Abstract
Studies of the aging brain have demonstrated that areas of the frontal cortex, along with their associated top–down executive control processes, are particularly prone to the neurodegenerative effects of age. Here, we investigate the effects of aging on brain and behavior using a novel task, which allows us to examine separate components of an individual's chosen strategy during routine problem solving. Our findings reveal that, contrary to previous suggestions of a specific decrease in cognitive flexibility, older participants show no increased level of perseveration to either the recently rewarded object or the recently relevant object category. In line with this lack of perseveration, lateral and medial regions of the orbito-frontal cortex, which are associated with inhibitory control and reward processing, appear to be functionally intact. Instead, a general loss of efficient problem-solving strategy is apparent with a concomitant decrease in neural activity in the ventrolateral prefrontal cortex and the posterior parietal cortex. The dorsolateral prefrontal cortex is also affected during problem solving, but age-related decline within this region appears to occur at a later stage.
INTRODUCTION
As people progress from adulthood into old age, there are changes throughout the brain at the molecular, cellular, and structural level, with concomitant changes in cognitive ability. The brain undergoes a global decline in terms of thinning of the cerebral cortex (Salat et al., 2004; Uylings & De Brabander, 2002), reduction in gray matter (Good et al., 2001), sulcal depth (Rettmann, Kraut, Prince, & Resnick, 2006), increased ventricular volume (Resnick et al., 2000), dysmorphology of neurons, and loss of dendritic spines (Raz & Rodrigue, 2006). However, anatomically distinct subregions of the human brain are not uniform in their susceptibility to age-related decline.
Regions of the frontal cortex appear to be particularly susceptible to age-related degeneration, with increased atrophy relative to the temporal lobe (Salat et al., 2004) and increased signs of white matter degeneration (Aine et al., 2006; Nordahl et al., 2006; Salat et al., 2005). The frontal cortex is typically associated with executive tasks such as the maintenance of items in working memory and the control of the attentional focus (Duncan, 2001; Miller & Cohen, 2001; Norman & Shallice, 1980). It is unsurprising, therefore, that age-related differences have been reported in tasks that involve top–down executive control (e.g., Gunning-Dixon & Raz, 2003; Robbins et al., 1998). The executive control functions associated with the frontal cortex have a number of different cognitive components, and there is evidence that these components can be differentiated anatomically. For example, switching attention between different object dimensions (extradimensional set shifting) is associated with the lateral regions of the prefrontal cortex, whereas adapting selective responses in the face of changes of reward contingency is associated with orbito-frontal regions (Hampshire & Owen, 2006; Dias, Robbins, & Roberts, 1996). Finer dissociations have also been reported within the lateral prefrontal cortex, with the ventral subregion (ventrolateral prefrontal cortex [VLPFC]) associated with simpler executive functions such as maintaining objects actively on-line in working memory (Owen et al., 1999; Petrides, 1994, 1995), and the dorsal subregion (dorsolateral prefrontal cortex [DLPFC]) associated with more complex executive functions including the active monitoring and manipulation of items in working memory (Owen et al., 1999; Petrides, 1994, 1995). It has been proposed that the fronto-polar cortex is at the apex of this executive hierarchy due to its involvement in demanding executive functions such as the combining of multiple cognitive rules and switching between different subtasks when multitasking (Ramnani & Owen, 2004; Koechlin, Ody, & Kouneiher, 2003; Koechlin, Basso, Pietrini, Panzer, & Grafman, 1999). Finer functional dissociations have also been reported within the orbito-frontal cortex (OFC), with medial regions associated with positive reward (O'Doherty, Critchley, Deichmann, & Dolan, 2003; O'Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001; Elliott, Dolan, & Frith, 2000), and lateral regions associated with inhibitory control (Hampshire & Owen, 2006; Dias et al., 1996) and the reception of negative feedback (O'Doherty et al., 2001, 2003). Finally, the anterior cingulate cortex (ACC) is thought to play a role during tasks that require the top–down resolution of response conflict (Pardo, Pardo, Janer, & Raichle, 1990). The rate of age-related decline is not thought to be homogenous across these subregions of the frontal cortex (MacPherson, Phillips, & Della Sala, 2002; Tisserand et al., 2002; Salat & Kaye, 2001), but although it seems unlikely therefore, that aging affects all components of top–down control to the same extent and at the same rate, the exact nature of age-related executive dysfunction remains controversial.
