Abstract
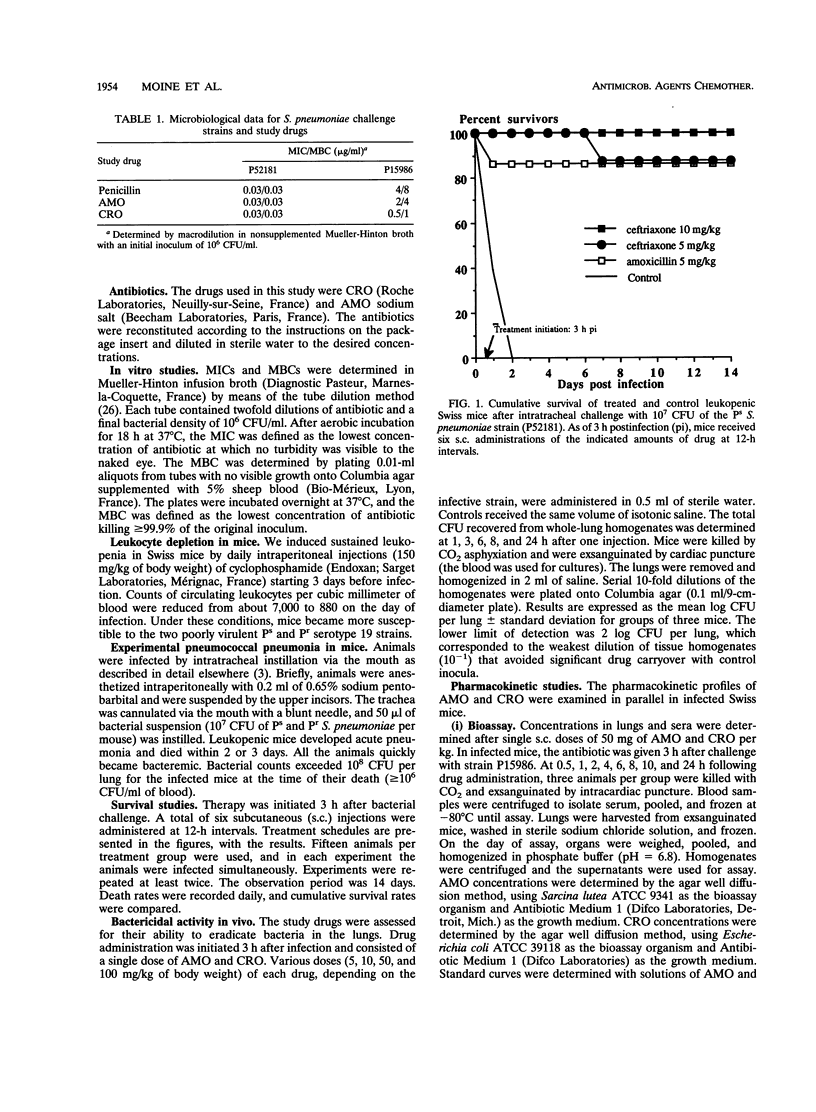
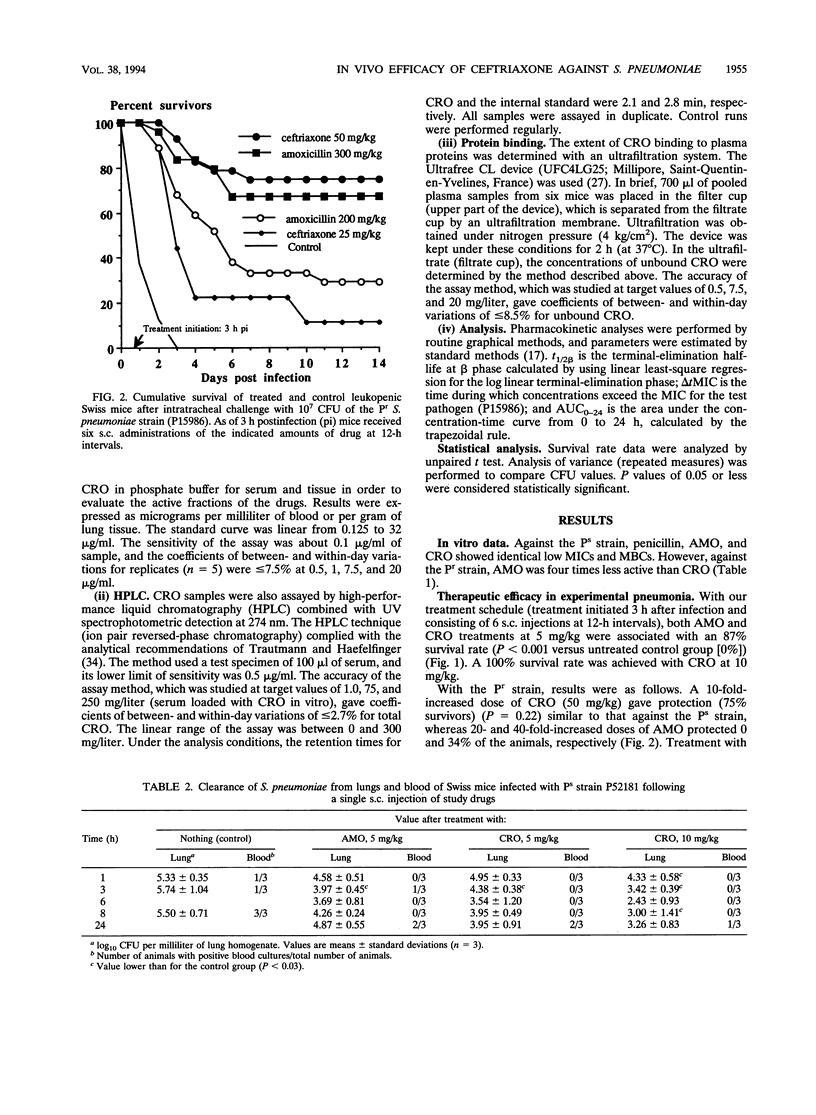
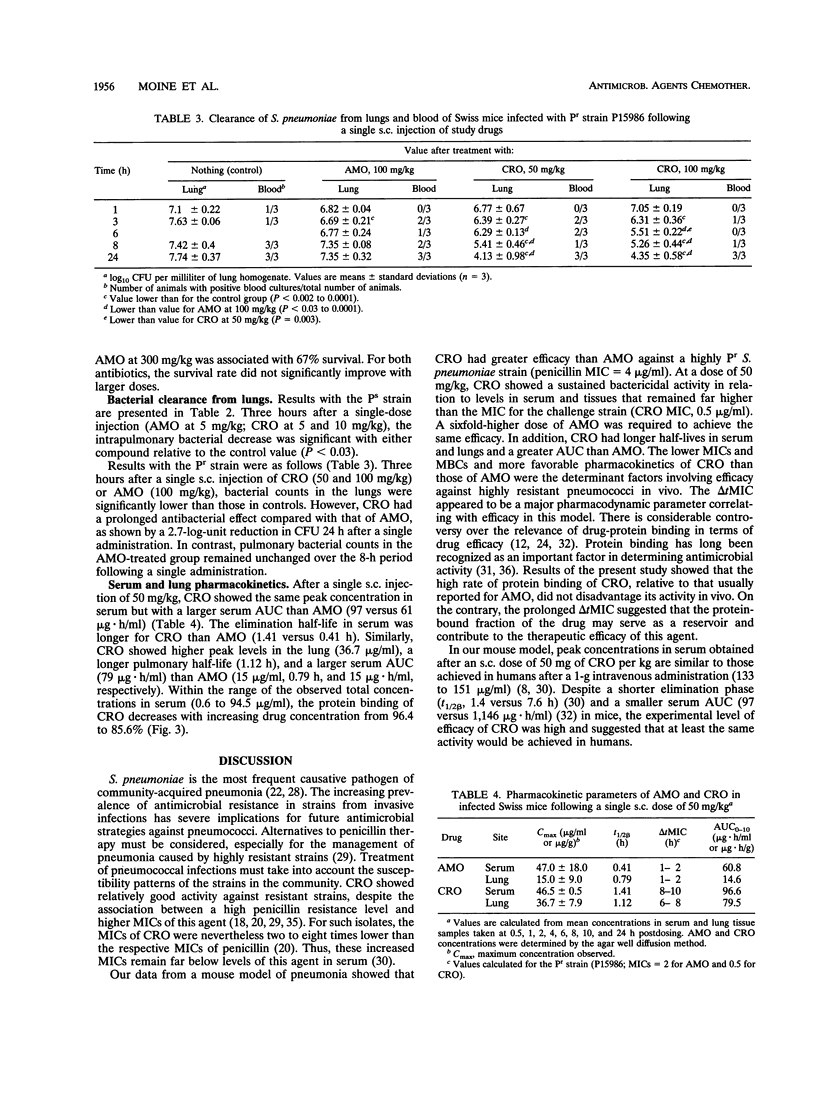
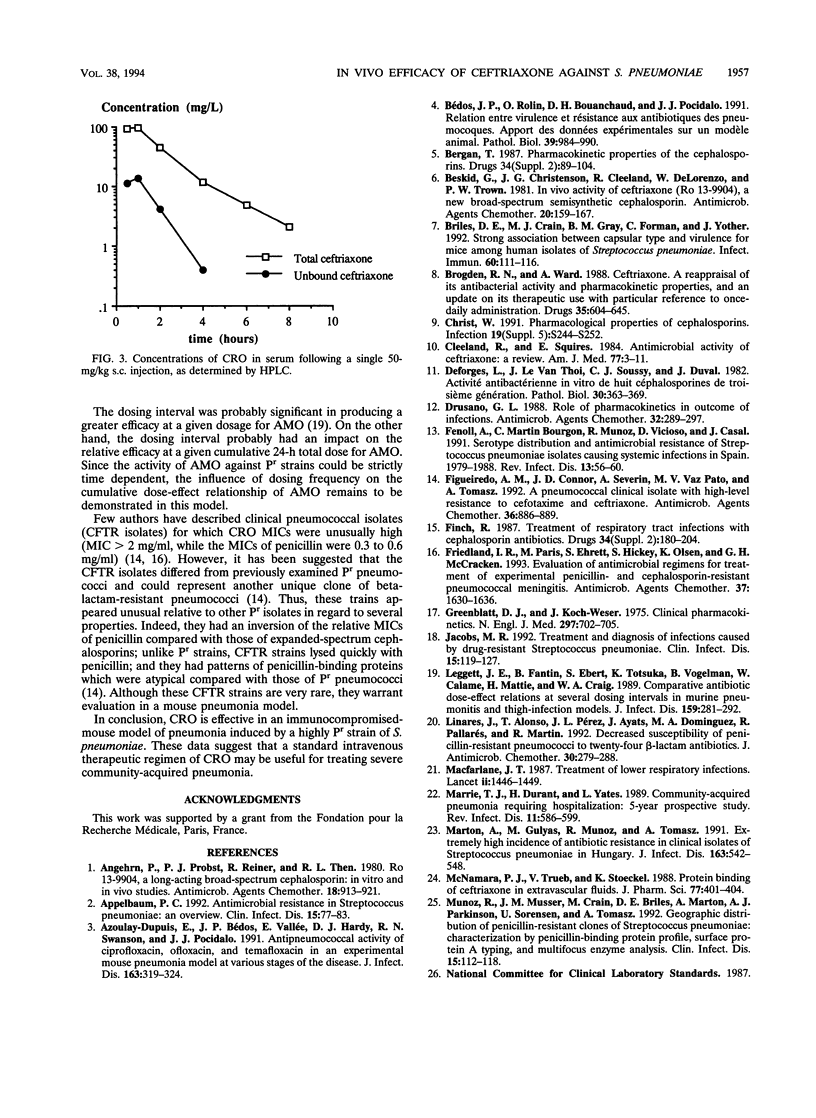
The increasing emergence of penicillin-resistant (Pr) strains of Streptococcus pneumoniae could pose a therapeutic problem in the next few years. Ceftriaxone (CRO), a broad-spectrum cephalosporin, exhibits a smaller increase in MICs against Pr S. pneumoniae strains than amoxicillin (AMO) (usually referred as to the "gold standard" therapy for pneumococcal infections). Therefore, we compared their respective efficacies in a leukopenic Swiss mouse model of pneumococcal pneumonia. Infection was induced with two serotype 19 strains: a penicillin-susceptible (Ps) strain (MICs of < 0.01 for penicillin, 0.03 for AMO, and 0.03 for CRO) and a Pr strain (MICs of 4 for penicillin, 2 for AMO, and 0.5 for CRO). Untreated mice died within 2 or 3 days. Against the Ps strain, the minimal protective dose (two subcutaneous injections at 12-h intervals for 3 days) for both CRO and AMO was 5 mg/kg of body weight (87% survivors). Ten-fold-increased doses of CRO (50 mg/kg) gave similar protection (75% survivors) against the Pr strain, whereas 20- and 40-fold-increased doses of AMO protected 0 and 34% of the animals, respectively, against the Ps strain. CRO had a marked and prolonged antibacterial effect in the lungs (2.7-log-unit reduction of CFU in 24 h after a single 50-mg/kg injection) against the Pr strain in comparison with AMO. A standard dosage of 50 mg of CRO per kg in mice resulted in peak levels in serum and protein binding comparable to those observed with 1 g given intravenously in humans. This dosage remained effective against a highly Pr S. pneumoniae strain in this model. The microbiological activity and pharmacodynamic and pharmacokinetic properties of CRO (time during which concentrations exceed the MIC for the test pathogen [delta t MIC], > or less than 8 h; and peak/MIC ratio, >90 for free active drug) accounted for its efficacy relative to AMO (50 mg/kg: delta t MIC, <2; peak/MIC ratio, <25) against the highly Pr S. pneumoniae strain used in this study.