Abstract
Inflammatory changes are a major component of the normal tissue response to ionizing radiation (IR) and increased NF-κB activity is an important mediator of inflammatory responses. Here, we used zebrafish embryos to assess the capacity of two different classes of pharmacological agents known to target NF-κB to modify radiation toxicity in the vertebrate organism. These were proteasome inhibitors including Lactacystin, MG132 and PS-341 (Bortezomib/VELCADE) and direct inhibitors of NF-κB activity, including ethyl pyruvate (EP) and the synthetic triterpenoid CDDO-TFEA (RTA401) among others. The proteasome inhibitors either did not significantly affect radiation sensitivity of zebrafish embryos (MG132, Lactacystin) or rendered zebrafish embryos more sensitive to lethal effects of IR (PS-341). Radiosensitization by PS-341 was reduced in fish with impaired p53 expression or function but not associated with enhanced expression of select p53 target genes. In contrast, the direct NF-κB inhibitors EP and CDDO-TFEA significantly improved overall survival of lethally irradiated zebrafish embryos. In addition, direct NF-κB inhibition reduced radiation-induced apoptosis in the central nervous system, abrogated aberrations in body axis development, restored metabolization and secretion of a reporter lipid through the gastrointestinal system and improved renal clearance compromised by radiation. In contrast to Amifostine, EP and CDDO-TFEA not only protected against but also mitigated radiation toxicity when given 1–2 h post-exposure. Finally, four additional IKK inhibitors with distinct mechanisms of action similarly improved overall survival of lethally irradiated zebrafish embryos. In conclusion, inhibitors of canonical pathways to NF-κB activation may be useful in alleviating radiation toxicity in patients.
Keywords: Genotoxic stress, zebrafish, NF-kappaB, radiation protective agents, proteasome inhibitor
Introduction
Normal tissue damage limits the dose of ionizing radiation (IR) that can be safely administered to treat neoplastic disease. A well-known example of this problem is inflammation of the oral mucosa and of the lining of the gastrointestinal tract in tumor patients receiving chemotherapy or radiation [1]. Depending on the area of the body treated with radiation, other organ sites including the lungs and the pericardium also manifest radiation-induced inflammation. A pervasive feature of IR-associated inflammation is the increased presence of pro-inflammatory cytokines including TNF-α and IL-6, both locally and in the circulation [2]. In contrast to intracellular regulators of the DNA damage response, these and other inflammatory mediators act in a paracrine fashion affecting diverse cell types in the tissue microenvironment or even at a distance [3]. This circumstance highlights the necessity to use animal models to investigate the relative contribution of inflammatory changes to the overall response to radiation-induced cell and tissue injury in a multicellular organism. In recognition of this need, we recently established zebrafish embryos as a facile vertebrate in vivo system to monitor the effects of radiation protectors on normal tissues during development [4].
The NF-κB family of transcription factors represents a diverse and shared signaling mechanism activated during cell stress responses [5]. In addition, deregulated NF-κB signaling has been implicated in the malignant phenotype and treatment resistance of select tumor forms [6–10]. The canonical pathway to NF-κB activation leads to IKKβ-dependent phosphorylation and subsequent proteasomal degradation of the NF-κB inhibitor IκB, increased nuclear presence of NF-κB dimers and enhanced NF-κB-dependent transcriptional activity [5].
Whole body radioprotection through anti-inflammatory agents has very recently been demonstrated in animal models. Specifically, certain triterpenoids (CDDO and derivatives thereof) have been shown to selectively protect normal mouse tissues against the deleterious effects of ionizing radiation [11]. Furthermore, ethyl pyruvate (EP), a derivative of the end product of glycolysis, similarly protects normal cells against the deleterious effects of radiation both in vitro and in mice [12]. Among other molecular targets, both drugs inhibit activation of NF-κB. EP inhibits NF-κB signaling through direct molecular interaction with a reactive cysteine of the p65 subunit of NF-κB [13] whereas CDDO-TFEA binds to a reactive cysteine (Cys179) of IKKα thus inhibiting its kinase activity [14]. However, these drugs also target other signaling molecules and pathways of potential relevance to the radiation response including STAT3 and Jaks [15,16]. In addition to these agents proteasome inhibitors have been shown to inhibit NF-κB-dependent transcription and one of these (PS-341; Bortezomib; VELCADE) has been FDA-approved for clinical use in patients afflicted with multiple myeloma (for review see [17,18]). It is presently unknown whether and how proteasome inhibitors affect whole body radiation sensitivity.
Collectively, these results raised the question whether inhibition of NF-κB activity by different pharmacological agents contributes to protection of normal cells and tissues against damage induced by ionizing radiation. Here, we addressed this issue using zebrafish embryos as an in vivo model system. We observed that the NF-κB inhibitors EP and CDDO-TFEA afforded protection to zebrafish embryos against lethal effects of radiation in the pre- and post-exposure settings, i.e. when administered hours after radiation exposure. Radiation protection extended to multiple organ sites including the GI system and, importantly, was also observed when using additional IKK inhibitors with different modes of action. In contrast, several proteasome inhibitors including PS-341, did not protect against, but rather moderately exacerbated radiation-associated normal tissue toxicity in zebrafish embryos. These results predict a favorable therapeutic index for the use of inhibitors of canonical pathways to NF-κB activation in combination with radiation therapy.
Materials and Methods
Embryo harvesting and maintenance
Zebrafish were mated in embryo collection tanks. Viable embryos were washed and sorted (25 embryos per 60-mm dish) at the one- to two-cell developmental stage, and maintained under normoxic conditions at 28.5 °C to enable normal development. Embryo medium (EM) was changed at 24, 72, 120 hours post fertilization (hpf). All procedures using live zebrafish were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University. In select experiments, embryos (24 hpf) were dechorionated by placement in EM supplemented with 50 µg/mL pronase (Sigma, St. Louis, MO) for approximately 10 min at room temperature, then gently agitating them with a plastic pipette until embryos were liberated from the disrupted chorions. After dechorionation, embryos were rinsed thoroughly with EM, and placed in fresh EM.
Dechorionation of zebrafish embryos
Zebrafish embryos at 24 hpf were placed in EM with 50 µg/mL pronase (Sigma, St. Louis, MO) for approximately 10 min at room temperature, then gently agitated with a plastic pipette until embryos were liberated from the disrupted chorions. Dechorionated embryos were rinsed thoroughly with EM, and placed in fresh EM and incubated at 28.5°C.
Radiation exposure and drug treatments
Pharmacological agents (EP, kindly provided by Mike Eperly; CDDO-TFEA, Reata Pharmaceutical, Irving, TX; IKK inhibitors: IKK Inhibitor 2 (Weldelolactone), IKK Inhibitor 3 (BMS-345541), IKK-2 Inhibitor 4 and IKK-2 Inhibitor 5(IMD-0354), Calbiochem; MG132, Sigma; PS-341, Millennium Pharmaceuticals, and Lactacystin, Calbiochem) were dissolved in EM containing <0.1% DMSO. EM was used as a vehicle control in all experiments. Unless stated otherwise embryos were exposed to ionizing radiation (IR) ranging in dose from 0–20 Gy at 24 hpf using an X-ray machine (Gulmay Medical, Bethel, CT) and a 137Cs radiation source. Toxicity analyses for EP (≤10 mM), CDDO-TFEA (≤10 µM), PS-341 (≤10 µM), MG132 (≤50 µM) or Lactacystin (≤10 µM) were conducted by monitoring survival and development of zebrafish embryos for 7 days in the absence of radiation. To determine modulation of radiation-induced toxicity, EP (1 mM) or CDDO-TFEA (1 µM) were added to embryos either 1h before or up to 3 h after radiation exposure at 24 hpf. The proteasome inhibitors were added to zebrafish embryos 1 h prior to IR. After irradiation, zebrafish embryos were maintained at 28.5 °C for up to 7 days post fertilization (dpf) to monitor effects of treatments on survival, morphology and organ-specific toxicity.
