Abstract
Alzheimer’s disease will reach epidemic proportions within the next 20–30 years if left unchecked. Currently, there are no treatments that prevent or slow Alzheimer’s disease but many are being developed. Parallel efforts to develop biomarkers to aid in disease diagnosis and prognosis, and assess disease risk are currently underway. Clinicopathological and biomarker studies have demonstrated that Alzheimer’s disease pathology can be detected preclinically. Using biomarkers to identify affected individuals prior to the onset of clinical symptoms and associated synaptic/neuronal loss should enable novel clinical trial design and early mechanism-based therapeutic intervention. This article summarizes the most promising cerebrospinal fluid biomarkers, highlights novel applications and current challenges, and provides a prediction on how the field may evolve in 5–10 years.
Keywords: Alzheimer’s disease, amyloid-β, biomarkers, cerebrospinal fluid, preclinical Alzheimer’s disease, tau
Alzheimer’s disease (AD) is a progressive and fatal neurodegenerative disease that currently affects approximately 10.6 million people in the USA and Europe, with projected estimates reaching 15.4 million by the year 2030 (according to the Alzheimer’s Association) [101]. If left untreated, AD will soon become a public health crisis with significant personal and economic ramifications. At present, there are no effective treatments that will prevent the disease, halt its progression or delay its onset, although several therapeutic approaches are currently being developed and tested in clinical trials. Parallel efforts are being channeled into developing biomarkers (e.g., fluid and imaging) that would aid in disease diagnosis and prognosis, and assessing disease risk. Together, these combined endeavors have the potential to provide physicians with the tools to effectively diagnose and treat the disease, preferably even before the onset of cognitive decline.
AD pathologic hallmarks, clinical diagnosis & ‘preclinical AD’
At present, a definitive diagnosis of AD can only be obtained at autopsy via the histological quantification of two AD hallmarks: brain amyloid plaques (extracellular deposits composed predominantly of the amyloid-β [Aβ] peptide) and intraneuronal neurofibrillary tangles (NFTs, composed of hyperphosphorylated forms of the microtubule-associated protein, tau). These two hallmark histopathological features are used by three sets of diagnostic histological criteria (Khachaturian, Consortium to Establish a Registry for Alzheimer’s Disease and National Institute on Aging/Reagan) in defining a postmortem AD diagnosis [1–3]. However, in a typical clinical setting, individuals are diagnosed antemortem with possible or probable dementia of the Alzheimer type (DAT) if they meet National Institute of Neurological Disorders and Stroke/Diagnostic and Statistical Manual of Mental Disorders, version IV (NINDS/DSM-IV) clinical criteria. It is important to note that a diagnosis of DAT, as opposed to AD, is a diagnosis made purely on clinical grounds, without verification of neuropathological or biomarker evidence of the disease. Investigators at Washington University (MO, USA) developed a clinical dementia rating (CDR) scale with which cognition is rated (using an informant-based clinical assessment) as normal (CDR 0), very mildly demented (CDR 0.5), mildly demented (CDR 1), moderately demented (CDR 2) or severely demented (CDR 3) [4]. Clinicians then provide an additional diagnosis regarding the presumed underlying cause of the dementia (e.g., DAT, frontotemporal dementia or dementia with Lewy bodies). Most individuals meeting NINDS/DSM-IV clinical criteria for DAT are CDR 1 or greater. However, one important challenge has been to diagnose individuals at earlier stages, when clinical symptoms are less severe and, perhaps, more amenable to intervention. During these early stages (CDR 0.5; which often last 2–5 years or longer), the majority of individuals meet clinical criteria for mild cognitive impairment (MCI) [5] or even a stage of pre-MCI [6]. MCI is a diagnostic construct that defines impairment by comparing one’s performance on cognitive tests with that of age-matched normative values. Histopathologic studies have demonstrated that the majority of CDR 0.5 subjects meeting MCI criteria already exhibit florid AD pathology (amyloid plaques and NFTs), including significant neuronal loss [7]. Consistent with these findings, the vast majority of CDR 0.5, MCI, subjects evaluated at Washington University have been shown to develop dementia due to AD [8], suggesting that the clinical symptoms of MCI, in many cases, are indicative of the very earliest clinical stage of AD. Not surprisingly, diagnostic accuracy in clinical settings that do not specialize in dementia are much lower [9,10].
Even within specialized dementia centers and clinics, this diagnostic staging is not as simple as it appears for identifying individuals with AD pathology. Neuropathological studies involving large numbers of individuals have identified significant AD pathology in the brains of cognitively normal elders [11,12]. Neocortical cerebral amyloid deposits have been identified in approximately 50% of brains from individuals over 75 years of age [12]. By contrast, the prevalence of AD dementia (diagnosed clinically) does not reach 50% until age 85 or more [13]. It appears that the onset of very mild dementia is correlated best, not with plaque or NFT burden, but with significant synaptic and neuronal loss [14–16]. Together, these data support the concept of preclinical AD, a phase during which plaques, and subsequently NFT, accumulate for approximately 10–20 years before the onset of cognitive decline. This condition is analogous in many ways to cardiovascular disease, in which atherosclerosis begins many years prior to myocardial infarction or ischemic stroke, or Parkinson’s disease, in which clinical symptoms become apparent only after years of neuronal cell death in the substantia nigra. Together, these observations suggest an early and insidious pathogenesis of AD, the clinical manifestations of which become apparent only after substantial neuronal cell death and synapse loss have taken place. Such observations have important implications for the development of AD therapeutic and diagnostic strategies. Namely, it will be critical to identify individuals with pre-clinical AD, prior to the development of clinical symptoms and concomitant neuronal loss, since this is the group in which targeted therapies are likely to have the greatest chance of preserving normal brain function (Figure 1).
Figure 1. Biomarkers of Alzheimer’s disease.
Hypothesized changes in CSF biomarkers in relation to the time course of pathological and clinical stages, and other biomarker modalities. The clinical stages of AD, marked by progressive dementia described as very mild/MCI, mild, moderate, and severe, correspond with the CDR scores of 0.5, 1, 2, and 3, respectively. These stages are associated with abundant amyloid plaques (red line), the gradual accumulation of neurofibrillary tangles (blue line), synaptic and neuronal loss in certain brain regions (green line). In the preclinical stage of AD, Aβ42 peptide forms amyloid plaques in the brains of nondemented individuals (CDR 0) for approximately 10–20 years, and damages neuronal processes and synapses. Eventually, dramatic neuronal losses occur in association with dementia onset. AD biomarker research seeks to capture these changes in the brain, which might be useful for diagnosis and prognosis during this preclinical phase of AD, before irreversible neuronal loss occurs. These changes can be measured by biochemical examination of CSF, and/or a variety of radiological imaging modalities (e.g., CT, MRI, and PIB PET). The most promising CSF biomarkers to date have been Aβ42 and tau species, which show diagnostic and prognostic utility. BACE1 as an indicator of Aβ production warrants further study, as do panels of inflammatory markers and markers of oxidative stress. Genetic variations (e.g., SNPs) may also be considered biomarkers that allow the earliest possible estimation of risk.