A number of studies have sought to behaviorally isolate and anatomically localize those components of executive control that show a disproportionate susceptibility to the effects of aging. However, these previous studies often use tasks that confound multiple cognitive demands, making it difficult to fully interpret the findings. For example, Robbins et al. (1998) found that the largest difference between old and young participants was in their ability to carry out the extradimensional switch stage of an attentional set-shifting task (when they must switch their focus of attention from one object dimension/category to another). On this basis, it seems that switching attention between object dimensions may be one of the most vulnerable executive tasks to age-related degeneration, manifesting itself as impaired cognitive flexibility in older participants. A problem for this interpretation, however, is that the attentional set-shifting task used only examines the first novel shift to a previously irrelevant task dimension, and this manipulation clearly has multiple discrete cognitive components, and could, perhaps, be better described as measuring general problem-solving ability. For example, although it is true that the participant must switch their focus of attention between the different object dimensions, at the same time, they must overcome learning during the previous stages of the task that the dimension to switch to is irrelevant (learned irrelevance). In addition, as only one dimension is relevant in the task prior to the extradimensional switch, participants must identify it as being available as an option at all, and must also work out that switching attention across object dimensions may be a relevant operation in the task. Finally, if the participant keeps responding to exemplars from the previously rewarded object dimension (as seems likely), then picking an incorrect exemplar after an extradimensional switch will lead to a partial as opposed to a total reward contingency, and it is therefore more ambiguous whether or not a switch of attention is required at all (the participants may consider 50% positive feedback to be quite reasonable).
More direct evidence for an age-related deficit in attentional set shifting comes from Gunning-Dixon and Raz (2003), who used magnetic resonance imaging to relate changes in neuroanatomical structure to performance on the Wisconsin Card Sorting Task (WCST). The WCST is a cognitively heterogeneous task that has previously been reported as sensitive to the effects of age-related decline (e.g., Volkow et al., 1998). They found that a significant proportion of the age-related variance in the number of perseverative errors that participants made on the WCST could be explained by overall prefrontal cortex volume. Again, however, these structural changes may correlate with an increase in the number of errors during extra-dimensional shifting, but the exact executive component that is affected is poorly defined (see Barcelo & Knight, 2002). Indeed, Gunning-Dixon and Raz only reported perseverative errors, and as they state themselves, “perseveration on the WCST may occur for a host of reasons.”
Evidence that the effect of aging on executive control may be more complex than a simple problem in switching attention across different object dimensions has been provided by Ravizza and Ciranni (2002) using an oddman-out task. The effects of aging were examined during attentional set shifting when the extent of choice was manipulated by presenting cues which alerted participants to the currently relevant object dimension (either letters or shapes). Older participants were slower than younger controls when making an extradimensional shift, but in the cued (low choice) condition, this shifting deficit was attenuated such that there was no significant difference between the old and the young groups. On this basis, the authors (Ravizza & Ciranni, 2002) proposed that older adults do not have a deficit in the process of attentional shifting per se (Gunning-Dixon & Raz, 2003; Robbins et al., 1998), but rather, that the previously observed extradimensional shifting deficits in old age reflect an inability to maintain goal-oriented information in memory. However, this task again poorly defines the exact executive component responsible, as the deficit could also be caused by a problem inhibiting the currently attended dimension, identifying candidate object dimensions, or making decisions where there are multiple conflicting choices available. In any of these situations, using a dimension cue would overcome the deficit by providing a bottom–up biasing signal.
In fact, in support of the idea of a deficit related to the extent of choice, a meta analysis (Verhaeghen & Cerella, 2002) of different types of executive task, pointed toward tasks with multiple parallel components, for example, dual-task performance, as being the most vulnerable to age-related decline. Little effect of age upon simple attentional switching, inhibition, or working memory was observed. This finding suggests that aging is accompanied by a loss of ability to make logical and structured decisions in the face of increased choice. It seems likely that older participants tend to have difficulty learning and strategically structuring the different subcomponents in more complex executive tasks as opposed to undertaking the specific processes within that overall task structure.