Analysis of treatment effects on zebrafish survival and gross morphology
Dechorionated embryos at 72 hpf were anesthetized with a 1:100 dilution of 4 mg/ml tricaine methanesulfonate (Sigma, St. Louis, MO) and immobilized by placing them on 3% methylcellulose on a glass depression slide. Morphology was assessed visually using a light transmission microscope (Olympus BX51; Olympus, Melville, NY) at 40–100X magnification, and representative images recorded using a QIMAGING camera and QIMAGING Advanced software (QIMAGING Diagnostic Instruments, Canada). Similarly, survival of embryos was assessed visually at 24-h intervals up to 168 hpf by light microscopy. The criterion for embryonic survival was the presence of cardiac contractions.
Apoptosis assay
Zebrafish embryos were incubated for 1 h in EM containing modifiers of the radiation response and exposed to 20 Gy at 24 hpf. Six hours after radiation exposure, embryos were stained for 15 minutes using 5 µg/ml of Acridine Orange dye (Sigma) and rinsed five times with EM as described previously [19]. Zebrafish embryos were imaged with QIMAGING camera, iVision software, the images were analyzed using ImageJ software.
Detection of ROS
ROS levels were measured in dechorionated zebrafish embryos in 96-well plates. Embryos (1 embryo/well) were treated with either vehicle (EM) or EP (1 mM) or CDDO-TFEA (1 µM) in the presence of 5-(and-6)-chloromethyl-2`,7`-dihydrodichlorofluorescein diacetate (CM-H2DCFA; 500 ng/ml; Molecular Probes, Eugene, OR) followed by radiation exposure at 24 hpf. The average fluorescence emission at 530 nm following excitation at 490 nm was detected immediately and 2h after IR exposure using a microplate fluorescent reader (BIO-TEK FL 600; BIO-TEK Instruments Inc.; Winooski, VT). To account for radiation-induced reactive oxygen species in the embryo medium results were corrected by subtraction of values obtained in wells containing fish in the presence and absence of pharmacological agents.
Renal function assay
Time-dependent clearance of tetramethylrhodamine-labeled 10-kDa dextran (Molecular Probes, Eugene, OR) was determined as described previously with minor modifications [20]. Briefly, zebrafish embryos at 24 hpf were exposed to IR and maintained in EM. At 72 hpf embryos were anesthetized using a 1:100 dilution of 4 mg/ml tricaine methanesulfonate (Sigma, St. Louis, MO) and dorsally positioned on 3% methylcellulose gel. Tetramethylrhodamine-labeled 10-kDa dextran was injected into the cardiac venous sinus; embryos were kept at 28.5°C, and imaged at 1 and 24 h following microinjection. The average fluorescence emission at 590 nm following excitation at 570 nm was detected at the center of the cardiac area, and the relative intensity was measured using a Leica microscope (Leica Mikroskopie & Systeme GmbH, Wetzlar, Germany). Images were transformed into grayscale and evaluated with National Institutes of Health ImageJ software as described by [20].
Morphological analysis of the gastrointestinal system
The functional and morphological integrity of the developing gastrointestinal system were assessed in zebrafish embryos using PED6, a fluorescent reporter of phospholipase A2 (PLA2) activity. PED6 is a fluorogenic substrate for PLA2, which contains a BODIPY FL dye–labeled acyl chain and a dinitrophenyl quencher group [21]. The cleavage of the dye-labeled acyl chain by PLA2 within cells lining the intestine unquenches the dye and leads to detectable fluorescence in the lumen of the developing gastrointestinal tract. PED6 was added to zebrafish embryos at day 5 followed by imaging the fish at day 6 with the average fluorescence emission at 540 nm following excitation at 505 nm. Images were taken at day 6 using a Leica microscope and analyzed with the help of the ImageJ software.
Histopathology and evaluation of tissue morphology
Zebrafish embryos were evaluated histopathologically for morphologic alterations induced by radiation exposure and potential radioprotective effects of EP and CDDO-TFEA with special emphasis on the gastrointestinal morphology. Briefly, embryos at 24 hpf were exposed to 0 or 12 Gy in the presence or absence of either CDDO-TFEA or EP administered 1 h prior to IR. Embryos were sacrificed, fixed by immersion in 4 % paraformaldehyde for 24 hours and then rinsed and placed in 10X PBS for another 24 h. Sections were embedded in paraffin, and coronal, transverse and sagittal whole-body sections (4 µm thickness) generated. All sections were stained with hematoxylin and eosin, mounted on glass slides, and examined by light microscope; representative images were taken using a QIMAGING camera and iVision software.
NF-κB reporter assay
This assay was performed as described by us previously [22] with minor modifications. HeLa cells were seeded at 7.5 × 104/ml in DMEM medium supplemented with 10% FBS. The cells were co-transfected with the pSEAP2- NF-κB vector (BD BioSciences) encoding a secreted form of human placental alkaline phosphatase driven by a NF-κB-responsive promoter and a β-galactosidase expression vector for control purposes. Forty-eight hours post transfection, different NF-κB inhibitors (Velcade, 0.5 µ M; MG-132, 5 µM; EP, 1mM; CDDO, 1 µM) were added to the cells in serum-free media for 24 hours. NF-κB-dependent transcription in the absence and presence of recombinant TNF-α (10 ng/ml; R&D Systems) was determined 72 hours post transfection using the Great EscAPe SEAP Reporter System 3, which is based on detection of secreted alkaline phosphatase in cell supernatants normalized to β-galactosidase activity using the luminescent β -gal detection kit (BD Biosciences).
Reverse transcription polymerase chain reaction (RT-PCR) analysis
Zebrafish total RNA was isolated from 100 embryos per experimental condition at 30 hpf (6 h post radiation) using the RNeasy mini kit (QIAGEN Sciences, Maryland, USA) and stored at - 80 °C. For reverse transcription, total RNA was annealed with Oligo(dT) primer (Roche) at 70°C for 5 min followed by the incubation at 42°C for 1 h. Reverse transcription reaction products were boiled for 2 min followed by incubation on ice for 2 min before use. Primer sequences used for amplification of bax, mdm2, p21/waf-1 and β-actin zebrafish sequences are provided in Supplementary Table 1. PCR reaction conditions were 94 °C, 60 °C, 72 °C for 30 sec, 30 sec, 1 min, respectively and 35 cycles with 7 min. extension time after the last cycle. Thermo Fisher Scientific Taq-polymerase was used in 50 µL PCR reaction mix containing 1 µL RT reaction. PCR reactions were analyzed by 1.5% agarose gel electrophoresis
Statistical analysis
All experiments were performed at least three times with at least 75 embryos total per experimental group. To determine statistically significant differences between groups Chi square tests were performed.
