a with PMA, prior to lysis, led to phosphorylation of Ser1674 and Ser1928 (Fig. 5B, C), but not Ser528 (Fig 5A). The PMA-induced phosphorylation of Ser1674 was inhibited by bisindolylmaleimide (Bis), indicating that the phosphorylation was mediated by PKC (Fig 5B). The PMA-induced phosphorylation of Ser1674 and Ser1928 was increased by the over-expression of PKCβ1 in HEK cells (Fig. 5B, C). In contrast, PMA-induced Ser528 phosphorylation was not detected with the endogenous PKC isoforms expressed in HEK cells (data not shown) or after PKCβI overexpression (Fig 5A). Taken together, these results suggest that in HEK cells, Ser1674 and Ser1928 can be phosphorylated in a cellular context.
Figure 5. Reconstitution of PMA/PKC mediated phosphorylation of Ser1674 and Ser1928 in HEK cells.
A, Recombinant WT α1c was transiently co-expressed with β2a and PKCβ1 in HEK293 cells. Cells were exposed to PMA (1 μM) and calyculin A (Cal: 50 nM) for 10 min. Bisindolylmaleimide (Bis, 0.5 μM) was pre-incubated for 1 hr at 37°C. Cells were harvested 24-48 hours after transfection, and lysed in the presence of phosphatase inhibitors. Lysates were size-fractionated on SDS-polyacrylamide gel, transferred to nitrocellulose membranes, and blotted with anti-phospho-Ser528 (pS1928; upper panel) or α1c (middle panel) antibodies. Arrowhead indicates size of anticipated phosphorylated band. Representative of 3 similar experiments. Lower panel, Activation of PKC by PMA had no effect on Ser528 phosphorylation. Mean ± SD. B, Recombinant WT or Ala-substituted Ser1674 (S1674A) α1c was transiently co-expressed with β2a in HEK293 cells, in the absence or presence of PKCβ1 as indicated. Methodology is identical to (A) except nitrocellulose membranes were blotted with anti-phospho-Ser1674 (pS1674; upper panel) or α1c (middle panel) antibodies. The specificity of the pS1674 antibody is shown using the S1674A α1c mutant. Representative of 3 similar experiments. Lower panel, Activation of PKC by PMA increased phosphorylation of α1c Ser1674. Phosphorylation of Ser1674 was caused by PKC because bisindolylmaleimide (Bis) prevented Ser1674 phosphorylation. C, Recombinant WT or Ala-substituted Ser1928 (S1928A) α1c was transiently co-expressed with β2a in HEK293 cells, in the absence or presence of PKCβ1 as indicated. Methodology is identical to (A) except nitrocellulose membranes were blotted with anti-phospho-Ser1928 (pS1674; upper panel) or α1c (middle panel) antibodies. Representative of 3 similar experiments. Lower panel, Activation of PKC by PMA increased phosphorylation of α1c Ser1928. *, p<0.05
α1c Ser1674 is phosphorylated by PKC in cardiomyocytes
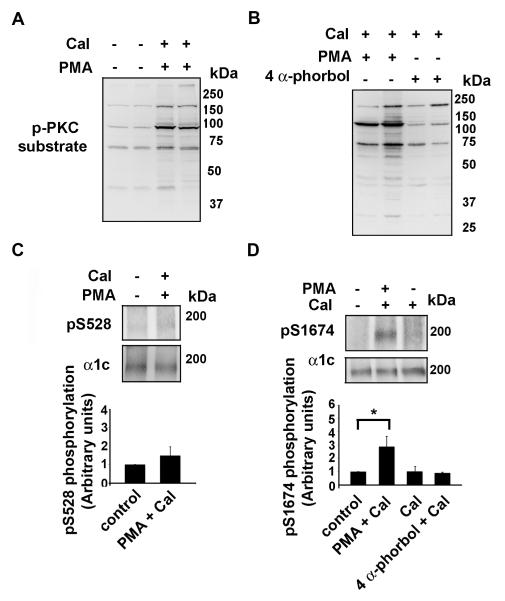
We have previously shown that α1c Ser1928 is phosphorylated by PKC in cardiomyocytes (23). We tested whether Ser528, which is not PKC phosphorylated in HEK cells (Fig.5A) but can be phosphorylated by PKC isoforms in vitro, and Ser1674 could be phosphorylated by PKC isoforms. To induce PKC phosphorylation, we mounted rat hearts on a Lagendorff apparatus and perfused through the aortic root for 15 minutes calyculin A, calyculin A and PMA or calyculin A and 4 α-phorbol (which does not activate PKC). The hearts were then frozen