One way to decompose a cognitively heterogeneous task such as the WCST is to compare the behavioral responses and neural activity at discrete points in time when the cognitive demands are differentially varied (e.g., Monchi et al., 2004; Monchi, Petrides, Petre, Worsley, & Dagher, 2001). Here, we examined the effects of aging using a novel analog of the WCST which, by using a partial feedback paradigm, allowed us to precisely calculate which particular exemplar was chosen by the participant at any given response (Hampshire & Owen, 2006). This increased precision enabled us to break down the time course of each individual's chosen problem-solving strategy into its constituent components. Errors made could therefore be categorized according to whether they were due to perseveration to a previously rewarded object (inhibitory control) or perseveration to a previously rewarded dimension (attentional set shifting). This allowed us to test the hypotheses that age-related decline is characterized by a loss of inhibitory control, or an inflexible top–down attentional set. Errors could also be categorized according to whether they occurred during the target search phase of the task (goal directed strategy), or when the target had been correctly identified (target maintenance in working memory). This allowed us to test the hypothesis that age-related decline is characterized by inefficient problem-solving strategy, or poor maintenance of the current target identity. Because the same two dimensions were used repeatedly, these executive components could be examined free from other confounding factors such as learnt irrelevance, and the novelty of the currently relevant task manipulations and object dimensions. The design also allowed us to localize and compare across age groups those brain regions that were differentially activated during problem solving, extradimensional and intradimensional attentional switching, reversal learning, and the processing of positive and negative feedback.
METHODS
Subjects
Two groups of 16 healthy right-handed participants, younger (mean age = 24 years, oldest = 31 years, youngest = 20 years) and older (mean age = 60 years, oldest = 77 years, youngest = 46 years), were recruited from the volunteer panel at the MRC Cognition and Brain Sciences Unit. They had no history of neurological or psychiatric disease, good vision and, where necessary, were provided with MR-compatible glasses. Permission for this study was obtained from the local research ethics committee and all subjects consented to participation.
Experimental Design
A shifting task was used (Hampshire & Owen, 2006) in which participants had to work out which object was the target in a stimulus set consisting of two faces and two buildings (Figure 1). The stimulus set was presented as two compound object pairs appearing on the left and right of the screen. Both compound object pairs consisted of a face and a building superimposed on top of each other. Each stimulus subtended a visual vertical angle of 6° and a horizontal angle of 6.2°, with a total combined horizontal angle of 15°.
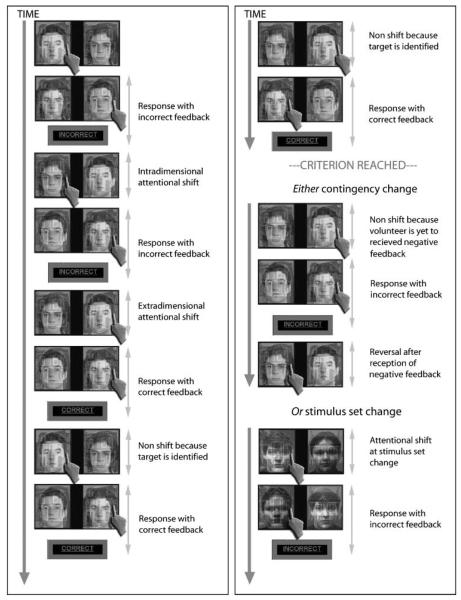
Figure 1.