Aβ: Amyloid-β;AD: Alzheimer’s disease; BACE: β-site amyloid precursor protein-cleaving enzyme; CDR: Clinical Dementia Rating; CSF: Cerebrospinal fluid; CT: Computed tomography; MCI: Mild cognitive impairment PIB: Pittsburgh compound-B; p-tau: Phosphorylated tau; SNP: Single nucleotide polymorphism.
Adapted with permission from [83].
Even in specialized dementia centers it is very difficult to diagnose AD at its earliest clinical stages. Furthermore, clinical measures, by definition, will not be able to identify preclinical cases. Thus, there is an urgent need for biomarkers (fluid, imaging and others) that will aid in identifying the disease at its most early clinical stages as well as preclinically. To date, biomarker studies have concentrated on two kinds of markers: diagnostic and prognostic. A diagnostic biomarker is a molecule, structure or other measure that defines the presence of a particular condition (e.g., dementia or amyloid deposition in the brain). A prognostic biomarker is a molecule, structure or other measure that is predictive of clinical outcome (e.g., conversion from MCI to DAT or from nondemented to demented). As disease-modifying therapies are being developed and treatments become available, there will be a greater need for diagnostic and prognostic biomarkers to decide who should enter particular clinical trials, for determining who should or should not receive a particular therapy, for determining the likelihood of disease progression and for tracking disease progression.
Potential of cerebrospinal fluid analytes as biomarkers for AD pathology
Short of a brain biopsy or directly placing a microdialysis catheter into the brain [17], cerebrospinal fluid (CSF), given its contiguity with the brain interstitial space, represents the most direct means to study the biochemical changes occurring in the CNS. To date, the major protein constituents of the pathology of AD (Aβ, tau and phosphorylated forms of tau) have emerged as the current leading diagnostic (and prognostic) CSF biomarkers in the research arena.
CSF Aβ42
Amyloid-β is a secreted peptide of unknown physiological function that is cleaved from the amyloid precursor protein by the sequential activities of β- and γ-secretase enzymes. The majority of Aβ is produced in the brain, and it is present in the CSF and plasma, appearing at relatively high and low levels, respectively. Aβ occurs in multiple forms ranging from 15 to 16 to up to 43 amino acids in length. Among these, Aβ40 is the most abundant species, but Aβ42 appears to be essential for initiating Aβ aggregation, and is considered central to the amyloid cascade hypothesis of AD [18]. The amyloid cascade hypothesis postulates a central initiating role for Aβ42 (in the form of oligomers, fibrils and/or plaques) in the subsequent pathological features of AD, including glial activation and neuroinflammation, synapse and neuritic dysfunction, tau hyperphosphorylation and development of NFTs, and ultimate synaptic and neuronal cell death, with resultant cognitive deficits. Reflecting their roles in the pathogenesis of AD, Aβ42 has emerged as a more useful biomarker for AD than its shorter counterpart, Aβ40. In addition, the ratio of CSF Aβ40:Aβ42 has also demonstrated promise by improving the reliability of clinical diagnosis compared with either analyte on its own [19]. CSF Aβ38 has also been evaluated in a few studies. The presence of Aβ38 in CSF was initially demonstrated by immunoblotting-based methods, and additional studies from the same group confirmed that there appeared to be an increase in this species compared with samples obtained from non-AD dementias [20–23]. The ratios of Aβ42:Aβ38 appeared to provide higher specificities for distinguishing AD from non-AD dementias than either species on its own. However, in many independent studies, comprising approximately 2000 subjects across many different research centers, the mean levels of Aβ42 (but not Aβ40) in CSF have been consistently reported to be decreased (by ~30–50%) in individuals who, on clinical grounds, have a diagnosis of DAT, compared with age-matched, nondemented controls [24–29]. However, in all studies, there is considerable overlap between individual values in the clinical groups, likely due to possible misdiagnoses of DAT in individuals with other, non-AD pathological substrates for their dementia (e.g., Lewy bodies), especially in the early clinical stages, as well as possible contamination of the nondemented control groups by preclinical AD cases. Estimates of the prevalence of preclinical AD in this age group (>60 years old) range from approximately 25 to 40% [11,14,16]. Mean CSF Aβ42 has been shown to be decreased in individuals diagnosed with very mild and mild DAT [24,25,30], as well as a subset of MCI individuals (e.g., typically ‘amnestic MCI’) [26]), consistent with earlier studies. However, values for MCI subjects demonstrate a substantial overlap with control values. β-secretase (BACE 1), one of the primary enzymes involved in the cleavage of amyloid precursor protein to Aβ, has also recently been quantified in the CSF. BACE 1 levels and activity have been reported to be increased in the CSF of MCI subjects compared with normal controls and subjects with DAT, suggesting that it may have utility as a biomarker of early-stage AD and as a predictor of subsequent cognitive decline [31]. Additional studies of this putative biomarker is warranted. CSF BACE 1 levels have also been shown to be influenced by the APOE genotype, the strongest genetic risk factor for AD [32], thus providing insight into possible mechanistic effects of the ε4 allele of APOE on levels of CNS Aβ.
While at first glance these data appear to be less than supportive of CSF Aβ42 being a clinically viable AD biomarker, providing adequate sensitivity (the ability to accurately identify people with AD pathology) and specificity (the ability to discriminate individuals with AD from those with other dementing disorders) above and beyond what can be diagnosed upon expert clinical evaluation, our group has recently demonstrated a very strong inverse relationship between CSF Aβ42 levels and in vivo cortical amyloid load as measured by PET imaging with Pittsburgh Compound B (PIB) [33]. Our findings demonstrated that a low level of CSF Aβ42 is an excellent marker of cortical amyloid deposition, regardless of clinical diagnosis [34,35]. Others have since reported this same relationship between PIB retention and CSF Aβ42 in MCI [36–38] and in DAT [38,39]. Importantly, we observed several cognitively normal elderly individuals in our original cohort who had PIB-positive cortical amyloid and low CSF Aβ42 levels, as one would predict given the prevalence of preclinical AD in this older age group. We have since gone on to validate this finding in a much larger cohort of nondemented individuals [40]. Thus, CSF Aβ42 can be considered a diagnostic biomarker for cortical amyloid deposition. In this larger cohort, 25 of the 189 CDR 0 subjects fell into this putative preclinical AD category (high PIB binding, low CSF Aβ42) (Figure 2). This finding provides support for the idea that the inadequate sensitivity and specificity of CSF Aβ42 for distinguishing clinical groups likely reflects contamination of the control group with preclinical cases of AD [41] and probably misdiagnoses of non-AD dementias in early-stage DAT groups. Whether low CSF Aβ42 is a good prognostic biomarker, identifying nondemented individuals who will go on to dement, remains to be determined. However, a very recent study has reported initial evidence that cognitively normal individuals with elevated cortical PIB retention are at a significantly greater risk for developing the symptomatic stages of AD than individuals with little or no PIB retention [42].