in liquid nitrogen and extracts prepared. To ensure that under these conditions infusion of PMA induced PKC activation and subsequent phosphorylation of targets within cardiomyocytes, we first examined the phosphorylation of multiple targets using a phospho-(Ser) PKC substrate antibody, which detects many cellular proteins only when phosphorylated at serine residues surrounded by Arg or Lys at the −2 and +2 positions and a hydrophobic residue at the +1 position. PMA (Fig. 6A), but not 4 α-phorbol (Fig. 6B), increased the phosphorylation of many PKC targets in the heart, assessed using the PKC phospho-Ser antibody. Of six hearts treated with PMA, we excluded two hearts because a significant increase in phospho-proteins was not observed (data not shown). α1c immunoprecipitates of the untreated, calyculin A, PMA and 4 α-phorbol treated heart extracts were probed with pS528 and pS1674 antibodies. Exposure of the heart to the combination of PMA and calyculin A induced phosphorylation of Ser1674 (p=0.02, n=4), whereas Ser528 demonstrated only a modest increase in signal (p= NS, n=4). Calyculin A and the combination of 4 α-phorbol and calyculin A had no effect on the phosphorylation of Ser1674 (Fig. 6D).
Figure 6. Ser1674 is phosphorylated in cardiomyocytes.
Rat hearts were perfused on a Langendorff apparatus with tyrode solution in the absence or presence of PMA (0.5 μM) + calyculin A (Cal, 50 nM) or 4 α-phorbol (0.5 μM) + calyculin A as indicated. Hearts were flash-frozen in liquid nitrogen and lysates prepared. A-B, Lysates were size-fractionated on SDS-PAGE, transferred to nitroceullose and blotted with anti-phospho-PKC substrate antibody. C-D, α1c immunoprecipates were size-fractionated on SDS-PAGE, transferred to nitrocellulose and blotted with anti-pS528 (upper panel) and anti-pS1674 antibodies (upper panel) and anti-α1c antibody (middle panels). Lower panels, bar graphs of densitometric quantification of Ser528 and Ser1674 phosphorylation (normalized to control; n=3-5). *, p < 0.025 PMA + calyculin compared to control; p<0.05 PMA + calyculin compared to calyculin A.
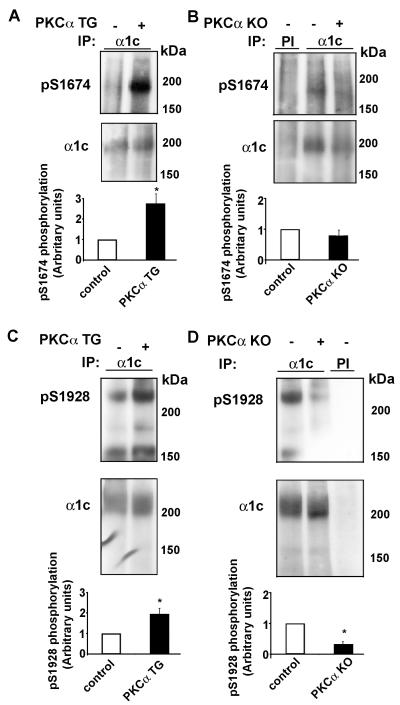
To further support that Ser1674 is phosphorylated in heart, we studied the hearts from PKCα knock-out mice and transgenic mice over-expressing PKCα (25). There are no compensatory changes in the expression and function of other PKC isoforms in the PKCα null mouse (25). Heart extracts were prepared from littermate control, knock-out and TG mice. α1c immunoprecipitates were size-fractionated on SDS-PAGE and probed with pS1674 and pS1928 antibodies. PKCα TG mice demonstrated markedly increased Ser1674 phosphorylation compared to littermate controls (Fig. 7A, p<0.05, n=3). Similarly, PKCα TG mice had increased phosphorylation of Ser1928 compared to littermate control mice (Fig. 7C, p<0.05, n=3). Ser1928 is phosphorylated under basal conditions (Fig. 7C, D); the basal phosphorylation may be due to PKC phosphorylation, since the PKCα null mice has decreased Ser1928 phosphorylation compared to the littermate control (Fig. 7D). In comparison, Ser1674 demonstrates minimal basal phosphorylation (Fig. 7A,B). Ser528 phosphorylation is not present in these mice (data not shown).