Experimental design. This figure illustrates a typical series of trials. The participant must work out by trial and error which of the two faces and two buildings is the target item. In this example, the participant initially chooses the face in the left superimposed face-building pair and so indicates left with the button box. When the response is made, the stimuli are removed from the screen and reappear after a short interval rearranged with the chosen face on the right of the screen superimposed on the other building; the participant therefore indicates right. Because the face-building combinations swap from one trial to the next, the program can compute which item was selected and because (in this example) it is not the target, negative feedback is given. Subsequently, the stimuli reappear on the screen and the participant selects the other face (intradimensional shift). Following the second response, negative feedback is given and the participant switches to select the building on the right of the screen (extradimensional shift). Following the second response to the building, positive feedback is given because the participant has correctly identified the target item. When the stimuli reappear on the screen, the participant responds to the same building, as they now know that it is the target (early correct response). They receive positive feedback on the second response, and so continue to select the same building (late correct response). After responding correctly again, they receive positive feedback and have now reached the criteria of six correct responses in a row. One of two things then happens: either a new stimulus set is presented, in which case the participant starts searching for the new target (set change). Alternatively, the reward contingency changes, in which case the participant responds twice more to the same building (because they have no way of knowing that anything has changed) before receiving negative feedback. They must then inhibit their responses to the recently rewarded target object and start trying to identify which of the other three possible items has become the target (reversal). It is important to note that the extradimensional and intradimensional shift events, along with the feedback, do not always occur in the sequence shown because the order in which the stimuli are tested is determined entirely by the choices made by the participants.
On each trial, the participants were required to indicate, using a button box, which side of the screen they thought the target was located on, and at the point of response the stimuli were removed from the screen. Every second response, feedback was presented on the screen for 0.6 sec, indicating whether the object they had chosen was the target or not. The feedback given was the word “CORRECT” in green if the last two responses were both correct. Otherwise, the feedback was the word “INCORRECT” in red.
After six correct responses to the target (that is, three positive feedback events), criterion was reached, and the rule was changed such that there was a new target object. The change could be in the form of a “set change,” in which two new face–building pairs were displayed so that the participant could not respond to the previous target object, effectively removing any response suppression component. Alternatively, the rule change could be in the form of a “reversal.” In a reversal, the stimulus set stayed the same but the reward contingency changed, such that a previous nontarget became the target, whereas the previous target became a nontarget. Thus, a negative feedback event to the previous target occurred, and the participant was required to inhibit their response to the previously rewarded target object and initiate searching for the new target. Maximum uncertainty was ensured in both cases, as the new target could be either an object from the same category (intradimensional [ID]) or an object from the alternative category (extradimensional [ED]). Importantly, as the face–house combinations comprising the compound stimuli were reversed on every trial, it was possible to calculate exactly which object was being attended to by examining consecutive responses.
Before entering the scanner, the participants were clearly instructed to keep responding to the correct object until informed that it was no longer the target. Participants were also asked to respond “as quickly and accurately as possible.”
Behavioral Analysis
The novel experimental design allowed a number of different behavioral measures to be taken at increasingly fine degrees of process specificity. Initially, to generate a rough overall measure of performance, the total number of targets that each individual correctly identified was measured collapsed across all four target change conditions. The average number of errors the individual made when trying to identify the target was then calculated separately for each of the four types of target change (ED shift, ID shift, ED reversal, ID reversal). This gave a measure of whether there were any specific age-related differences due to perseveration to the previously relevant object, or to the previously relevant object category. To better define the nature of any general increase in the number of errors, the sequence of responses made by the individual was then examined in detail, and the number of occurrences of several different types of erroneous response was calculated. Errors made after two or four correct responses were first counted, and these were termed “early known errors” and “late known errors,” as they occurred after it appeared that the participant had correctly identified the target object. These errors could be due either to an accidental incorrect button press, mistaking one object for another, or failing to maintain the target identity in working memory. Next, the total number of times that a participant continued to respond to the previous target object after receiving negative feedback at reversal was measured in order to give a measure of direct perseveration. An increase in the number of perseverative errors could be caused by either a problem with inhibitory control or difficulty in processing and making judgments on the basis of abstract negative feedback. Finally, the number of repetitive responses to the same nontarget object and the number of times that the participants went back and double checked an incorrect object were calculated to give a measure of how efficiently the non-target items were eliminated.
The final behavioral analysis examined the response times for the different events defined in the event-related functional magnetic resonance imaging (fMRI) model as described below.