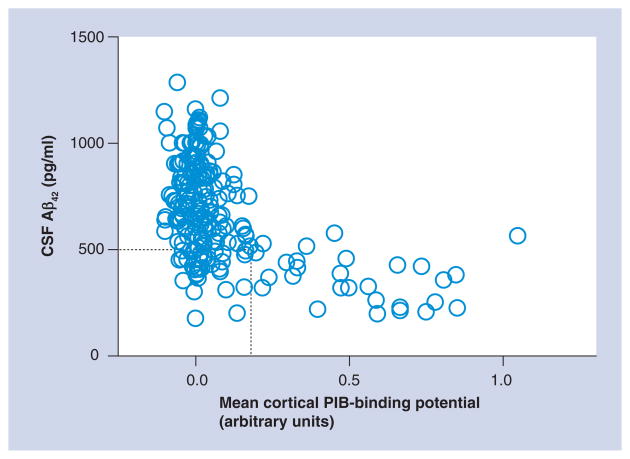
Figure 2. Levels of cerebrospinal fluid amyloid-β42 in cognitively normal individuals as a function of cortical amyloid load as assessed by the amyloid-binding agent Pittsburgh Compound B.
The majority of individuals in this cohort (n = 164) exhibited low or no cortical PIB binding (PIB-binding potentials clustering around zero). However, a subset (n = 25) of individuals exhibited positive binding for PIB, similar in level and distribution to what is observed in subjects with dementia of the Alzheimer type (data not shown). All of the PIB-positive individuals had relatively low levels of CSF Aβ42, whereas PIB-negative individuals had high levels of CSF Aβ42, indicating that low CSF Aβ42 is an excellent marker of cortical amyloid. A subset of individuals exhibited low CSF Aβ42 in the absence of cortical PIB binding (within the dashed box). Longitudinal follow-up of these individuals will be required to determine whether they eventually become PIB-positive or if they represent a different subgroup (e.g., those with diffuse, PIB-negative, plaques or are just reflective of the low end of the normal CSF Aβ42 spectrum).
Aβ: Amyloid-β; CSF: Cerebrospinal fluid; PIB: Pittsburgh Compound B.
Adapted with permission from [84].
Low CSF Aβ42 may also serve as a harbinger of amyloid deposition itself. Recently, we have identified a unique class of nondemented individuals who exhibit low CSF Aβ42 but show no evidence of amyloid on PET PIB scans [40] (Figure 2, sector outlined by dotted line). Longitudinal PIB follow-up of the participants in this lower quadrant (low PIB, low CSF Aβ42) will be required to understand whether the low CSF Aβ42 represents Aβ aggregation in diffuse (PIB-negative) plaques, oligomeric forms prior to substantial fibrillar (PIB-positive) Aβ deposition or simply reflects the low end of the normal spectrum of CSF Aβ42 levels. In support of the first hypothesis, one of these individuals was recently autopsied and demonstrated widespread diffuse – but minimal fibrillar – amyloid plaque deposits [43], suggesting low CSF Aβ42 may also be a marker of diffuse (nonfibrillar) plaques in addition to PIB-positive fibrillar plaques. Longitudinal follow-up (with clinical, PIB and CSF measures) of such subjects will be important for understanding the biological relevance of this pattern. However, taken together, these observations demonstrate that CSF Aβ42 may have utility as a biomarker for diagnosis, plaque burden and prognosis, and indeed, may provide the very earliest clue to identify preclinical AD (as defined by the emergence of Aβ deposition).
CSF total tau & phosphorylated tau
A great number of studies have reported elevated levels of CSF total tau (and hyperphosphorylated forms) in AD [24,26,28,29]. However, similar to Aβ42, there is a significant overlap between individual tau values in MCI/AD and control groups, especially in the early disease stages. Furthermore, the increase in tau is not specific for AD (although increases in phosphorylated tau [p-tau] appear relatively specific for AD) [44–46]. Unlike Aβ, which is a secreted peptide, tau is a microtubule-associated protein found within the cytosol of neurons. In AD, tau becomes hyperphosphorylated and twists to form paired helical filaments, constituents of NFTs. Elevations in CSF p-tau levels have been shown to be correlated with NFT load in autopsy studies [27,47], thus it appears to correlate with the presence of NFTs. However, it is unclear whether increases in CSF total tau in AD reflect NFT burden or whether it is due to an increase in the release of this structural protein in the presence of damaged axons, dendrites, synapses or even with cell death. Consistent with this latter idea, CSF tau has been shown to increase after head trauma [48,49] and acute stroke [48,50]. However, since tau is normally found in the CSF at appreciable levels in all ages, it is likely that there are normal pathways for its metabolism that have yet to be elucidated.
Clinicopathological studies have demonstrated that AD pathology, both plaques and NFTs, develop very early in the disease process, many years prior to the onset of clinical dementia. Although tangles increase normally with age [11], CSF studies and amyloid imaging have suggested that changes in Aβ metabolism and its aggregation into insoluble amyloid plaques precedes the acceleration of NFT pathology characteristic of AD [40]. This finding is consistent with the amyloid cascade hypothesis. However, these markers (CSF Aβ42 and tau), once they are altered, generally remain stable throughout the course of the disease and do not change appreciably with disease progression [51,52]. Instead, dementia severity is correlated best with synaptic and neuronal cell death [53]. While CSF Aβ42 and tau correlate with global and regional measures of brain atrophy in cross-sectional studies of nondemented aging and DAT [54], results have been conflicting in the few longitudinal CSF studies looking at these analytes as markers of disease severity [51]. Thus, there is a great need for additional markers that correlate with dementia severity and ongoing neurodegeneration.