Results
Proteasome inhibitors radiosensitize zebrafish embryos
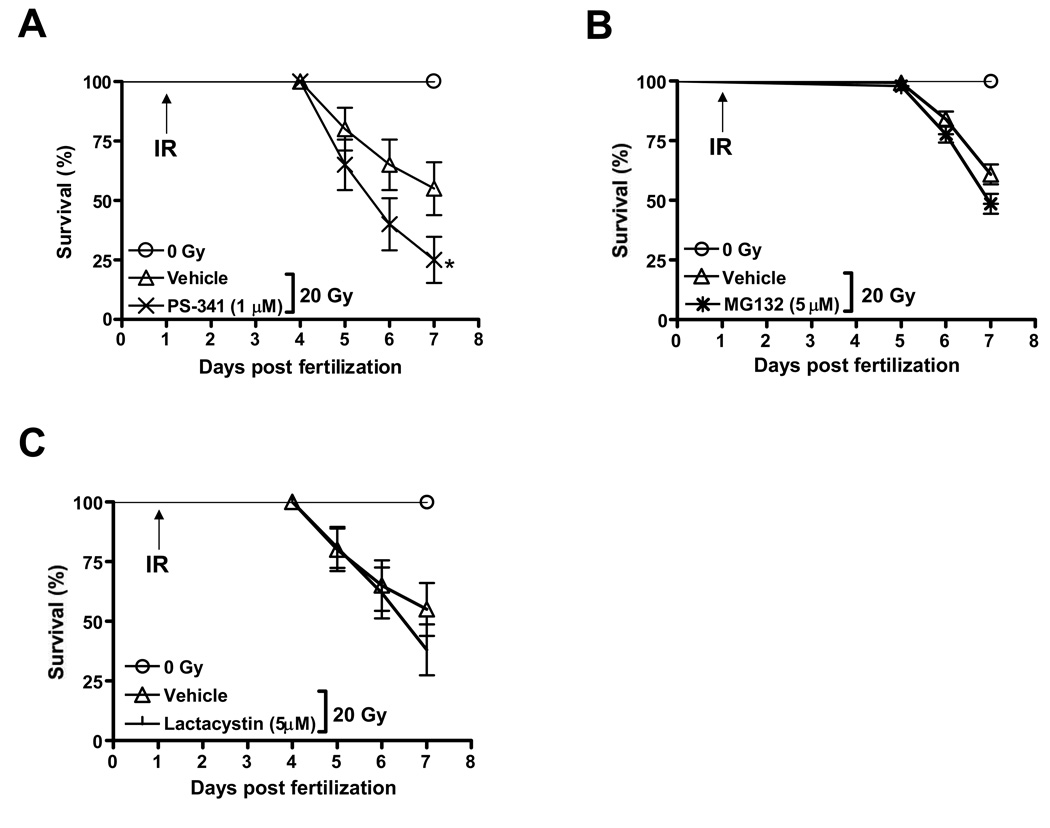
The proteasome inhibitor PS-341 (Bortezomib/VELCADE) is presently the only FDA-approved drug with well-characterized inhibitory effects on NF-κB activity [18]. PS-341 is a small, cell-permeable molecule inhibiting proteasome activity in a reversible manner. In addition to reducing the activation state of NF-κB by inhibiting proteasomal degradation of IκB, PS-341 affects many other pathways and targets, leading to high expression levels of several proapoptotic proteins in certain experimental conditions [23]. In vitro, PS-341 has been found to enhance anti-tumor cell effects of select chemotherapeutic agents [6,24], tumor cell targeting antibodies [25] and ionizing radiation [26]. Yet, little is known about the combined effects of PS-341 and ionizing radiation on normal cells and tissues of vertebrate organisms. To address this issue we used PS-341 in zebrafish embryos exposed to high doses of ionizing radiation as described by us previously [4,27,28]. We first established that treatment of zebrafish with PS-341 alone (dose range 0–10 µM) was non-toxic as assessed by embryo survival and gross morphology during the first 7 days after fertilization (Supplemental Fig. 1). In contrast, PS-341 (1 µM) markedly sensitized zebrafish embryos to the lethal effects of IR when administered 1 h prior to radiation (Fig. 1A). In these experiments zebrafish embryos were exposed at 24 hpf to 20 Gy, previously determined to kill 50% of irradiated zebrafish embryos by day 7 of development [27]. In HeLa cells, at the same concentration (1 µM) PS-341 abrogated the TNF-α induced NF-κB activity, while it did not significantly affect the basal activity (Supplemental Fig. 2A and B).
Figure 1.
Effects of the proteasome inhibitors PS-341 (A), MG132 (B) and Lactacystin (C) on radiation sensitivity of zebrafish embryos. Embryos were irradiated at 24 hpf and survival was scored every day up to 7 dpf. Results shown represent m±SD of triplicate experiments. Asterix indicates statistically significant difference in survival at 6–7 dpf.
To ascertain whether radiation sensitization by PS-341 could be replicated using other inhibitors of the proteasome we next tested the effects of MG132, a non-boronated small molecule inhibitor of the 26S proteasome [29], on zebrafish survival in the presence and absence of IR. Similar to PS-341, MG132 was remarkably nontoxic when applied as a single agent to zebrafish embryos (dose range 0–50 µM) yet efficiently inhibited TNF-α-induced but not the baseline NF-κB activity in HeLa cells when used at 5 µM (Supplemental Fig. 2A and B). At this concentration, however, MG132 marginally sensitized zebrafish embryos to the lethal effects of 20 Gy IR albeit to a lesser degree than PS-341 (Fig. 1B). An irreversible proteasome inhibitor (Lactacystin) at a non-toxic concentration (5 µM) also slightly radiosensitized zebrafish embryos in a manner similar to MG132 (Fig 1C).
These results demonstrate that several proteasome inhibitors do not protect normal cells and tissues in the developing fish larvae against the deleterious effects of radiation. As p53 is a major target of proteasomal degradation and enhances IR-associated tissue damage in mice [30–32] and zebrafish [33,34] we asked whether the deleterious effects of proteasome inhibitors could be linked to p53 stabilization and subsequent induction of target genes. Consistent with our earlier observations, ablating p53 expression by antisense morpholino oligodeoxynucleotide [35] or p53 function by PFT-α (1 µM) given to zebrafish embryos at 24 hpf [34] markedly improved zebrafish survival after radiation either alone or in combination with PS-341 (not shown). However, RT-PCR analysis did not reveal increased steady-state mRNA levels of the p53 targets p21/WAF1, bax or the zebrafish ortholog of mdm2 in PS-341-treated embryos whereas ionizing radiation led, as expected, to elevated transcript levels for these genes (Supplemental Fig. 3). Thus, the molecular target(s) responsible for radiosensitization by PS-341 and their relationship, if any, to the p53 response remain to be identified.
Radiation protection of zebrafish embryos by the NF-κBp65 inhibitor ethyl pyruvate
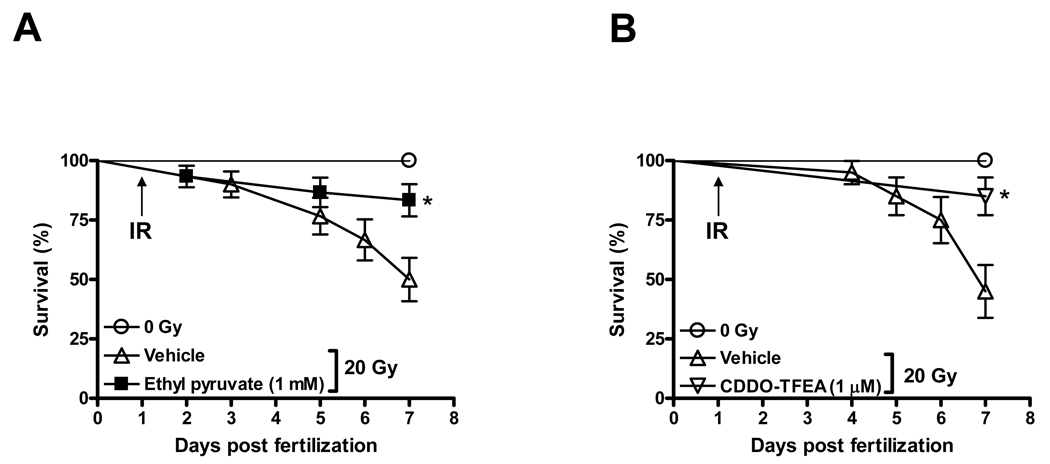
In consideration of the fact that proteasome inhibitors affect multiple intracellular pathways in addition to NF-κB and to pinpoint the functional contribution of NF-κB to the radiation response of zebrafish embryos we tested the effects of a series of pharmacological inhibitors of NF-κB activity with different mechanisms of action on the radiation response of zebrafish embryos. Reducing NF-κB activity by expression of upstream regulator IκB has previously been shown to cause severe embryonal malformations in zebrafish [36,37] and, thus, was not further considered. In addition, knockdown of the NF-κBp65 subunit by antisense morpholinos similarly caused severe morphological defects (no tail phenotype) during the first 3 days of development (Supplemental Fig. 4) consistent with published results [37] and, thus, was not informative in the context of assaying the radiation response. Instead, we used pharmacological inhibitors which disrupt the canonical pathway to NF-κB activation and could be used at concentrations which do not interfere with embryonal development. First, we tested ethyl pyruvate (EP), an ROS scavenger and inhibitor of NF-κBp65 [38]. EP inhibits the DNA binding activity of NF-κBp65 by binding to a reactive cysteine in the DNA binding site (Cys 38) of NF-κBp65 [13] which is shared between humans and zebrafish (Supplemental Fig. 2C). EP has very recently been shown to mitigate deleterious effects of total body irradiation in mice [12]. We observed that EP similarly not only protected against but also mitigated lethality associated with whole body irradiation of zebrafish embryos (Fig. 2A, C and F). EP was administered at various time points ranging from 1 h prior to radiation exposure to 3 h post irradiation. The ROS scavengers Amifostine and DF-1 served as positive controls in these experiments as we observed marked protection of embryos by these two compounds in earlier work [4,27]. Whereas Amifostine and DF-1 afforded protection against deleterious effects of IR when administered prior to or concurrent with radiation, neither compound could mitigate lethal effects of radiation when given beyond 15 min after IR [27]. In marked contrast, EP administered up to 2 h after radiation significantly reduced IR-associated lethality (Fig. 2F).