Event Modeling
The event modeling focused on individual types of participant response, and these were defined according to which objects were currently and previously selected (Figure 1).
Two of the events related to the period when the participant was actively trying to work out which object was the target: one was termed an “extradimensional switch,” because the focus of attention changed between objects of different types (for example, from a face to a building), and the other an “intradimensional switch,” because the focus of attention changed between objects of the same type (e.g., from one face to another face). Although each of these events involved multiple components (e.g., response suppression and attended stimulus change), the only way in which they differed from one another was with respect to the change of attention to object type, so subtraction of one from the other isolated this extradimensional switch component.
Two additional switch events were defined at the point when the participant had correctly identified the previous target and a different object became the new target. In one of these switch events, the stimulus set was changed so the participant could not respond to the previous target but had to switch to a new object that had not been seen previously. This effectively removed any response suppression component and was called a “set change.” In the other switch event, the stimulus set stayed the same but the reward contingency changed. Thus, a negative feedback event occurred after a response to the previous target, and the participant was required to search for the new target object. Because the new target was a previous nontarget and because the previous target was still present (but as a nontarget), this manipulation was termed a “reversal.” Although these two events had multiple components, subtraction of switching with stimulus set change from switching with reward contingency allowed examination of the reversal aspect of attentional shifting.
Responses when the target was known on the basis of prior positive feedback were divided into the first (early) and subsequent (late) correct responses (at the early correct responses, an important behavioral change occurred as the participant stopped trying to work out which object was the target).
Finally, the positive and negative feedback events were compared directly to localize those brain regions that were activated during the reception of abstract positive and negative reward.
Regions of Interest
In the fMRI analysis, we focused on the same functionally defined subregions of the fronto-parietal network that were examined in the original version of our task. These regions were selected as they have been implicated previously in attentional switching and problem solving, and an in-depth discussion of their involvement in this task is published elsewhere (Hampshire & Owen, 2006).
Both the VLPFC and the DLPFC have been implicated in a wide variety of tasks requiring attention. ROIs (10 mm) were defined bilaterally in the DLPFC and in the VLPFC, based upon averaged coordinates taken from an analysis, in which multiple and diverse parametrically varied cognitive tasks requiring attention were compared (Duncan & Owen, 2000). Mean coordinates were at x = −38, y = 30, z = 22and x = 38, y = 30, z = 22 for the DLPFC, and x = −39, y = 20, z = 2 and x = 39, y = 20, z = 2 for the VLPFC.
Posterior parietal cortex (PPC) activity has typically been observed in association with lateral prefrontal activity, and mean coordinates were again taken from Duncan and Owen (2000) to define bilateral 10-mm spherical ROIs for this region (x = −31, y = −53, z = 40 and x = 34, y = −52, z = 41).
Multiple regions of the OFC have been implicated in reward-based control of behavior (Rogers, Andrews, Grasby, Brooks, & Robbins, 2000; Rogers et al., 1999). A distinction has been drawn between the lateral and medial surfaces, which are thought to be involved in processing negative and positive rewards, respectively (O'Doherty et al., 2001; Elliott et al., 2000). The coordinates used to define the orbital ROIs in Hampshire and Owen (2006) were taken from a study by O'Doherty et al. (2001), in which a distinct right lateral area was shown to be involved in processing negative reward at reversal of a reward contingency, with a medial orbital region shown to be involved in the reception of positive feedback. Accordingly, bilateral 10-mm radius spherical ROIs were defined at the reported peak right lateral coordinate, and this coordinate mirrored for the left hemisphere. Similarly, the mean coordinates of the medial orbital activation were used to define a 10-mm spherical ROI. Several of the participants had signal loss at the peripheral loci of these orbital ROIs, and they were therefore shifted 10 mm vertically, further into the OFC (left OFC: x = −36, y = 58, z = −2; right OFC: x = 36, y = 58, z = −2; medial OFC [MOFC]: x = −3, y = 37, z = −11).