CSF biomarker combinations
While a number of studies have demonstrated that CSF Aβ40 is unchanged in AD [35,55–57], the ratio of Aβ42:Aβ40, rather than either marker alone, has been demonstrated to better distinguish AD subjects from controls or other dementias, and to identify incipient AD in subjects with MCI [19,57,58]. More recent studies have reported an added benefit in discriminating DAT from controls when combining CSF tau, p-tau, Aβ42 and Aβ38 (and their various ratios) [59,60]. These changes in CSF biomarker profiles have also been shown to have a prognostic value at very early disease stages. Ratios of CSF tau (and p-tau) to Aβ42 have been reported to strongly predict further cognitive decline in MCI patients [61] and those with very mild DAT [62], as well as predicting future dementia in nondemented cohorts [35,63].
The need for markers of CNS inflammation
Neuroinflammation, in the way of glial hypertrophy and hyperplasia (especially in the vicinity of amyloid plaques), is a robust but nonspecific feature of AD. A number of reports published in the 1990s and early 2000s describe alterations in the levels of various cytokines, markers of oxidative stress and inflammatory molecules (e.g., α1-antichymotrypsin, isoprostane, the interleukins, TNF-α, IFN-γ, complement C1q and TGF-β) in DAT CSF. However, results were very inconsistent, likely owing to methodological differences, differences in CSF collection and processing, assay differences and differences in subject ascertainment, prevalence of comorbidities and methods of diagnosis. Unbiased proteomics methods have more recently been used to identify molecules that differ between DAT and control CSF (as well as serum and plasma). These studies have consistently identified a plethora of inflammatory markers that differ in abundance between clinical groups [64–67]. However, even in these unbiased screens, the direction of reported difference in abundance has not been consistent. Ongoing studies by our group, designed to assay a large panel of inflammatory markers in the CSF and plasma from a large cohort of nondemented and early-stage AD subjects should provide a rich dataset from which to draw some definitive conclusions regarding the potential of neuroinflammatory molecules as biomarkers of AD. It is conceivable (and probable) that adding markers of neuroinflammation to the other CSF markers (e.g., Aβ42, tau and p-tau) will further strengthen the diagnostic and prognostic capability.
The potential power of combinations of biomarker modalities
Although the studies discussed above have demonstrated that combinations of CSF biomarkers can discriminate DAT from control groups better than the individual CSF measures on their own and can predict future cognitive decline in nondemented cohorts, analyses of combinations of biomarker modalities are just beginning to be reported. Such combinations may prove to be more accurate than single modalities on their own and may also provide information regarding pathophysiological mechanisms of disease. To date, studies have focused mainly on correlations between biomarker modalities as a way to better understand the pathophysiology of the disease. As mentioned above, studies looking at the relationship between PIB amyloid imaging and CSF markers have shown that a low level of CSF Aβ42 is a robust marker of amyloid in the brain, regardless of clinical diagnosis [34,35,63]. In addition, cortical PIB binding has been demonstrated to correlate with cerebral atrophy (via MRI) in subjects clinically diagnosed with DAT, suggesting that amyloid deposition is associated with neuron loss in AD [68]. A recent study by our group suggests that reductions in CSF Aβ42 (and Aβ aggregation/amyloid deposition, by extension) are also associated with neurodegeneration in the preclinical phase [54]. Other CSF/MRI studies have demonstrated correlations between longitudinal increases in CSF p-tau levels, and increased rates of hippocampal atrophy in MCI [69] and DAT [70], suggesting that increases in p-tau may be indicative of progressive neurodegeneration in AD. This is consistent with our findings of inverse correlations between levels of CSF tau and p-tau and whole-brain volume in subjects diagnosed with DAT [54]. Together, these data suggest that Aβ aggregation and deposition are associated with brain atrophy in the preclinical phase, whereas changes in CSF tau and accelerated brain atrophy are later events in the disease that occur with or just prior to cognitive decline and subsequent clinical progression (Figure 1). Thus, combinations of biomarker modalities may have utility for determining where a person is in this progressive neurodegenerative cascade. Consistent with this possibility, recent studies of a small number of subjects reported that baseline CSF isoprostane level, a proposed marker of oxidative stress, and its change over time, added to the diagnostic accuracy of hippocampal volume measures in classifying MCI (n = 6) versus normal controls (n = 11) [69,71].
Novel applications of CSF biomarkers in AD studies
Use of CSF markers as endophenotypes in genetic studies
Just as CSF biomarker data from well-characterized, longitudinally followed cohorts of study participants may be used to guide diagnosis and estimate prognosis, it can also be used to identify genetic markers that are associated with AD risk. Compared with typical genetic studies of AD that rely on less precise clinical diagnoses, genetic studies based on quantitative endophenotype data can provide more power. In support of this approach, recent studies have demonstrated that elevated CSF tau and p-tau levels are associated with single nucleotide polymorphisms in the MAPT gene (from which tau protein is produced) [72]. Likewise, CSF Aβ levels have been found to associate with polymorphisms in several genes [73]. In this way, by converting endophenotype data derived from fluid biomarkers to novel genetic risk factors, it may be possible to identify individuals at a greater risk of developing AD and, in the near future, provide treatment options prior to the development of any AD pathology.
Investigating CNS protein metabolism via analysis of CSF in real time
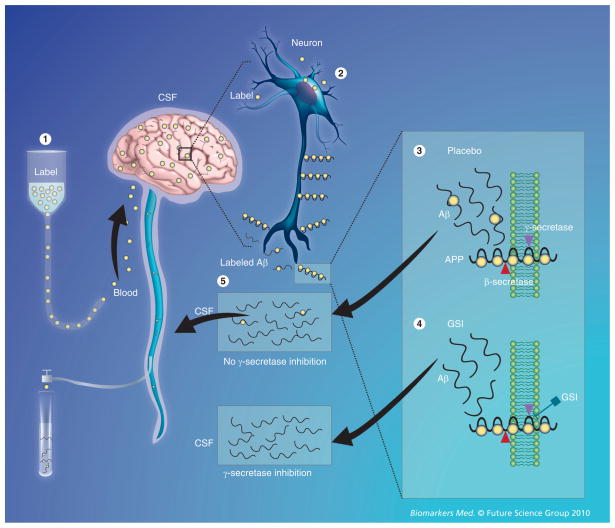
Recently, a new in vivo technique has been developed to measure the production and clearance rates of CNS proteins in human subjects. In this technique, a stable (nonradioactive) isotope-labeled amino acid (e.g., 13C6-leucine) is administered intravenously and becomes incorporated into newly synthesized proteins. CSF (and plasma) can then be sampled over time via intrathecal and intravenous catheters. Using mass spectrometry to compare labeled versus unlabeled proteins over time, very precise synthesis, clearance and dose–response curves can be developed. This technique was first applied to determine the synthesis and clearance rates of Aβ in the CNS [74]. The fractional production and clearance rates of Aβ in vivo was found to be extremely rapid (7.6 and 8.3% per h, respectively), with absolute concentrations in the CSF varying widely between sampling times. This technique was used more recently in a randomized, double-blind, placebo-controlled study to demonstrate the pharmacokinetic/pharmodynamic relationship between an Aβ synthesis inhibitor and the absolute rate of CNS Aβ synthesis (Figure 3) [75]. Since this technique automatically labels all newly synthesized proteins, its potential lies in the fact that it allows for the evaluation of other proteins relevant to AD, other neurodegenerative diseases and the metabolism of multiple biomarkers simultaneously.