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992 Jul;15(1):77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- Azoulay-Dupuis E., Bedos J. P., Vallée E., Hardy D. J., Swanson R. N., Pocidalo J. J. Antipneumococcal activity of ciprofloxacin, ofloxacin, and temafloxacin in an experimental mouse pneumonia model at various stages of the disease. J Infect Dis. 1991 Feb;163(2):319–324. doi: 10.1093/infdis/163.2.319. [DOI] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetic properties of the cephalosporins. Drugs. 1987;34 (Suppl 2):89–104. doi: 10.2165/00003495-198700342-00008. [DOI] [PubMed] [Google Scholar]
- Beskid G., Christenson J. G., Cleeland R., DeLorenzo W., Trown P. W. In vivo activity of ceftriaxone (Ro 13-9904), a new broad-spectrum semisynthetic cephalosporin. Antimicrob Agents Chemother. 1981 Aug;20(2):159–167. doi: 10.1128/aac.20.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Crain M. J., Gray B. M., Forman C., Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992 Jan;60(1):111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R. N., Ward A. Ceftriaxone. A reappraisal of its antibacterial activity and pharmacokinetic properties, and an update on its therapeutic use with particular reference to once-daily administration. Drugs. 1988 Jun;35(6):604–645. doi: 10.2165/00003495-198835060-00002. [DOI] [PubMed] [Google Scholar]
- Bédos J. P., Rolin O., Bouanchaud D. H., Pocidalo J. J. Relation entre virulence et résistance aux antibiotiques des pneumocoques. Apport des donnée expérimentales sur un modèle animal. Pathol Biol (Paris) 1991 Dec;39(10):984–990. [PubMed] [Google Scholar]