ACC is known to play a role in top–down executive control (Pardo et al., 1990). We therefore included additional anatomically defined ACC ROIs (Tzourio-Mazoyer et al., 2002). In another recent study (Gruszka et al., unpublished data), significant differences were observed in the caudate nucleus using the same task as in the current study. Therefore, anatomical ROIs were also included bilaterally in the caudate nucleus (Tzourio-Mazoyer et al., 2002).
Imaging Acquisition
Scanning was undertaken at the Wolfson Brain Imaging Centre using a 3-T Bruker Medspec scanner (Bruker s300, Ettingen, Germany) with 21 slices (4 mm slices with 1 mm interslice gap) per image and a TR of 1.1 sec and an in-plane resolution of 3.125 × 3.125 mm. Eight hundred fifty T2-weighted echo-planar images, depicting blood oxygenation level-dependent contrast, were acquired per run, and the first 18 were discarded to avoid T1 equilibrium effects. Images were slice-time acquisition corrected, reoriented, subject motion corrected, geometrically undistorted using phase maps (Cusack, Brett, & Osswald, 2003), spatially normalized to the standard Montreal Neurological Institute EPI template, smoothed with an 8-mm full width at half maximum Gaussian kernel, and modeled using Statistical Parametric Mapping 2 (SPM2, Wellcome Department of Cognitive Neurology). The time series were high-pass filtered. The hemodynamic response was modeled to the stimulus onsets and durations. For switch events, durations were up until the time of response at which stage the stimuli were removed from the screen, whereas for feedback events, durations were up until the point of removal of the feedback from the screen. The contrasts of interest were extracted, and the con images for the critical contrasts were exported and analyzed in a second-level group analysis in SPM5. ROIs were then modeled for this higher-level analysis using MARSBAR (Brett, Anton, Valabregue, & Poline, 2002) with correction for multiple comparisons. These contrasts were also examined in a less constrained whole-brain analysis with false discovery rate (FDR) correction at p = .05.
The experimental acquisition consisted of two 15-min runs. As the timing was response driven, the number of switches completed varied for each participant. The interstimulus interval was randomly jittered from 0.6 to 1.6 sec. Participants also underwent a prescanner training session to ensure that they understood and were capable of performing the task. Responses were made using the first and second fingers of the right hand on a button box and were recorded throughout the experimental acquisition.
RESULTS
Behavioral Analysis
An independent-samples t test was carried out to examine the effect of age on the total number of targets identified. There was a significant effect of age group (t = 2.46, p < .05), with the older participants correctly identifying fewer targets than the younger participants over the course of the experiment.
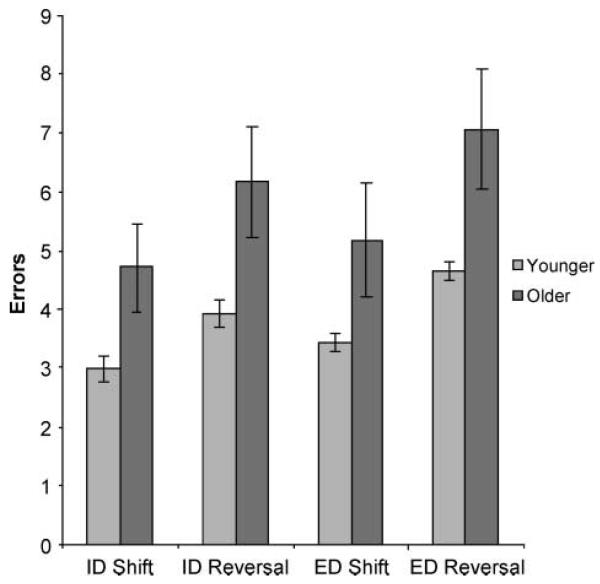
The effects of the four types of target change were then compared by analyzing the number of errors committed before correct target identification using a 2 × 2 × 2 multiway repeated measures analysis of variance (ANOVA) in SPSS. The first factor was dimension change (whether the target changed across- or within-object category). The second factor was reversal (whether when the target changed there was a reward contingency change or a stimulus set change). Age group was included as a between-subject factor. There was a significant main effect of dimension change [F(1, 30) = 7.71, p < .01], with more errors made when an extradimensional switch was required, and a significant main effect of reversal [F(1, 30) = 41.2, p < .001], with more responses made when the reward contingency changed and the stimulus set stayed the same. There was also a significant main effect of age group [F(1, 30) = 5.76, p < .05], with older participants making more errors, and no significant interactions, suggesting that this difference was general across the four target change conditions (Figure 2).