Figure 3. In vivo CNS stable isotope-labeled kinetics of Aβ production and the effect of its inhibition by a γ-secretase inhibitor. (1).
A stable isotope-labeled amino acid is infused into the bloodstream and is transported to the brain. (2) The labeled amino acid is incorporated into newly synthesized proteins (e.g., APP in neurons). (3) Labeled APP is cut by β- and γ-secretases to produce labeled Aβ, or in the presence of a GSI (4) labeled Aβ production is inhibited. (5) Labeled and unlabeled Aβ is transported and cleared through the CSF, which is in direct communication with the extracellular space of the brain, where sampling occurs over time to measure the production and clearance of Aβ.
Aβ: Amyloid-β; APP: Amyloid precursor protein; CSF: Cerebrospinal fluid; GSI: γ-secretase inhibitor.
Reproduced with permission from [85].
Challenges in CSF biomarker studies
The search for CSF biomarkers has been fraught with many challenges that have contributed to slow progress. First, the identification of reliable CSF (or any) biomarkers has been hindered by the fact that patient/subject classification relies on clinical diagnosis, which is not always accurate, especially during early stages of the disease. Second, obtaining postmortem confirmation of disease diagnosis, while serving as the gold standard, is very impractical, especially for biomarkers of early clinical and preclinical stages. Third, as mentioned earlier, clinical measures, by definition, will not be able to identify those individuals with preclinical disease; therefore, control groups will always contain individuals with preclinical AD, thus adversely influencing estimates of biomarker sensitivity and specificity. Fourth, limited patient/subject sample size and the lack of adjustment for covariates, such as age, gender, ethnicity and APOE genotype, have restricted the application of results from some studies to the general population. Fifth, protocols for sample collection, preparation and analysis often vary widely between laboratories, thus contributing additional methodological variability and limiting the ability to compare studies directly. Indeed, preanalytical sample handling procedures have been shown to significantly affect CSF Aβ42 values [76]. Furthermore, a recent multicenter study demonstrated high intersite assay variability in measurements of CSF Aβ42, tau and p-tau181, highlighting the importance of method standardization for biomarker assessment and validation [26]. These issues have also stymied the defining of cut-off values, a metric that will have to be employed once CSF biomarkers become a reality in the clinical arena. Initiation of large-scale, standardized studies, such as AD Neuroimaging Initiative (ADNI and related ADNI-type studies in Europe and Japan) and the German Dementias Competence Network study are direct responses to this challenge. Adopting standardized protocols for clinical assessment, sample analysis and statistical evaluation between individual laboratories would also help overcome many of these shortcomings, and efforts are underway to do just this. Sixth, efforts must be directed towards educating the public, as well as the scientific and medical communities, regarding the true safety of the lumbar puncture procedure for obtaining CSF [77]. Lack of information and frank misinformation has resulted in difficulties with patient/subject enrollment into research studies in the USA and, thus, eventually into clinical trials. This barrier has not been experienced by our European colleagues since the majority of CSF biomarker studies are carried out on patients seen in memory clinics where lumbar puncture is a standard practice of care. Indeed, in some European countries (e.g., Sweden and Germany), CSF biomarkers are not considered simply as analytes for research purposes but instead are routinely obtained and used in the actual diagnostic procedure, even in nonresearch hospitals.
Future perspective
As a first step, greater numbers of standardized, large-scale, longitudinal biomarker studies are required to validate the core analytes that have demonstrated the most promise to date (e.g., Aβ42, tau and p-tau). Such studies are currently underway. Additional unbiased and targeted proteomic screens using CSF samples from subjects with defined pathology (e.g., from subjects with autopsy confirmation soon after CSF collection, or amyloid imaging or known CSF Aβ42 and tau levels) will be useful for novel biomarker discovery, which will augment current markers providing the results from such screens can be validated with independent quantitative methods (e.g., ELISA) in additional cohorts. Given the multifactorial nature of the disease, it is unlikely that a single biomarker will meet the needs for clinical diagnosis; however, a panel of biomarkers may offer the appropriate sensitivity, specificity, and positive and negative predictive values. Large, longitudinal studies employing multiple biomarker modalities within the same individual, such as ADNI [78], European ADNI [28,79], Japanese ADNI [80], the Adult Children Study [81], the German Dementias Competence Network [82] and Dominantly Inherited Alzheimer Network (DIAN) [102], are currently underway and should prove to be incredibly powerful in identifying such biomarker panels.
Therapy development and biomarker discovery must occur in parallel. The real-time in vivo analysis of CNS protein production and clearance pioneered by Bateman and colleagues can be used to directly determine drug effects in the CNS in early-phase human trials [74]. Utilization of this technique will greatly accelerate drug/therapy development by allowing one to determine the efficacy of its effect on the defined target, as well as determining the optimal dose and timing, thus increasing the probability of success in later Phase III trials [75].
In the next 5–10 years, the evolving appreciation of the preclinical stage of AD will likely lead to a paradigm shift in the way Alzheimer researchers and clinicians design and implement clinical trials of promising disease-modifying therapies. To date, all therapeutic trials have utilized clinical criteria (MCI or early DAT) for patient enrollment despite the fact that by the time any clinical symptoms are apparent, significant AD pathology has already developed, including substantial synaptic and neuronal loss. Therefore, it comes as no surprise that no treatment to date has demonstrated a disease-modifying effect. Instead, prevention trials are required. Such trials, under the current paradigm, would be too costly in the terms of time (years of clinical follow-up), money and resources (numbers of nondemented patients to be enrolled to provide adequate statistical power). To circumvent such roadblocks, CSF biomarkers can be used to design and evaluate such a prevention trial. The use of biomarkers would allow one to enroll individuals who are cognitively normal but who are in the preclinical stages of the disease and, importantly, are within a few years of developing cognitive symptoms. Such a strategy would not only benefit from, but would necessitate, the use of biomarkers as inclusion/exclusion criteria (e.g., low CSF Aβ42 for identifying preclinical AD and high CSF tau/Aβ42 for identifying those who are within a few years of developing symptoms). CSF biomarkers, in combination with sensitive cognitive outcome measures, could also be used to monitor response to therapy, especially for therapies designed to directly influence Aβ42 and/or tau (either as direct targets or as possible surrogates for amyloid and/or neurodegeneration, respectively). Ultimately, such biomarkers could be used to assist in making treatment decisions as more invasive and potentially harmful disease-modifying treatments for AD become available.