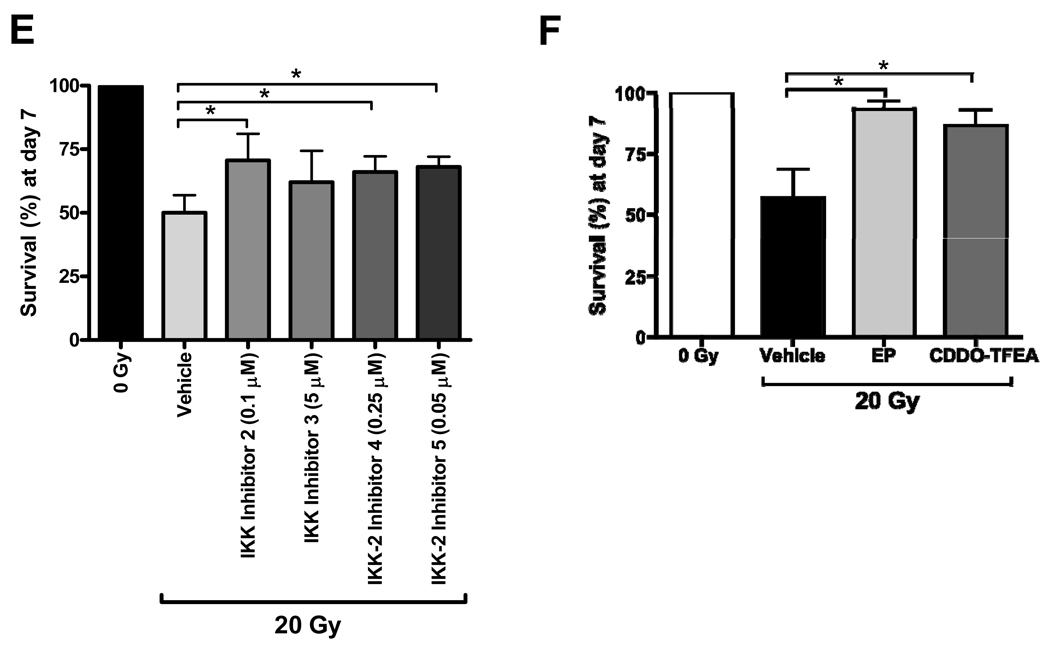
Figure 2.
Protection against and mitigation of lethal effects of radiation by direct NF-κB inhibitors. Differential survival of zebrafish embryos pretreated with EP (panels A and C) or CDDO-TFEA (panels B and D) and exposed to either 20 or 40 Gy as indicated. (E) Radiation protection of zebrafish embryos exposed to 20 Gy and treated for 1 h prior to irradiation with different IKK inhibitors. (F) Increased survival of irradiated (20 Gy) zebrafish embryos treated with EP (1mM) or CDDO-TFEA (1 µM) 2 h post radiation at 24 hpf. Survival was scored at 7 dpf. All results shown represent m±SD of triplicate experiments. Asterices indicate statistically significant differences in survival at 7 dpf between drug-treated and vehicle-treated groups.
The IKK inhibitor CDDO-TFEA mitigates radiation effects in zebrafish embryos
To further address the relevance of NF-κB activation in modulating radiation sensitivity of zebrafish embryos we used CDDO-TFEA which inhibits NF-κB signaling by interacting with Cys179 of IKKβ, inhibiting its activity and, preventing phosphorylation and proteasomal degradation of IκB [39] and, thus, through a molecular mechanism distinct from EP. The amino acid sequence around this reactive Cys179 is also highly conserved in zebrafish (Supplemental Fig 2C). CDDO-TFEA protected against and mitigated overall lethal effects of radiation in zebrafish embryos in a manner similar to EP (Fig. 2 B, D and F). We next determined whether mitigation of radiation effects cosegregated with the capacity of the compounds under investigation to act as ROS scavengers. This was based on the findings that, in addition to directly binding to IKKβ, CDDO has been described to induce expression of enzymes catalyzing antioxidant reactions in PBMCs due to increased nuclear accumulation of Nrf2, an oxidant-responsive bZIP transcription factor [40,41]. Whereas EP is an effective ROS scavenger in irradiated zebrafish embryos, CDDO-TFEA did not reduce ROS levels measured 2 h after radiation exposure (Supplemental Fig. 5). Thus, at least the effect of CDDO-TFEA on radiation mitigation cannot be ascribed to ROS scavenging whereas, in the case of EP, ROS scavenging and NF-κB inhibition may be jointly responsible for the beneficial effects of EP in the mitigation setting. Of note, the ROS scavengers with no known effect on NF-κB signal transduction (Amifostine and DF-1) do not mitigate radiation effects if administered beyond 15 min after IR [27]. To further probe whether IKK inhibition is radioprotective we tested four additional small molecule IKK inhibitors, i.e. Wedelolactone (IKK inhibitor II), BMS-345541 (IKK inhibitor III) and IKK-2 inhibitors IV and V. All four agents protected zebrafish embryos against the lethal effects of radiation in a manner similar to CDDO-TFEA and EP (Fig. 2E). Moreover, unlike EP or CDDO-TFEA, these agents are not known to have antioxidant properties and primarily inhibit IκBα phosphorylation by IKKβ (i.e. IKK-2). On balance, these results suggest that prolonged and excessive activation of the canonical NF-κB pathway is a major contributor to radiation toxicity in the developing vertebrate organism and that inhibiting this pathway may protect the organism against deleterious effects of radiation.