Figure 2.
Errors for different target changes. This figure illustrates the effects on the number of errors made while searching for the target when within and between dimension shifts are required, and when the change in target is cued by reward contingency change and stimulus set change. Significantly more errors were made for both extradimensional shifting and reversal at contingency change at p < .01. There was also a significant main effect of age at p < .05, with no significant interactions.
This general increase in the number of errors made by the older participants was analyzed further by categorizing the different types of response during target search and by comparing the total number of occurrences across age groups in a series of independent-samples t tests. Both age groups performed equivalently when the target had been identified, with neither the younger nor the older group making many early or late errors when the target was known (so after two and four correct responses, respectively) (early, mean younger = 4.19, mean older = 5.75, t = 0.90, p = 0.38; late, mean younger = 2.94, mean older = 2.31, t = 0.79, p = .44). Both groups performed close to ceiling at reversal, with no tendency to perseverate to the previous target object after the reception of negative feedback (mean younger = 1.94, mean older = 1.56, t = 0.52, p = .60). However, there were age-related effects when the identity of the target object was being derived. More specifically, an age-related increase was observed in the number of consecutive responses that were made to a nontarget object despite the reception of negative feedback (mean younger = 6.63, mean older = 15.63, t = 2.12, p < .05). In conjunction with this increase in repetitive incorrect responding, an increase was observed in the number of times that a nontarget object was tried, eliminated, switched away from, and then subsequently re-examined in the older group (mean younger = 13.19, mean older = 26.0, t = 2.10, p < .05).
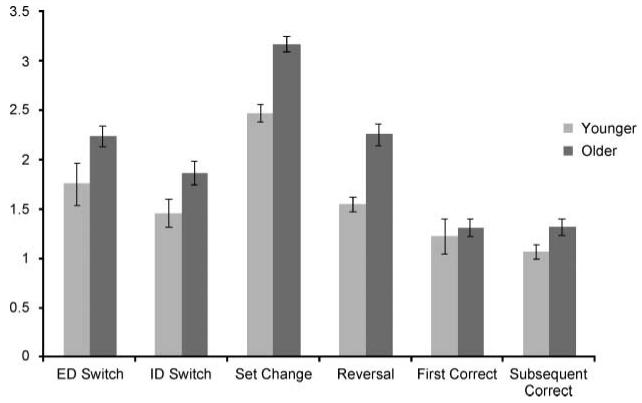
Response times were then compared for different event types defined in the fMRI linear model using a one-way repeated measures ANOVA with age group included as a between-subject factor. The main factor was switch type, and the conditions were extradimensional switch, intradimensional switch, reward contingency change, stimulus set change, first known correct response, and subsequent known correct response. There was a significant main effect of switch type [F(1, 30) = 17.1, p < .001], and a small but significant effect of age group [F(1, 30) = 5.04, p < .05] with no interaction, indicating that the older participants were marginally slower at responding in general (Figure 3).
Figure 3.
Response time data. This figure illustrates the response times for the two age groups. The older age group displayed generally slower response times.
Pairwise comparisons of the response times, collapsed across group for the different contrasts in the event-related fMRI design, revealed that participants were slower when they decided to switch their attention between (extradimensional switch) rather than within (intra-dimensional switch) object dimensions (t = 4.9, p < .01), and were slower when moving attention within dimensions than when routinely responding to the known target (late correct responses) (t = 6.9, p < .001). Shifts of attention due to set change were compared with those due to reward contingency change. In direct contrast to the error data described above (where more errors were made in the blocks following reward contingency change), the results revealed a significantly greater response time for the set change condition (t = 4.7, p < .001), presumably due to the time spent taking in the new stimulus set. There were no significant response time differences between the early and late correct responses.