Executive summary
Introduction
There are currently no proven treatments that will prevent or delay the onset of Alzheimer’s disease (AD), although many are under development.
Parallel efforts are being channeled into developing biomarkers to aid in disease diagnosis, and prognosis and assessing disease risk.
AD pathologic hallmarks, clinical diagnosis & ‘preclinical AD’
A definitive diagnosis of AD can only be determined at autopsy by quantifying amyloid plaques and neurofibrillary tangles, the hallmark pathologic lesions of AD.
Diagnoses based on clinical criteria are not always accurate, especially in the early disease stages.
AD pathology is estimated to begin 10–20 years prior to the appearance of any cognitive signs or symptoms (preclinical AD).
The potential of cerebrospinal fluid analytes as biomarkers for AD pathology
Levels of cerebrospinal fluid (CSF) amyloid-β42 (Aβ42) are reduced in AD, even in early and preclinical stages of the disease.
A low level of CSF Aβ42 is an excellent marker of cortical amyloid deposition in the brain (as assessed by in vivo amyloid imaging with Pittsburgh Compound-B).
Levels of CSF total tau and phosphorylated tau (p-tau) increase in AD and accelerate during later disease stages, concomitant with neurofibrillary tangle formation and synapse and neuron loss.
The ratios of CSF tau/Aβ42 and p-tau/Aβ42 have been shown to be predictive of cognitive decline in individuals with mild cognitive impairment as well as in the cognitively normal elderly.
CNS inflammation (glial hypertrophy and hyperplasia) is a nonspecific feature of AD, but CSF inflammatory markers have been under-investigated.
The potential power of combinations of biomarker modalitie
Analyses of combinations of biomarker modalities are just beginning to be reported.
Such combinations (e.g., CSF and imaging) may prove to be more accurate for AD diagnosis and prognosis than single modalities on their own.
Novel applications of CSF biomarkers in AD studies
Using CSF markers as endophenotypes provides more power for identifying novel genetic markers than the typical clinical diagnosis.
Elevated CSF tau and p-tau levels are associated with single nucleotide polymorphisms in the MAPT gene (from which tau protein is produced).
CSF Aβ levels have been found to associate with polymorphisms in several genes.
A novel in vivo technique has been developed to measure the production and clearance rates of CNS proteins in humans.
The fractional production and clearance rates of Aβ in vivo were found to be extremely rapid, with absolute concentrations in the CSF varying widely between sampling times.
Challenges in CSF biomarker studies
Identification of reliable CSF biomarkers has been hindered by the fact that patient/subject classification relies on clinical diagnosis, which is not always accurate, especially at early stages of the disease.
Obtaining postmortem confirmation of disease diagnosis, while serving as the gold standard, is very impractical, especially for biomarkers of early clinical and preclinical stages.
Clinical measures, by definition, will not be able to identify those individuals with preclinical disease; therefore, ‘control groups’ will always be ‘contaminated’ with individuals with preclinical AD.
Limited patient/subject sample size and lack of adjustment for covariates have restricted the application of results from some studies to the general population.
Protocols for sample collection, preparation and analysis often vary widely between laboratories, thus contributing additional methodological variability and limiting the ability to compare studies directly.
Efforts must be directed towards educating the public, as well as the scientific and medical communities, regarding the true safety of the lumbar puncture procedure for obtaining CSF.
Future perspective
Greater numbers of standardized, large-scale, longitudinal biomarker studies are required to validate the core analytes that have demonstrated the most promise to date (e.g., Aβ42, tau and p-tau).
Additional unbiased and targeted proteomic screens using CSF samples from subjects with defined pathology will be useful for novel biomarker discovery that will augment current markers.
Panels of biomarkers may offer the appropriate sensitivity, specificity, and positive and negative predictive values required for ultimate clinical utility.
The novel in vivo CSF sampling technique can be used to directly determine drug effects in the CNS in early-phase human trials.
CSF biomarkers can be used to design and evaluate prevention trials by allowing one to enroll individuals who are cognitively normal but who are in the preclinical stages of the disease and, importantly, are within a few years of developing cognitive symptoms.
CSF biomarkers, in combination with sensitive cognitive outcome measures, could also be used to monitor response to therapy.
Ultimately, such biomarkers could be used to assist in making treatment decisions as more invasive and potentially harmful disease-modifying treatments for AD become available.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
AM Fagan and DM Holtzman are supported by grants P50 AG05681, P01 AG03991, P01 AG026276 and P30 NS057105 from the National Institute of Aging of the National Institutes of Health; UL1 RR024992, National Center for Research Resources, NIH Roadmap for Medical Research, and the Charles and Joanne Knight Alzheimer Research Initiative. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Mirra S, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 2.Khachaturian Z. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 3.Hyman B, Trojanowski J. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Morris J. The clinical dementia rating (CDR). Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 5.Petersen R, Smith G, Waring S, Ivnik R, Tabgalos E, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.Storandt M, Grant E, Miller J, Morris J. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 7.Price J, Ko A, Wade M, Tsou S, McKeel D, Morris J. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer’s disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 8.Morris J, Storandt M, Miller J, et al. Mild cognitive impairment represents early-stage Alzheimer’s disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 9.Cummings J, Jeste D. Alzheimer’s disease and its management in the year 2010. Psychiatr Serv. 1999;50:1173–1177. doi: 10.1176/ps.50.9.1173. [DOI] [PubMed] [Google Scholar]
- 10.Teel C. Rural practitioners’ experiences in dementia diagnosis and treatment. Aging Ment Health. 2004;8:422–429. doi: 10.1080/13607860410001725018. [DOI] [PubMed] [Google Scholar]
- 11▪.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. A classic neuropathological study that defines the neuroanatomical development of Alzheimer’s disease (AD) neuropathological lesions with aging. [DOI] [PubMed] [Google Scholar]