Figure 7. Ser1674 and Ser1928 phosphorylation is altered in PKCα TG and KO mice.
Heart extracts were prepared from PKCα TG and KO mice and α1c or preimmune (PI) immunoprecipitates were size-fractionated on SDS-PAGE, transferred to nitrocellulose and blotted with (A-B) anti-pS1674 or (C-D) anti-pS1928 antibodies (upper panels) or anti-α1c antibody (middle panel). Bar graphs of densitometric quantification of relative pS1674 and pS1928 phosphorylation (normalized to α1c immunoprecipitation; littermate control normalized to 1; n=3, *, p<0.05).
DISCUSSION
The regulation of Ca2+ influx through Cav1.2 phosphorylation is important for the modulation of excitation-contraction coupling in the heart. Despite prior electrophysiological characterization of the modulation of Cav1.2 by phosphorylation, the underlying molecular mechanisms remain largely unknown (2). This has been exemplified recently by the findings that an Ala-substitution at Ser1928 knock-in mouse retained β-adrenergic agonist up-regulation of Ca2+ current (27). Ser1928 has been postulated to be one of the residues in Cav1.2 responsible for PKA up-regulation of channel activity (28-31). The scarcity of this transmembrane protein, the difficulties performing biochemical experiments and reconstituting regulation in heterologous expression systems (oocyte and mammalian cells) have limited progress (2).
We have identified several new PKC phosphorylation sites within the α1c subunit of the L-type Ca2+ channel. These sites are distinctly phosphorylated by PKC isoforms, suggesting that the L-type Ca2+ channel function may be differentially regulated. The rabbit α1c subunit has many consensus PKC phosphorylation site in the intracellular, transmembrane and extracellular domains. Using GST fusion proteins incorporating only the intracellular regions, which are exposed to cellular kinases and phosphatases, we avoided studying sites that cannot be modulated in a cellular context. The disadvantage of this approach is that the fusion proteins may not fold correctly. For these in vitro kinase assays, we used 9 PKC isoforms, representing conventional, novel and atypical forms, as well as PKD. We found that the first portion of the amino-terminal segment of the rabbit α1c (residues 1-80) was not phosphorylated by any PKC isoform; two residues within this segment were proposed to be responsible for PKC-induced inhibition of channel activity, based upon cellular electrophysiology experiments (22). We found that the second portion of the amino-terminal segment of the α1c subunit could be weakly phosphorylated by several PKC isoforms; mutagenesis of all potential sites, either as single, double or triple Ala-substitutions, failed to significantly reduce phosphorylation. This suggests that residues within this fragment do not represent significant phosphorylation sites in vitro. Significant phosphorylation by several PKC isoforms was found for the I-II loop and for two segments within the C-terminus, 1509-1905 and 1905-2170.
Within the I-II loop, we identified Ser528 and Ser533 as PKC phosphorylation sites. In addition to Ser1928 in the 1905-2170 fusion protein (23), we identified residues within the 1509-1905 fusion protein, Ser1517, Ser1674 and Ser1842/1843 which are PKC phosphorylated. We generated phospho-epitope specific antibodies for each of these sites and found that Ser1674 and Ser1928 are phosphorylated in HEK cells and cardiomyocytes in response to direct PKC activators. Whereas Ser1928 is strongly phosphorylated by all PKC isoforms tested except PKCη, Ser1674 demonstrates variable PKC phosphorylation, primarily phosphorylated by PKCα, βI, βII, γ and Θ.