- Christ W. Pharmacological properties of cephalosporins. Infection. 1991;19 (Suppl 5):S244–S252. doi: 10.1007/BF01645535. [DOI] [PubMed] [Google Scholar]
- Cleeland R., Squires E. Antimicrobial activity of ceftriaxone: a review. Am J Med. 1984 Oct 19;77(4C):3–11. [PubMed] [Google Scholar]
- Deforges L., Le Van Thoi J., Soussy C. J., Duval J. Activité cantibactérienne in vitro de huit céphalosporines de troisième génération. Pathol Biol (Paris) 1982 Jun;30(6):363–369. [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoll A., Martín Bourgon C., Muñz R., Vicioso D., Casal J. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979-1989. Rev Infect Dis. 1991 Jan-Feb;13(1):56–60. doi: 10.1093/clinids/13.1.56. [DOI] [PubMed] [Google Scholar]
- Figueiredo A. M., Connor J. D., Severin A., Vaz Pato M. V., Tomasz A. A pneumococcal clinical isolate with high-level resistance to cefotaxime and ceftriaxone. Antimicrob Agents Chemother. 1992 Apr;36(4):886–889. doi: 10.1128/aac.36.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch R. Treatment of respiratory tract infections with cephalosporin antibiotics. Drugs. 1987;34 (Suppl 2):180–204. doi: 10.2165/00003495-198700342-00014. [DOI] [PubMed] [Google Scholar]
- Friedland I. R., Paris M., Ehrett S., Hickey S., Olsen K., McCracken G. H., Jr Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1993 Aug;37(8):1630–1636. doi: 10.1128/aac.37.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Kock-Weser J. Drug therapy. Clinical Pharmacokinetics (first of two parts). N Engl J Med. 1975 Oct 2;293(14):702–705. doi: 10.1056/NEJM197510022931406. [DOI] [PubMed] [Google Scholar]