- 12.Price J, Mckeel D, Buckles V, et al. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans D, Funkenstein H, Albert M, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 14▪.Morris J, Price J. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. Clinicopathological study correlating AD lesions with different stages of aging and development of AD dementia. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Isla T, Price J, McKeel D, Morris J, Growdon J, Hyman B. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markesbery W, Schmitt F, Kryscio R, Davis D, Smith C, Wekstein D. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 17▪.Brody D, Magnoni S, Schwetye K, et al. Amyloid-β dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. Elegant study using intracerebral microdialysis in living patients suffering from acute brain injury to measure analytes in serial samples of brain interstitial fluid and demonstrates a correlation between changes in amyloid-β (Aβ) concentration and neurological status. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy J, Selkoe D. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 19.Wiltfang J, Esselmann H, Bibl M, et al. Amyloid β peptide ratio 42/40 but not Aβ 42 correlates with phospho-tau in patients with low- and high-CSF Aβ 40 load. J Neurochem. 2007;101:1053–1059. doi: 10.1111/j.1471-4159.2006.04404.x. [DOI] [PubMed] [Google Scholar]
- 20.Wiltfang J, Esselmann H, Bibl M, et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Aβ peptides 1–37/38/39 in addition to 1–40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J Neurochem. 2002;81:481–496. doi: 10.1046/j.1471-4159.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 21.Bibl M, Mollenhauer B, Esselmann H, et al. CSF amyloid-β-peptides in Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease dementia. Brain. 2006;129:1177–1187. doi: 10.1093/brain/awl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bibl M, Mollenhauer B, Esselmann H, et al. CSF diagnosis of Alzheimer’s disease and dementia with Lewy bodies. J Neural Transm. 2006;10 doi: 10.1007/s00702-006-0537-z. [DOI] [PubMed] [Google Scholar]
- 23.Bibl M, Mollenhauer B, Lewczuk P, et al. Validation of amyloid-β peptides in CSF diagnosis of neurodegenerative dementias. Mol Psychiatry. 2007;12:671–680. doi: 10.1038/sj.mp.4001967. [DOI] [PubMed] [Google Scholar]
- 24.Sunderland T, Linker G, Mirza N, et al. Decreased β-amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer’s disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 25.Craig-Schapiro R, Fagan A, Holtzman D. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35:128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 27.Tapiola T, Alafuzoff I, Herukka S, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 28.Buerger K, Frisoni G, Uspenskaya O, et al. Validation of Alzheimer’s disease CSF and plasma biological markers: the multicentre reliability study of the pilot European Alzheimer’s Disease Neuroimaging Initiative (E-ADNI) Expl Gerontol. 2009;44:579–585. doi: 10.1016/j.exger.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Visser P, Verhey F, Knol D, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 30.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Z, Ewers M, Teipel S, et al. Levels of β-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry. 2007;64:718–726. doi: 10.1001/archpsyc.64.6.718. [DOI] [PubMed] [Google Scholar]
- 32.Ewers M, Zhong Z, Bürger K, et al. Increased CSF-BACE 1 activity is associated with APOE-ε4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain. 2008;131:1252–1258. doi: 10.1093/brain/awn034. [DOI] [PubMed] [Google Scholar]
- 33▪▪.Klunk W, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. Representing the first human study of amyloid-imaging PET tracer compound Pittsburgh Compound-B (PIB), this report describes retention of PIB in areas of AD brains known to contain amyloid, and additionally describes an inverse relationship between PIB PET signal and cerebral glucose metabolism, measured by FDG-PET. [DOI] [PubMed] [Google Scholar]
- 34▪▪.Fagan A, Mintun M, Mach R, et al. Inverse relation between in vivo amyloid imaging load and CSF Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. Illustrates an inverse relationship between mean cortical retention of amyloid-imaging PET tracer compound PIB and cerebrospinal fluid (CSF) Aβ42 levels among demented and nondemented subjects alike, suggesting that brain amyloid deposition results in low CSF Aβ42, and that amyloid imaging and CSF Aβ42 might serve as antecedent biomarkers of preclinical AD. [DOI] [PubMed] [Google Scholar]
- 35.Fagan A, Roe C, Xiong C, Mintun M, Morris J, Holtzman D. Cerebrospinal fluid tau/Aβ42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 36.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 37.Koivunen J, Pirttilä T, Kemppainen N, et al. PET amyloid ligand [11C]PIB uptake and cerebrospinal fluid β-amyloid in mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;26:378–383. doi: 10.1159/000163927. [DOI] [PubMed] [Google Scholar]
- 38.Jagust W, Landau S, Shaw L, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimmer T, Riemenschneider M, Förstl H, et al. β amyloid in Alzheimer’s disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagan A, Mintun M, Shah A, et al. Cerebrospinal fluid tau and p-tau181 increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aizenstein H, Nebes R, Saxton J, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪▪.Morris J, Roe C, Grant E, et al. Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. Demonstrates that the presence of brain amyloid in cognitively normal individuals is not benign but rather is predictive of future cognitive decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cairns N, Ikonomovic M, Benzinger T, et al. Absence of Pittsburgh Compound B detection of cerebral amyloid β in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease. Arch Neurol. 2009;66(12):1557–1562. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh N, Arai H, Urakami K, et al. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer’s disease. Ann Neurol. 2001;50:150–156. doi: 10.1002/ana.1054. [DOI] [PubMed] [Google Scholar]
- 45.Buerger K, Teipel S, Zinkowski R, et al. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002;59:627–629. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- 46.Hampel H, Mitchell A, Blennow K, et al. Core biological marker candidates of Alzheimer’s disease – perspectives for diagnosis, prediction of outcome and reflection of biological activity. J Neural Transm. 2004;111:247–272. doi: 10.1007/s00702-003-0065-z. [DOI] [PubMed] [Google Scholar]
- 47.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129(Pt 11):3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 48.Ost M, Nylén K, Csajbok L, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injuries. Neurology. 2006;67:1600–1604. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- 49.Blennow K, Nellgård B. Amyloid β 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2004;62:159. doi: 10.1212/wnl.62.1.159. [DOI] [PubMed] [Google Scholar]
- 50.Yao C, Williams A, Ottens A, et al. Detection of protein biomarkers using high-throughput immunoblotting following focal ischemic or penetrating ballistic-like brain injuries in rats. Brain Inj. 2008;22:723–732. doi: 10.1080/02699050802304706. [DOI] [PubMed] [Google Scholar]
- 51.Zetterberg H, Pedersen M, Lind K, et al. Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimers Dis. 2007;12:255–260. doi: 10.3233/jad-2007-12307. [DOI] [PubMed] [Google Scholar]