Organ-specific radiation protection by CDDO-TFEA and EP
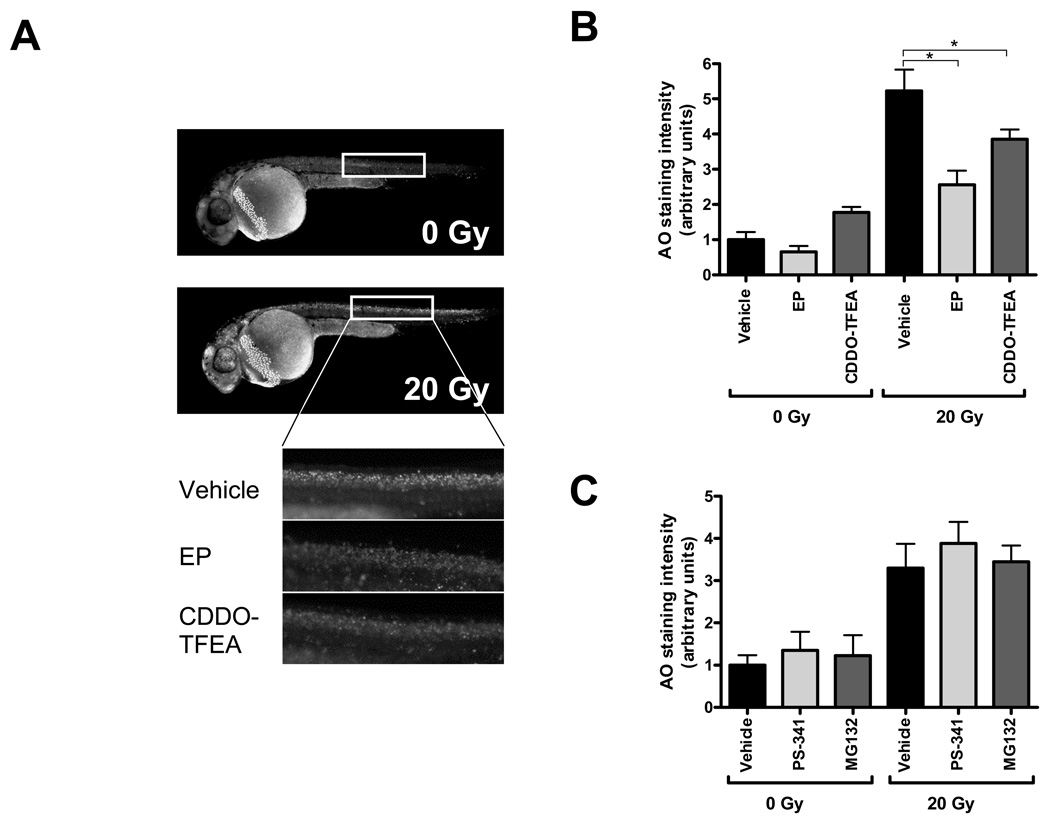
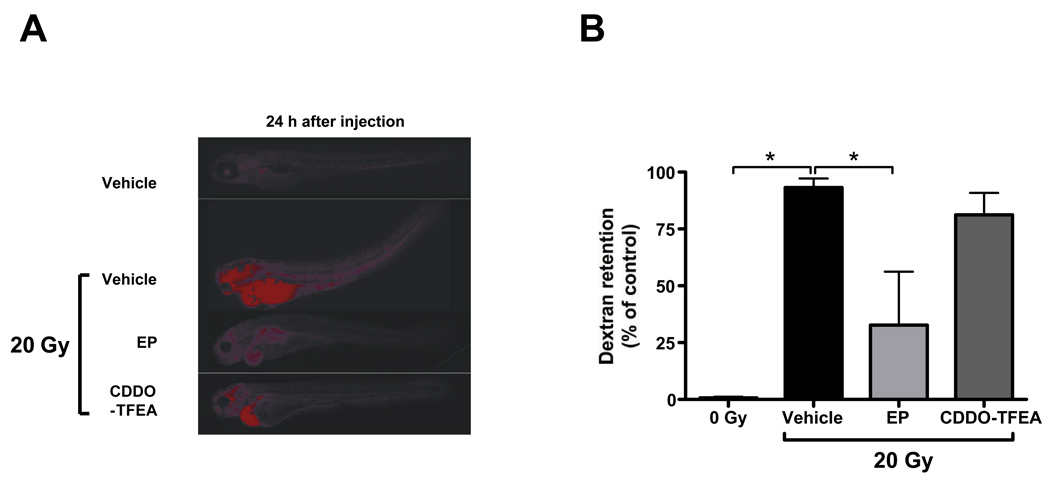
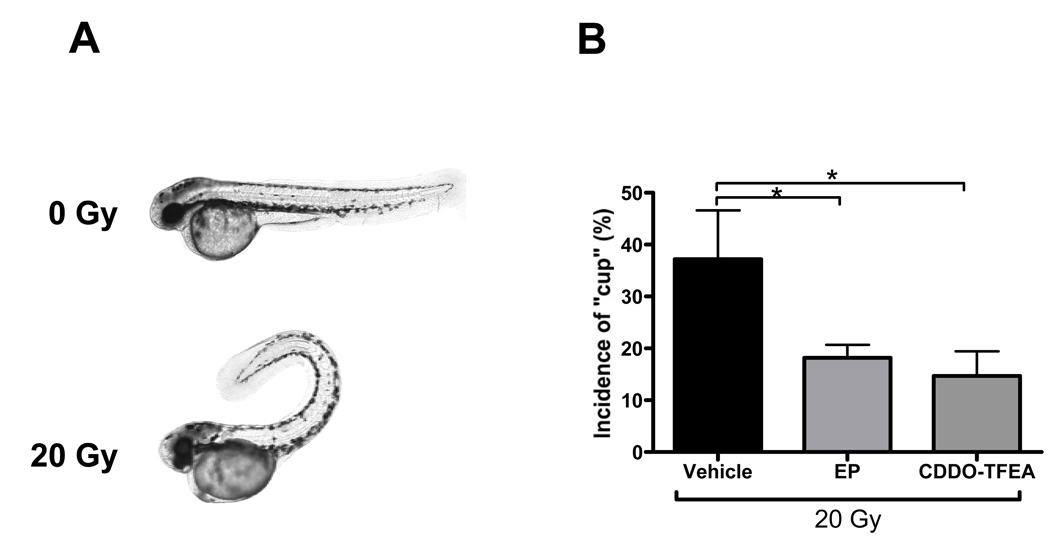
Having established that EP and CDDO-TFEA provide whole body protection against lethal doses of radiation and in consideration of the fact that these compounds are in preclinical development we next determined organ-specific radiation protective effects of these two NF-κB inhibitors. First, we assessed, by acridine orange staining, organism-wide apoptosis in zebrafish embryos determined 6 h after radiation. Consistent with earlier reports [33], we observed increased AO staining in the central nervous system and along the body axis of irradiated embryos. Both NF-κB inhibitors markedly reduced radiation-induced AO staining (Fig. 3). We previously reported that IR compromised zebrafish kidney function as determined by delayed excretion of a fluorescent dextran injected intracardially [27]. Treatment with EP but not CDDO-TFEA significantly reversed the effect of IR on dextran clearance of irradiated embryos to near normal levels suggesting protection against IR-induced kidney damage (Fig. 4). It is currently unknown whether this effect reflects differences in ROS scavenging capacity between the two compounds as described above (Supplemental Fig. 5) or is due to differences in pharmacokinetics or pharmacodynamics. In addition, radiation of zebrafish embryos is associated with a high incidence of a body axis malformation dubbed “curly-up” to describe aberrant dorsal curvature of the fish tail. Both, CDDO-TFEA and EP reduced the incidence of curly-up significantly (Fig. 5).
Figure 3.
Reduction of organism-wide apoptosis by EP and CDDO-TFEA treatment pre- and post-radiation. Acridine orange (AO) staining of whole embryos was performed 6 hours post radiation at 30 hpf. (A) Representative examples of control or irradiated fish revealing strong AO staining in the central nervous system and along the body axis induced by radiation (20 Gy). Regions selected for quantitative evaluation are boxed. (B) Reduced AO staining in CDDO-TFEA and EP-treated embryos exposed to IR. (C) PS-341 or MG132 treatment does not significantly affect AO staining of embryos exposed to IR.
Figure 4.
Effects of EP and CDDO-TFEA on radiation-induced kidney damage as determined by dextran clearance. (A) Representative images of dextran retention in irradiated embryos and effects of EP and CDDO-TFEA on this phenomenon. (B) Quantitative representation of dextran retention in embryos treated with either EP or CDDO-TFEA as indicated; results are expressed relative to vehicle-treated, non-irradiated controls. Asterices denote statistically significant differences between the experimental groups indicated by brackets.
Figure 5.
EP and CDDO-TFEA alleviate radiation-induced malformations of the body axis (A) Representative micrographs showing dorsal curvature (‘curly-up’; cup) in irradiated (20 Gy) embryos at 72 hpf relative to control non-irradiated fish embryos. (B) Quantification of “curly-up’ incidence in CDDO-TFEA- and EP-treated fish relative to vehicle-treated controls. Asterices denote statistically significant differences between the experimental groups indicated by brackets.