- Jacobs M. R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1992 Jul;15(1):119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- Leggett J. E., Fantin B., Ebert S., Totsuka K., Vogelman B., Calame W., Mattie H., Craig W. A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989 Feb;159(2):281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- Liñares J., Alonso T., Pérez J. L., Ayats J., Domínguez M. A., Pallarés R., Martín R. Decreased susceptibility of penicillin-resistant pneumococci to twenty-four beta-lactam antibiotics. J Antimicrob Chemother. 1992 Sep;30(3):279–288. doi: 10.1093/jac/30.3.279. [DOI] [PubMed] [Google Scholar]
- Macfarlane J. T. Treatment of lower respiratory infections. Lancet. 1987 Dec 19;2(8573):1446–1449. doi: 10.1016/s0140-6736(87)91140-8. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Durant H., Yates L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis. 1989 Jul-Aug;11(4):586–599. doi: 10.1093/clinids/11.4.586. [DOI] [PubMed] [Google Scholar]
- Marton A., Gulyas M., Munoz R., Tomasz A. Extremely high incidence of antibiotic resistance in clinical isolates of Streptococcus pneumoniae in Hungary. J Infect Dis. 1991 Mar;163(3):542–548. doi: 10.1093/infdis/163.3.542. [DOI] [PubMed] [Google Scholar]
- McNamara P. J., Trueb V., Stoeckel K. Protein binding of ceftriaxone in extravascular fluids. J Pharm Sci. 1988 May;77(5):401–404. doi: 10.1002/jps.2600770509. [DOI] [PubMed] [Google Scholar]
- Munoz R., Musser J. M., Crain M., Briles D. E., Marton A., Parkinson A. J., Sorensen U., Tomasz A. Geographic distribution of penicillin-resistant clones of Streptococcus pneumoniae: characterization by penicillin-binding protein profile, surface protein A typing, and multilocus enzyme analysis. Clin Infect Dis. 1992 Jul;15(1):112–118. doi: 10.1093/clinids/15.1.112. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Johno I., Kitazawa S. Comparative evaluation of microultrafiltration devices for determination of protein binding. Ther Drug Monit. 1988;10(3):310–315. doi: 10.1097/00007691-198803000-00013. [DOI] [PubMed] [Google Scholar]