Both Ser1674 and Ser1928 are modulated in the PKCα TG and KO mice. Mouse animal models with altered cardiomyocyte PKC isoforms, induced by either transgenic or gene ablation approaches, have demonstrated important roles for PKC isoforms in the regulation of cardiac contractility, and development of cardiac hypertrophy (reviewed in (5)). Hemodynamic overload can produce significant changes in PKC activity (32, 33); for instance, aortic banding in Sprague-Dawley rats caused ~3 fold increased expression of PKCα, which correlated with the degree of left ventricular hypertrophy (LVH). PKCε levels increased ~6 fold at 24 weeks and its autophosphorylation increased in LVH and heart failure (34). In the failing human heart, the expression and activity of Ca2+ sensitive PKCα and β isoforms are elevated (35). Postnatal cardiac specific expression of PKCβ2 caused a cardiomyopathy characterized by LVH, myocardial fibrosis and reduced LV function (36). In contrast, mice with cardiac specific PKCε over-expression demonstrated concentric hypertrophy with normal cardiac function, implying that PKC isoforms may play different roles in cardiac hypertrophy and failure(37). Whereas transgenic over-expression of PKCα causes a reduced cardiac contractility, PKCα deficient mice demonstrated enhanced cardiac contractility (25). Therefore, PKCα, the most highly expressed of the myocardial PKC isoforms, may be more important as a regulator of myocardial contractility than cardiac hypertrophy.
L-type Ca2+ channel currents recorded from the PKCα null mice cardiomyocytes demonstrated a rightward shift in the current-voltage, compared to littermate control mice (25). The molecular mechanism responsible for this shift in channel characteristics is not clear. PKCα directly phosphorylates protein phosphatase inhibitor -1 (I-1), which alters its inhibitory activity protein phosphatase 1 (PP1). PKCα null mice have a >30% decrease in PP1-specific activity, but no change in PP2A activity (25). In contrast, the PKCα TG mice have an increase in PP1 activity in the heart. Thus, our findings of a decreased phosphorylation of Ser1674 and Ser1928 in the PKCα null hearts and an increased phosphorylation of these residues in the PKCα TG hearts are not due to a change in phosphatase activity, but rather most likely due to a direct effect of PKCα phosphorylation of the channel.
The role of phosphorylation of Ser1928 in mediating β-adrenergic agonist up-regulation of L-type Ca2+ current has recently been explored using adenoviral mediated over-expression in cardiomyocytes and a knock-in mouse. Ala-substitution of Ser1928 did not prevent β-agonist up-regulation of current, implying that other molecular mechanisms are responsible. It is not known whether Ser1928 plays a role in mediating PKC-modulation of L type Ca2+channel function in the heart. The assessment of the functional effects of PKC phosphorylation of these newly identified sites will likely require either over-expression in cardiomyocytes or generation of knock-in animals.
The L-type Ca2+ channel α and β subunits are phosphorylated by several kinases, including Ca2+/calmodulin-dependent kinase (CamKII) (38, 39), PKA (40), PKC (23, 40) and PKG (24, 41). Many of the phosphorylation sites for these kinases have been identified although in some cases, the sites can be phosphorylated by several of these kinases (Ser1928 can be phosphorylated by PKA (42), PKC (23) and PKG (24)). Further work is required to determine whether in a cellular context, specificity can be imparted by differential phosphorylation of these sites. Taken together, our findings identify additional PKC regulatory sites within the α1c subunit of the L-type Ca2+ channel. The sites are differentially phosphorylated by PKC isoforms, suggesting a molecular mechanism that could lead to highly specific fine-tuning of channel activity.
ACKNOWLEDGEMENT
We thank Jeffrey Molkentin for the PKCα KO and TG mice hearts.
This work was supported in part by NIH research grant R01 HL68093 and the Arlene and Arnold Goldstein Family Foundation. Darshan Doshi is supported by a Glorney-Raisbeck Medical Student Fellowship from the NY Academy of Medicine and a Heritage Affiliate-American Heart Association Medical Student Fellowship. S.O.M. is an Established Investigator of the AHA.