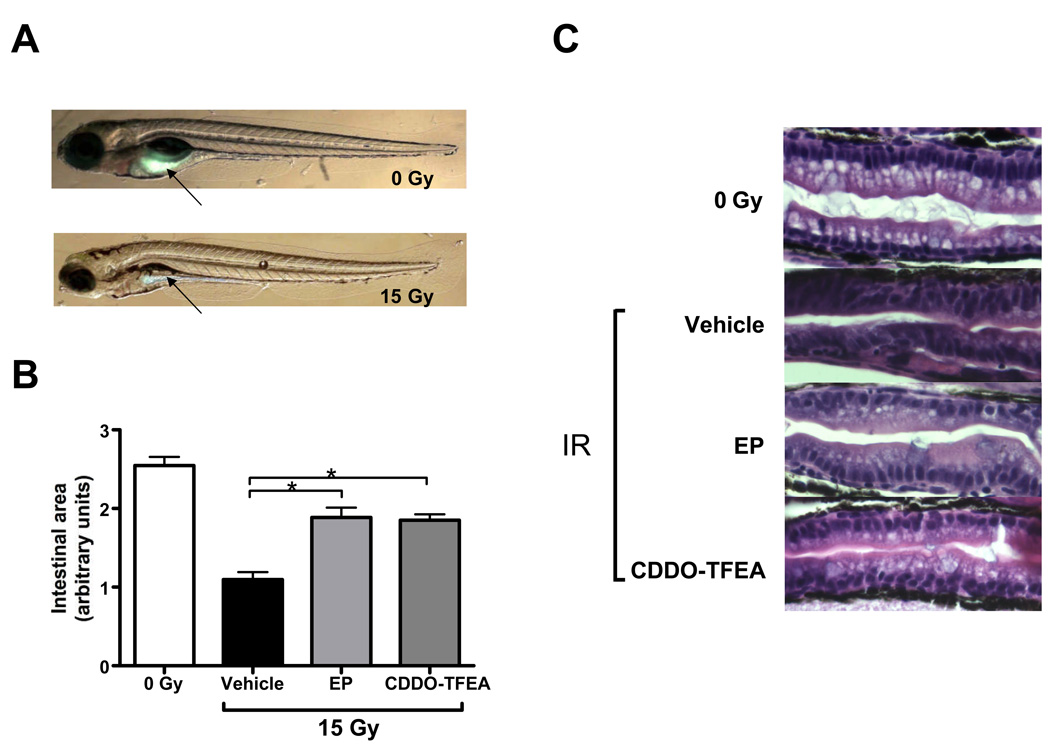
Finally, we determined the effects of radiation on the developing GI system. This was done in consideration of several prior reports suggesting that NF-κB activation protects the gastrointestinal tract of higher vertebrates against acute radiation damage [42,43]. Radiation protection of the GI system was determined in several ways. First, we assayed overall gastrointestinal function by scoring ‘long-term’ survival of fish irradiated in the presence and absence of EP or CDDO-TFEA up to 15 dpf. Fish larvae become dependent on external food sources at approximately 6 dpf when the contents of the yolk sac are depleted. Significant functional damage to the GI system will thus lead to death by starvation within 10 days after conception [19]. Conversely, survival of fish beyond two weeks indicates establishment of a functionally adequate gastrointestinal system. Both, EP and CDDO-TFEA increased extended survival of zebrafish larvae (Supplemental Fig. 6) although this effect was statistically significant only in case of CDDO-TFEA. To address the combined effects of radiation and EP or CDDO-TFEA treatment on the developing GI system further we determined gastrointestinal lumen formation by use of a fluorescent reporter (PED6) [21] which is metabolized and excreted through the GI system. This analysis revealed severely impaired lumen formation of the GI system induced by IR (15 Gy) and partial restoration of lumen formation and fluorescent dye excretion by treatment with either EP or CDDO. These functional results were complemented by examining the histological appearance of the GI system 5 days after radiation exposure in the presence and absence of the NF-κB inhibitors under investigation (Fig. 6). The hindgut mucosal epithelium immediately proximal to the cloaca revealed distinct cellular changes associated with sublethal IR exposure (12 Gy) including irregular shape and disorganization of the columnar absorbing cells with redistribution of nuclei away from the basal orientation. In addition, decreased goblet cell numbers were observed. By contrast, EP and CDDO-TFEA pretreatment of irradiated embryos restored, in part, the columnar structure of absorbing cells and basal location of nuclei.
Figure 6.
Effects of EP and CDDO-TFEA on radiation-induced alterations of the gastrointestinal system. (A) Improved PLA2 activity and gastrointestinal lumen formation in irradiated zebrafish treated with either EP or CDDO-TFEA. Dequenched fluorescence reflects endogenous PLA2 activity and transport of cleavage products through the lumen of the developing gastrointestinal system. (B) Quantitative evaluation of gastrointestinal lumen formation as determined by fluorescent dye content at 6 dpf. (C) Representative histological sections of hindgut proximal to the cloaca at 6 dpf (5 days post radiation (12 Gy)).
Discussion
Our results show that 6 out of 6 pharmacological inhibitors with different chemical structures and mode of actions inhibit the canonical pathway of NF-κB activation (consisting of IKKβ/IκB/NF-κBp65) and provide protection against radiation-induced overall lethality and damage to multiple organ systems of the developing zebrafish. By contrast, 3 out of 3 proteasome inhibitors did not afford radiation protection, but radiosensitized zebrafish embryos to lethal effects of ionizing radiation. Taking into account that each of the pharmacological agents used in this study is likely to affect targets other than NF-κB it is remarkable that radioprotection co-segregated with interference with activation of the canonical pathway to NF-κB. This observation suggests that NF-κB may be the relevant target for radiation protection by pharmacological IKK/NF-κB inhibition.
Currently, there is no consensus about the functional contribution of NF-κB activation to the radiation response [44]. Abundant reports of radiosensitization of tumor cells in vitro and in vivo by NF-κB inhibition are contrasted by relatively few such reports dealing with normal cells. The use of genetically engineered mouse models to monitor NF-κB dysfunction in normal tissues has been limited due to embryonal lethality observed in IKKβ [45] and NF-κBp65 [46] knockout animals. In cases where either conditional knockouts were made or transgenic mice were generated by forced expression of dominant negative regulators to modulate NF-κB activation, the interpretation of results is further complicated by compensatory adjustments of homeostasis (for review see [47]). The present study sidesteps the problems inherent to using genetic models by examining the effects of pharmacological agents used at concentrations which reduce but do not abrogate NF-κB activity. The ease of our “assay system”, i.e., observation of overall effects of ionizing radiation on zebrafish survival as well as effects on specific target organs allowed us to monitor effects of drug classes grouped according to target specificity and mechanisms of action. This approach had the advantage to minimize confounding effects due to unknown, off-target effects of any pharmacological agent. By contrast and, as expected, ablating NF-κB activity by targeting IKKβ or NF-κBp65 expression using antisense approaches produced a dramatically different outcome as these interventions were associated with embryonic lethality even in the absence of genotoxic stress (see Supplementary Fig. 4) and [46]). This result is consistent with the view that inhibition of excess NF-κB activity after lethal irradiation is beneficial whereas blocking NF-κB expression and / or activation altogether as in genetic knockout/knockdown models, is deleterious (even in the absence of radiation). This contention is further supported by our finding that EP and CDDO-TFEA at the non-toxic concentration used here disrupted TNF-α-induced NF-κB activation but not basal NF-κB activity in HeLa cells in vitro (see Supplemental Fig. 2). Importantly, CDDO-TFEA and EP not only protected against but also mitigated lethal effects of radiation. This result is of interest as it points to the importance of sustained NF-κB activation consistent with inflammatory responses rather than the burst of NF-κB activity observed immediately after radiation exposure. It remains to be seen whether other anti-inflammatory agents may be used to mitigate radiation damage to normal tissues in the developing embryo.
Interestingly, radiation protection of zebrafish embryos by NF-κB inhibitors extended to the GI system whereas previous work using genetically modified mice [42] and the TLR5 ligand flagellin [43] has implicated NF-κB activation in radiation protection of GI stem cells. The reason(s) for this difference are unclear at this point. However, the TLR5 ligand flagellin exerts pleiotropic stimulatory effects on multiple signaling pathways which include NF-κB but also p38, Erk/MAPK and, potentially, STATs [48]. It has not been reported which of these multiple effects alone or in combination is at cause for radioprotection provided by flagellin [43]. In addition, the NF-κB inhibitory effects of both EP and CDDO-TFEA are completely reversible, while genetic ablation is not and this circumstance could affect outcomes of NF-κB activation in reference to GI function. Our findings are further consistent with the view that excessive NF-κB activation, as observed in the context of chronic inflammation, is potentially deleterious to the gastrointestinal system [49] and, thus, downmodulating NF-κB activity but not ablating it altogether can be advantageous in certain settings [50]. While the details of these diverse outcomes in different model systems remain to be sorted out our results clearly demonstrate that reducing NF-κB activity with a variety of compounds with different mechanisms of action diminishes radiation-induced damage to several organ systems in the developing zebrafish embryo.