- Ortqvist A., Hedlund J., Grillner L., Jalonen E., Kallings I., Leinonen M., Kalin M. Aetiology, outcome and prognostic factors in community-acquired pneumonia requiring hospitalization. Eur Respir J. 1990 Nov;3(10):1105–1113. [PubMed] [Google Scholar]
- Pallares R., Gudiol F., Liñares J., Ariza J., Rufi G., Murgui L., Dorca J., Viladrich P. F. Risk factors and response to antibiotic therapy in adults with bacteremic pneumonia caused by penicillin-resistant pneumococci. N Engl J Med. 1987 Jul 2;317(1):18–22. doi: 10.1056/NEJM198707023170104. [DOI] [PubMed] [Google Scholar]
- Patel I. H., Kaplan S. A. Pharmacokinetic profile of ceftriaxone in man. Am J Med. 1984 Oct 19;77(4C):17–25. [PubMed] [Google Scholar]
- Rolinson G. N. The significance of protein binding of antibiotics in antibacterial chemotherapy. J Antimicrob Chemother. 1980 May;6(3):311–317. doi: 10.1093/jac/6.3.311. [DOI] [PubMed] [Google Scholar]
- Scaglione F., Raichi M., Fraschini F. Serum protein binding and extravascular diffusion of methoxyimino cephalosporins. Time courses of free and total concentrations of cefotaxime and ceftriaxone in serum and pleural exudate. J Antimicrob Chemother. 1990 Sep;26 (Suppl A):1–10. doi: 10.1093/jac/26.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Scully B. E., Fu K. P., Neu H. C. Pharmacokinetics of ceftriaxone after intravenous infusion and intramuscular injection. Am J Med. 1984 Oct 19;77(4C):112–116. [PubMed] [Google Scholar]
- Viladrich P. F., Gudiol F., Liñares J., Rufi G., Ariza J., Pallares R. Characteristics and antibiotic therapy of adult meningitis due to penicillin-resistant pneumococci. Am J Med. 1988 May;84(5):839–846. doi: 10.1016/0002-9343(88)90061-7. [DOI] [PubMed] [Google Scholar]
- Wise R. Protein binding of beta-lactams: the effects on activity and pharmacology particularly tissue penetration. I. J Antimicrob Chemother. 1983 Jul;12(1):1–18. doi: 10.1093/jac/12.1.1. [DOI] [PubMed] [Google Scholar]