- 52.Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007;419:18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 53.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 54.Fagan A, Head D, Shah A, et al. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:175–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuyama R, Mizuno T, Mizuno T, et al. Age-dependent changes in the levels of Aβ40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ42 to Aβ40 level in cerebrospinal fluid from Alzheimer’s disease patients. Eur Neurol. 2000;43:155–160. doi: 10.1159/000008156. [DOI] [PubMed] [Google Scholar]
- 56.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid β proteins 1–40 and 1–42 in Alzheimer’s disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 57.Lewczuk P, Esselmann H, Otto M, et al. Neurochemical diagnosis of Alzheimer’s dementia by CSF Aβ42, Aβ42/Aβ40 ratio and total tau. Neurobiol Aging. 2004;25:273–281. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 58.Hansson O, Zetterberg H, Buchhave P, et al. Prediction of Alzheimer’s disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 59.Welge V, Fiege O, Lewczuk P, et al. Combined CSF tau, p-tau181 and amyloid-β 38/40/42 for diagnosing Alzheimer’s disease. J Neural Transm. 2009;116:203–212. doi: 10.1007/s00702-008-0177-6. [DOI] [PubMed] [Google Scholar]
- 60.Lewczuk P, Kornhuber J, Vanderstichele H, et al. Multiplexed quantification of dementia biomarkers in the CSF of patients with early dementias and MCI: A multicenter study. Neurobiol Aging. 2008;29:812–818. doi: 10.1016/j.neurobiolaging.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 61▪.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. First report to demonstrate the utility of CSF biomarkers Aβ42, tau and phospho-tau to predict progression of patients from mild cognitive impairment to dementia attributed clinically to AD. [DOI] [PubMed] [Google Scholar]
- 62▪.Snider B, Fagan A, Roe C, et al. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–645. doi: 10.1001/archneurol.2009.55. First study to correlate CSF biomarkers with the rate of future cognitive decline in individuals diagnosed with very early stage dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li G, Sokal I, Quinn J, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, Goodlett D, Quinn J, et al. Quantitative proteomics of cerebrospinal fluid from patients with Alzheimer’s disease. J Alzheimers Dis. 2005;7:125–133. doi: 10.3233/jad-2005-7205. [DOI] [PubMed] [Google Scholar]
- 65.Hu Y, Hosseini A, Kauwe J, et al. Identification and validation of novel CSF biomarkers for early stages of Alzheimer’s disease. Proteomics Clin Appl. 2007;1:1373–1384. doi: 10.1002/prca.200600999. [DOI] [PubMed] [Google Scholar]
- 66.Castano E, Roher A, Esh C, Kokjohn T, Beach T. Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer’s disease and non-demented elderly subjects. Neurol Res. 2006;28:155–163. doi: 10.1179/016164106X98035. [DOI] [PubMed] [Google Scholar]
- 67.Finehout E, Franck Z, Choe L, Relkin N, Lee K. Cerebrospinal fluid proteomic biomarkers for Alzheimer’s disease. Ann Neurol. 2006;61:120–129. doi: 10.1002/ana.21038. [DOI] [PubMed] [Google Scholar]
- 68.Archer H, Edison P, Brooks D, et al. Amyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography study. Ann Neurol. 2006;60:145–147. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- 69.De Leon Mj, Desanti S, Zinkowski R, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27(3):394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 70▪.Hampel H, Burger K, Pruessner J, et al. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol. 2005;62:770–773. doi: 10.1001/archneur.62.5.770. Demonstrates that levels of CSF ptau231 can predict progression of hippocampal atrophy in AD, providing a good example of the power of combining biomarker modalities. [DOI] [PubMed] [Google Scholar]
- 71.De Leon M, Mosconi L, Li J, et al. Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. J Neurol Sci. 2007;254(12):1666–1675. doi: 10.1007/s00415-007-0610-z. [DOI] [PubMed] [Google Scholar]
- 72▪.Kauwe J, Cruchaga C, Mayo K, et al. Variation in MAPT is associated with cerebrospinal fluid tau levels in the presence of amyloid-β deposition. Proc Natl Acad Sci USA. 2008;105:8050–8054. doi: 10.1073/pnas.0801227105. Demonstrates that CSF biomarkers can be used as endophenotypes for genetic studies of AD by providing evidence for a genetic–epigenetic interaction that predisposes some individuals to the development of tauopathy and accelerated AD progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kauwe J, Wang J, Mayo K, et al. Alzheimer’s disease risk variants show association with cerebrospinal fluid amyloid β. Neurogenetics. 2009;10:13–17. doi: 10.1007/s10048-008-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪.Bateman R, Munsell L, Morris J, Swarm R, Yarasheski K, Holtzman D. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. This elegant study describes a novel method to measure the production and clearance rates of CNS proteins in living humans and demonstrated a very fast turnover of Aβ in young (23–45 years) cognitively normal individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75▪▪.Bateman R, Siemers E, Mawuenyega K, et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. This study demonstrates the utility of an in vivo labeling method to test the effect of a drug on the pharmacokinetics of CNS protein (in this case the Aβ peptide) production and clearance in living humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewczuk P, Beck G, Esselmann H, et al. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid β peptides. Clin Chem. 2006;52:332–334. doi: 10.1373/clinchem.2005.058776. [DOI] [PubMed] [Google Scholar]
- 77.Peskind E, Nordberg A, Darreh-Shori T, Soininen H. Safety of lumbar puncture procedures in patients with Alzheimer’s disease. Curr Alzheimer Res. 2009;6:290–292. doi: 10.2174/156720509788486509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78▪.Mueller S, Weiner M, Thal L, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. Introduces the Alzheimer’s Disease Neuroimaging Initiative (ADNI), the first large-scale, multi-institutional study of its kind designed to obtain longitudinal biomarker and clinical data from individuals with mild cognitive impairment, AD and cognitively normal individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frisoni G, Henneman W, Weiner M, et al. The pilot European Alzheimer’s Disease Neuroimaging Initiative of the European Alzheimer’s Disease Consortium. Alzheimers Dement. 2008;4:255–264. doi: 10.1016/j.jalz.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ihara Y. Overview on Alzheimer’s disease. Rinsho Shinkeigaku. 2007;47:902–904. [PubMed] [Google Scholar]
- 81.Coats M, Morris J. Antecedent biomarkers of Alzheimer’s disease: The Adult Children Study. J Geriatr Psychiatry Neurol. 2005;18:242–244. doi: 10.1177/0891988705281881. [DOI] [PubMed] [Google Scholar]
- 82.Kornhuber J, Schmidtke K, Frolich L, et al. Early and differential diagnosis of dementia and mild cognitive impairment: design and cohort baseline characteristics of the German Dementia Competence Network. Dement Geriatr Cogn Disord. 2009;27:404–417. doi: 10.1159/000210388. [DOI] [PubMed] [Google Scholar]
- 83.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and p-tau181 increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1(8–9):371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bateman RJ, Siemers ER, Mawuenyega KG, et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Alzheimer’s Association. www.alz.org.
- 102.Dominantly Inherited Alzheimer Network (DIAN) www.dian-info.org.