Abbreviations
- (Ca2+)
calcium
- (E-C)
excitation-contraction
- (RAS)
Renin-angiotensin system
- (SNS)
Sympathetic nervous system
- (PKC)
Protein kinase C
- (PS)
Phosphatidylserine
- (PMA)
phorbol 12-myristate 13-acetate
- (IP)
immunoprecipitation
- (PKD)
Protein kinase D
- (Ser)
Serine
- (Thr)
Threonine
- (Ala)
Alanine
- (Asp)
Aspartic
- (GST)
Glutathione S-transferase
- (Bis)
Bisindolylmaleimide
- (Cal)
Calyculin
REFERENCES
- 1.Fabiato A, Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- 2.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 4.Dorn GW, 2nd, Tepe NM, Lorenz JN, Koch WJ, Liggett SB. Low- and high-level transgenic expression of beta2-adrenergic receptors differentially affect cardiac hypertrophy and function in Galphaq-overexpressing mice. Proc Natl Acad Sci U S A. 1999;96:6400–6405. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puceat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994;269:16938–16944. [PubMed] [Google Scholar]
- 7.Aiello EA, Cingolani HE. Angiotensin II stimulates cardiac L-type Ca(2+) current by a Ca(2+)- and protein kinase C-dependent mechanism. Am J Physiol Heart Circ Physiol. 2001;280:H1528–1536. doi: 10.1152/ajpheart.2001.280.4.H1528. [DOI] [PubMed] [Google Scholar]
- 8.Robu VG, Pfeiffer ES, Robia SL, Balijepalli RC, Pi Y, Kamp TJ, Walker JW. Localization of functional endothelin receptor signaling complexes in cardiac transverse tubules. J Biol Chem. 2003;278:48154–48161. doi: 10.1074/jbc.M304396200. [DOI] [PubMed] [Google Scholar]
- 9.He JQ, Pi Y, Walker JW, Kamp TJ. Endothelin-1 and photoreleased diacylglycerol increase L-type Ca2+ current by activation of protein kinase C in rat ventricular myocytes. J Physiol. 2000;524(Pt 3):807–820. doi: 10.1111/j.1469-7793.2000.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng TH, Chang CY, Wei J, Lin CI. Effects of endothelin 1 on calcium and sodium currents in isolated human cardiac myocytes. Can J Physiol Pharmacol. 1995;73:1774–1783. doi: 10.1139/y95-242. [DOI] [PubMed] [Google Scholar]
- 11.Thomas GP, Sims SM, Karmazyn M. Differential effects of endothelin-1 on basal and isoprenaline-enhanced Ca2+ current in guinea-pig ventricular myocytes. J Physiol. 1997;503(Pt 1):55–65. doi: 10.1111/j.1469-7793.1997.055bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O-Uchi J, Sasaki H, Morimoto S, Kusakari Y, Shinji H, Obata T, Hongo K, Komukai K, Kurihara S. Interaction of alpha1-adrenoceptor subtypes with different G proteins induces opposite effects on cardiac L-type Ca2+ channel. Circ Res. 2008;102:1378–1388. doi: 10.1161/CIRCRESAHA.107.167734. [DOI] [PubMed] [Google Scholar]
- 13.Dosemeci A, Dhallan RS, Cohen NM, Lederer WJ, Rogers TB. Phorbol ester increases calcium current and simulates the effects of angiotensin II on cultured neonatal rat heart myocytes. Circ Res. 1988;62:347–357. doi: 10.1161/01.res.62.2.347. [DOI] [PubMed] [Google Scholar]
- 14.Lacerda AE, Rampe D, Brown AM. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988;335:249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- 15.Tseng GN, Boyden PA. Different effects of intracellular Ca and protein kinase C on cardiac T and L Ca currents. Am J Physiol. 1991;261:H364–379. doi: 10.1152/ajpheart.1991.261.2.H364. [DOI] [PubMed] [Google Scholar]
- 16.Schreur KD, Liu S. 1,2-Dioctanoyl-sn-glycerol depresses cardiac L-type Ca2+ current: independent of protein kinase C activation. Am J Physiol. 1996;270:C655–662. doi: 10.1152/ajpcell.1996.270.2.C655. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZH, Johnson JA, Chen L, El-Sherif N, Mochly-Rosen D, Boutjdir M. C2 region-derived peptides of beta-protein kinase C regulate cardiac Ca2+ channels. Circ Res. 1997;80:720–729. doi: 10.1161/01.res.80.5.720. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 19.Puri TS, Gerhardstein BL, Zhao XL, Ladner MB, Hosey MM. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry. 1997;36:9605–9615. doi: 10.1021/bi970500d. [DOI] [PubMed] [Google Scholar]