In conclusion, the most salient finding of this study is that direct inhibitors of NF-κB activity provided effective protection and mitigation against overall lethality and specific organ damage caused by ionizing radiation in zebrafish embryos. Direct NF-κB inhibitors also exert antineoplastic effects in select model systems as demonstrated extensively for CDDO-TFEA and derivatives thereof [51–57]. These findings are consistent with a favorable therapeutic window for NF-κB inhibitors when used in combination with radiation and, potentially, chemotherapeutic drugs.
Supplementary Material
Figure S1: Toxicity of pharmacological agents used in the present study. PS-341 (≤ 10 µM), MG132 (≤ 50µM), Lactascystin (≤ 10 µM), EP (≤ 10 mM) , CDDO (≤ 10µM) were added to EM for 7 days and survival scored at 7dpf. All compounds were used in toxicity assay at ranges exceeding the concentration used to determine modulation of the radiation response by such agents as indicated by asterices.
Figure S2: Targets and effects of pharmacological agents used in the present study on NF-κB activity in vitro. (A) Effects of the proteasome inhibitors PS-341 and MG132 as well as EP and CDDO-TFEA on TNF-α-induced NF-κB activity in HeLa cells. (B) Effects of compounds on baseline NF-κB activity in HeLa cells. (C) Conservation of human and zebrafish amino acid sequences flanking reactive cysteines (highlighted in yellow), which are the molecular targets of CDDO (Cys179; IKKβ) and EP (Cys38; NF-κBp65), respectively. In these experiments compounds were used at the concentrations equal to those used in radiation protection experiments in zebrafish embryos.
Figure S3: Effects of IR and PS-341 on steady-state transcript levels of the p53 target genes p21/Waf1, mdm2 and bax as determined by semi-quantitative PCR standardized to β-actin. The effects of EP and CDDO-TFEA on target gene expression are shown for comparison.
Figure S4: Lethal effect of morpholino-mediated NF-κB knockdown on zebrafish embryos. (A) Morphology of NF-κB-MO-treated embryos (B) Time-dependent survival of control, control-MO (random control morpholino, Gene-Tools) and NF-κB-MO-treated embryos.
Figure S5: EP but not CDDO is an effective ROS scavenger in irradiated zebrafish embryos. Zebrafish embryos were exposed to IR (20 Gy) at 24 hpf and ROS levels determined at 26 hpf, i.e., 2 h after IR as described in Materials and Methods.
Figure S6: Extended survival of irradiated zebrafish embryos pretreated with EP and CDDO-TFEA as determined at 15 dpf.
Supplemental Table S1: Primer sequences used in RT-PCR analysis of zebrafish mRNA
Acknowledgements
The support of the Zebrafish Core Facility at the Department of Biochemistry and Molecular Biology Thomas Jefferson University is gratefully acknowledged.
Financial Support: This work was supported by grants from the National Institute of Health (CA10663, CA81008), the Tobacco Research Settlement Fund, the Commonwealth of Pennsylvania, USDA # 2006-03152 and, the Christine Baxter Fund.
Abbreviations
- dpf
days post fertilization
- EP
ethyl pyruvate
- hpf
hours post fertilization
- MO
morpholino deoxynucleotide
- NF-κB
Nuclear factor kappa B
- RT-PCR
Reverse transcriptase polymerase chain reaction
- TNF-α
Tumor necrosis factor-alpha
Footnotes
Conflict of interest: The authors declare no conflict of interest
References
- 1.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 2.Hall PD, Benko H, Hogan KR, Stuart RK. The influence of serum tumor necrosis factor-alpha and interleukin-6 concentrations on nonhematologic toxicity and hematologic recovery in patients with acute myelogenous leukemia. Exp Hematol. 1995;23:1256–1260. [PubMed] [Google Scholar]
- 3.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 4.McAleer MF, Davidson C, Davidson WR, et al. Novel use of zebrafish as a vertebrate model to screen radiation protectors and sensitizers. Int J of Radiat Oncol Biol Phys. 2005;61:10–13. doi: 10.1016/j.ijrobp.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 6.Cusack JC, Jr, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 7.Braunstein S, Formenti SC, Schneider RJ. Acquisition of stable inducible up-regulation of nuclear factor-kappaB by tumor necrosis factor exposure confers increased radiation resistance without increased transformation in breast cancer cells. Mol Cancer Res: MCR. 2008;6:78–88. doi: 10.1158/1541-7786.MCR-07-0339. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed KM, Li JJ, et al. ATM-NF-kappaB connection as a target for tumor radiosensitization. Curr Cancer Drug Targets. 2007;7:335–342. doi: 10.2174/156800907780809769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BY, Kim KA, Kwon O, et al. NF-kappaB inhibition radiosensitizes Ki-Ras-transformed cells to ionizing radiation. Carcinogenesis. 2005;26:1395–1403. doi: 10.1093/carcin/bgi081. [DOI] [PubMed] [Google Scholar]
- 10.Munshi A, Kurland JF, Nishikawa T, Chiao PJ, Andreeff M, Meyn RE. Inhibition of constitutively activated nuclear factor-kappaB radiosensitizes human melanoma cells. Mol Cancer Ther. 2004;3:985–992. [PubMed] [Google Scholar]
- 11.Meyer CJ, Sporn MB, Wigley WC, Sonis ST. RAT 402 (CDDO-Me) suppresses tumor and treatment induced inflammation, sensitizing tumors to and protecting normal tissue from radiation. Eur J Cancer (suppl.) 2006;4:162. [Google Scholar]
- 12.Epperly M, Jin S, Nie S, et al. Ethyl pyruvate, a potentially effective mitigator of damage after total-body irradiation. Radiat Res. 2007;168:552–559. doi: 10.1667/RR1009.1. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005;312:1097–1105. doi: 10.1124/jpet.104.079707. [DOI] [PubMed] [Google Scholar]
- 14.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12:1828–1838. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 15.Liby K, Voong N, Williams CR, et al. The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res. 2006;12:4288–4293. doi: 10.1158/1078-0432.CCR-06-0215. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)-->signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008;68:2920–2926. doi: 10.1158/0008-5472.CAN-07-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 18.Zavrski I, Kleeberg L, Kaiser M, et al. Proteasome as an emerging therapeutic target in cancer. Curr Pharm Des. 2007;13:471–485. doi: 10.2174/138161207780162908. [DOI] [PubMed] [Google Scholar]
- 19.Westerfield M. The zebrafish book. Eugene OR: University of Oregon Press; 1995. [Google Scholar]
- 20.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J of Physiol- Renal Fluid & Electrolyte Physiology. 2005;288:F923–F929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 21.Farber SA, Pack M, Ho SY, et al. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292:1385–1388. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- 22.Ren Q, Kari C, Quadros MR, et al. Malignant transformation of immortalized HaCaT keratinocytes through deregulated nuclear factor kappaB signaling. Cancer Res. 2006;66:5209–5215. doi: 10.1158/0008-5472.CAN-05-4158. [DOI] [PubMed] [Google Scholar]
- 23.Milano A, Iaffaioli RV, Caponigro F. The proteasome: a worthwhile target for the treatment of solid tumours? Eur J Cancer. 2007;43:1125–1133. doi: 10.1016/j.ejca.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 24.Neukirchen J, Meier A, Rohrbeck A, et al. The proteasome inhibitor bortezomib acts differently in combination with p53 gene transfer or cytotoxic chemotherapy on NSCLC cells. Cancer Gene Ther. 2007;14:431–439. doi: 10.1038/sj.cgt.7701029. [DOI] [PubMed] [Google Scholar]
- 25.Cardoso F, Durbecq V, Laes J-F, et al. Bortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER-2-positive breast cancer cells in a synergistic manner. Mol Cancer Ther. 2006;5:3042–3051. doi: 10.1158/1535-7163.MCT-06-0104. [DOI] [PubMed] [Google Scholar]
- 26.Russo SM, Tepper JE, Baldwin AS, Jr, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys. 2001;50:183–193. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 27.Daroczi B, Kari G, McAleer MF, Wolf JC, Rodeck U, Dicker AP. In vivo radioprotection by the fullerene nanoparticle DF-1 as assessed in a zebrafish model. Clin Cancer Res. 2006;12:7086–7091. doi: 10.1158/1078-0432.CCR-06-0514. [DOI] [PubMed] [Google Scholar]
- 28.McAleer MF, Duffy KT, Davidson WR, et al. Antisense inhibition of cyclin D1 expression is equivalent to flavopiridol for radiosensitization of zebrafish embryos. Int J Radiat Oncol Biol Phys. 2006;66:546–551. doi: 10.1016/j.ijrobp.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Rock KL, Gramm C, Rothstein L, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 30.Komarov PG, Komarova EA, Kondratov RV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 31.Komarova EA, Christov K, Faerman AI, Gudkov AV. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19:3791–3798. doi: 10.1038/sj.onc.1203717. [DOI] [PubMed] [Google Scholar]
- 32.Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 33.Berghmans S, Murphey RD, Wienholds E, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson W, Ren Q, Kari G, Kashi O, Dicker AP, Rodeck U. Inhibition of p73 function by Pifithrin-alpha as revealed by studies in zebrafish embryos. Cell Cycle. 2008;7:1224–1230. doi: 10.4161/cc.7.9.5786. [DOI] [PubMed] [Google Scholar]
- 35.Langheinrich U, Hennen E, Stott G, Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol. 2002;12:2023–2028. doi: 10.1016/s0960-9822(02)01319-2. [DOI] [PubMed] [Google Scholar]
- 36.Correa RG, Matsui T, Tergaonkar V, Rodriguez-Esteban C, Izpisua-Belmonte JC, Verma IM. Zebrafish IkappaB kinase 1 negatively regulates NF-kappaB activity. Curr Biol. 2005;15:1291–1295. doi: 10.1016/j.cub.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Correa RG, Tergaonkar V, Ng JK, Dubova I, Izpisua-Belmonte JC, Verma IM. Characterization of NF-kappa B/I kappa B proteins in zebra fish and their involvement in notochord development. Mol Cell Biol. 2004;24:5257–5268. doi: 10.1128/MCB.24.12.5257-5268.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fink MP. Ethyl pyruvate: a novel anti-inflammatory agent. J Intern Med. 2007;261:349–362. doi: 10.1111/j.1365-2796.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- 39.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5:3232–3239. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- 40.Thimmulappa RK, Fuchs RJ, Malhotra D, et al. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxid Redox Signal. 2007;9:1963–1970. doi: 10.1089/ars.2007.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yates MS, Tauchi M, Katsuoka F, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 42.Egan LJ, Eckmann L, Greten FR, et al. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ. 2006;13:773–784. doi: 10.1038/sj.cdd.4401843. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 46.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 47.Gerondakis S, Grumont R, Gugasyan R, et al. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006;25:6781–6799. doi: 10.1038/sj.onc.1209944. [DOI] [PubMed] [Google Scholar]
- 48.Vijay-Kumar M, Gewirtz AT. Guardians of the gut: newly appreciated role of epithelial toll-like receptors in protecting the intestine. Gastroenterology. 2008;135:351–354. doi: 10.1053/j.gastro.2008.06.064. [comment] [DOI] [PubMed] [Google Scholar]
- 49.Eckmann L, Nebelsiek T, Fingerle AA, et al. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groesdonk HV, Senftleben U. Modulation of inhibitor kappaB kinase/ nuclear factor kappaB signaling during critical illness: a double-edged sword. Crit Care Med. 2004;32:1239. doi: 10.1097/01.ccm.0000115255.43177.2c. [comment] author reply 1239–1240. [DOI] [PubMed] [Google Scholar]
- 51.Gao X, Deeb D, Jiang H, Liu Y, Dulchavsky SA, Gautam SC. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-kappaB and Notch1 signaling. J Neurooncol. 2007;84:147–157. doi: 10.1007/s11060-007-9364-9. [DOI] [PubMed] [Google Scholar]
- 52.Kress CL, Konopleva M, Martinez-Garcia V, et al. Triterpenoids display single agent anti-tumor activity in a transgenic mouse model of chronic lymphocytic leukemia and small B cell lymphoma. PLoS ONE [Electronic Resource] 2007;2:e559. doi: 10.1371/journal.pone.0000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyer ML, Croxton R, Krajewska M, et al. Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res. 2005;65:4799–4808. doi: 10.1158/0008-5472.CAN-04-3319. [DOI] [PubMed] [Google Scholar]
- 54.Chauhan D, Li G, Podar K, et al. The bortezomib/proteasome inhibitor PS-341 and triterpenoid CDDO-Im induce synergistic anti-multiple myeloma (MM) activity and overcome bortezomib resistance. Blood. 2004;103:3158–3166. doi: 10.1182/blood-2003-08-2873. [DOI] [PubMed] [Google Scholar]
- 55.Place AE, Suh N, Williams CR, et al. The novel synthetic triterpenoid, CDDO-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin Cancer Res. 2003;9:2798–2806. [PubMed] [Google Scholar]
- 56.Ito Y, Pandey P, Sporn MB, Datta R, Kharbanda S, Kufe D. The novel triterpenoid CDDO induces apoptosis and differentiation of human osteosarcoma cells by a caspase-8 dependent mechanism. Mol Pharmacol. 2001;59:1094–1099. doi: 10.1124/mol.59.5.1094. [DOI] [PubMed] [Google Scholar]
- 57.Ito Y, Pandey P, Place A, et al. The novel triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid induces apoptosis of human myeloid leukemia cells by a caspase-8-dependent mechanism. Cell Growth Differ. 2000;11:261–267. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Toxicity of pharmacological agents used in the present study. PS-341 (≤ 10 µM), MG132 (≤ 50µM), Lactascystin (≤ 10 µM), EP (≤ 10 mM) , CDDO (≤ 10µM) were added to EM for 7 days and survival scored at 7dpf. All compounds were used in toxicity assay at ranges exceeding the concentration used to determine modulation of the radiation response by such agents as indicated by asterices.
Figure S2: Targets and effects of pharmacological agents used in the present study on NF-κB activity in vitro. (A) Effects of the proteasome inhibitors PS-341 and MG132 as well as EP and CDDO-TFEA on TNF-α-induced NF-κB activity in HeLa cells. (B) Effects of compounds on baseline NF-κB activity in HeLa cells. (C) Conservation of human and zebrafish amino acid sequences flanking reactive cysteines (highlighted in yellow), which are the molecular targets of CDDO (Cys179; IKKβ) and EP (Cys38; NF-κBp65), respectively. In these experiments compounds were used at the concentrations equal to those used in radiation protection experiments in zebrafish embryos.
Figure S3: Effects of IR and PS-341 on steady-state transcript levels of the p53 target genes p21/Waf1, mdm2 and bax as determined by semi-quantitative PCR standardized to β-actin. The effects of EP and CDDO-TFEA on target gene expression are shown for comparison.
Figure S4: Lethal effect of morpholino-mediated NF-κB knockdown on zebrafish embryos. (A) Morphology of NF-κB-MO-treated embryos (B) Time-dependent survival of control, control-MO (random control morpholino, Gene-Tools) and NF-κB-MO-treated embryos.
Figure S5: EP but not CDDO is an effective ROS scavenger in irradiated zebrafish embryos. Zebrafish embryos were exposed to IR (20 Gy) at 24 hpf and ROS levels determined at 26 hpf, i.e., 2 h after IR as described in Materials and Methods.
Figure S6: Extended survival of irradiated zebrafish embryos pretreated with EP and CDDO-TFEA as determined at 15 dpf.
Supplemental Table S1: Primer sequences used in RT-PCR analysis of zebrafish mRNA