- 20.Wei X, Neely A, Olcese R, Lang W, Stefani E, Birnbaumer L. Increase in Ca2+ channel expression by deletions at the amino terminus of the cardiac alpha 1C subunit. Receptors Channels. 1996;4:205–215. [PubMed] [Google Scholar]
- 21.Shistik E, Ivanina T, Blumenstein Y, Dascal N. Crucial role of N terminus in function of cardiac L-type Ca2+ channel and its modulation by protein kinase C. J Biol Chem. 1998;273:17901–17909. doi: 10.1074/jbc.273.28.17901. [DOI] [PubMed] [Google Scholar]
- 22.McHugh D, Sharp EM, Scheuer T, Catterall WA. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci U S A. 2000;97:12334–12338. doi: 10.1073/pnas.210384297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101:465–474. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 25.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 26.Dobransky T, Doherty-Kirby A, Kim AR, Brewer D, Lajoie G, Rylett RJ. Protein kinase C isoforms differentially phosphorylate human choline acetyltransferase regulating its catalytic activity. J Biol Chem. 2004;279:52059–52068. doi: 10.1074/jbc.M407085200. [DOI] [PubMed] [Google Scholar]
- 27.Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008 Dec 12;283(50):34738–44. doi: 10.1074/jbc.M804981200. Epub 2008 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 29.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunemann M, Gerhardstein BL, Gao T, Hosey MM. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the beta(2) subunit. J Biol Chem. 1999;274:33851–33854. doi: 10.1074/jbc.274.48.33851. [DOI] [PubMed] [Google Scholar]
- 31.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 32.Jalili T, Takeishi Y, Song G, Ball NA, Howles G, Walsh RA. PKC translocation without changes in Galphaq and PLC-beta protein abundance in cardiac hypertrophy and failure. Am J Physiol. 1999;277:H2298–2304. doi: 10.1152/ajpheart.1999.277.6.H2298. [DOI] [PubMed] [Google Scholar]
- 33.Takeishi Y, Jalili T, Ball NA, Walsh RA. Responses of cardiac protein kinase C isoforms to distinct pathological stimuli are differentially regulated. Circ Res. 1999;85:264–271. doi: 10.1161/01.res.85.3.264. [DOI] [PubMed] [Google Scholar]
- 34.Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during the progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem. 2003;242:145–152. [PubMed] [Google Scholar]
- 35.Murphy S, Frishman WH. Protein kinase C in cardiac disease and as a potential therapeutic target. Cardiol Rev. 2005;13:3–12. doi: 10.1097/01.crd.0000124914.59755.8d. [DOI] [PubMed] [Google Scholar]
- 36.Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, Walsh RA, King GL. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci U S A. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeishi Y, Ping P, Bolli R, Kirkpatrick DL, Hoit BD, Walsh RA. Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy. Circ Res. 2000;86:1218–1223. doi: 10.1161/01.res.86.12.1218. [DOI] [PubMed] [Google Scholar]
- 38.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, Anderson ME, Colbran RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 41.Jiang LH, Gawler DJ, Hodson N, Milligan CJ, Pearson HA, Porter V, Wray D. Regulation of cloned cardiac L-type calcium channels by cGMP-dependent protein kinase. J Biol Chem. 2000;275:6135–6143. doi: 10.1074/jbc.275.9.6135. [DOI] [PubMed] [Google Scholar]
- 42.Hell JW, Yokoyama CT, Breeze LJ, Chavkin C, Catterall WA. Phosphorylation of presynaptic and postsynaptic calcium channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO J. 1995;14:3036–3044. doi: 10.1002/j.1460-2075.1995.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]