Imaging Analysis
To identify brain regions that were activated during solution search, all events where the target was known (early and late correct responses and feedback events whilst the target was known) were subtracted from all events where the participant was actively trying to derive the target (extradimensional and intradimensional switches, reversals, set change, and feedback events during solution search). The resultant brain maps, containing the weighted parameter estimates (con images), were examined at the group level, both collapsed across the age groups and contrasting between the age groups, using an independent-samples t test. In the ROI analysis, the DLPFC, the VLPFC, the PPC, and the lateral OFC were significantly activated and the MOFC was significantly deactivated, during solution search compared with when the target was known (corrected for multiple comparisons) (Table 1). ACC and caudate ROIs were not significantly activated for this contrast even when the data were examined uncorrected for multiple comparisons. The whole-brain analysis confirmed this result (Figure 4), with extensive activity in lateral prefrontal, lateral orbito-frontal, and PPC for solution search, and significant activity in the MOFC for the reverse contrast (FDR corrected for the whole brain mass at p = .05). In addition, the pre-supplementary motor area (pre-SMA) was significantly activated during solution search, and regions of the temporal cortex were significantly deactivated bilaterally.
Table 1.
Peak Activation Coordinates from the Group-level Whole-brain Analyses
| ROI Analysis |
Nearest Maxima (p < .05, FDR Corrected) |
||||||
|---|---|---|---|---|---|---|---|
| Contrast | ROI | t | p Corrected | x | y | z | t |
| Solution Search–Knowing the Target | |||||||
| Main effect | Left DLPFC | 7.01 | <.001 | −44 | 38 | 14 | 6.08 |
| Right DLPFC | 6.6 | <.001 | 38 | 34 | 24 | 6.89 | |
| Left VLPFC | 5.26 | <.001 | −32 | 24 | −8 | 6.99 | |
| Right VLPFC | 4.38 | <.001 | 36 | 28 | −6 | 6.75 | |
| Left PPC | 7.86 | <.001 | −26 | −56 | 40 | 8.16 | |
| Right PPC | 7.55 | <.001 | 34 | −50 | 44 | 8.48 | |
| Left OFC | 4 | .002463 | −22 | 42 | −12 | 2.72 | |
| Right OFC | 3.98 | .00261 | 26 | 46 | −14 | 3.43 | |
| Age effect | Left DLPFC | 2.72 | .067884 | −38 | 32 | 18 | 3.24 |
| Right DLPFC | 2.26 | .183711 | 50 | 28 | 28 | 2.9 | |
| Left VLPFC | 3.25 | .018228 | −34 | 20 | −18 | 6.59 | |
| Right VLPFC | 4.04 | .002217 | 36 | 28 | −6 | 5.25 | |
| Left PPC | 3.56 | .008207 | −26 | −56 | 38 | 4.38 | |
| Right PPC | 3.35 | .014018 | 34 | −48 | 46 | 4.35 | |
| Knowing the Target–Solution Search | |||||||
| Main effect | Medial OFC | 3.27 | .017527 | −2 | 42 | −12 | 3.19 |
| ED–ID Switching | |||||||
| Main effect | Left VLPFC | 2.92 | .042071 | −36 | 28 | −2 | 4.41 |
| Right VLPFC | 3.75 | .004956 | 28 | 18 | −20 | 4.96 | |
| Left ACC | 3.59 | .00752 | 4 | 26 | 38 | 4.72 | |
| Right ACC | 2.98 | .035987 | 6 | 24 | 40 | 4.67 | |
| Age effect | Left VLPFC | 1.82 | .040* | ||||
| Right VLPFC | 2.07 | .024* | |||||
| Left ACC | 2.01 | .027* | |||||
| Right ACC | 1.77 | .044* | |||||
| Reversal–Stimulus Set Change | |||||||
| Main effect | Left PPC | 5.13 | <.001 | −34 | −56 | 36 | 7.05 |
| Right PPC | 3.05 | .030432 | 52 | −46 | 40 | 5.1 | |
| Left OFC | 4.54 | <.001 | −32 | 50 | −8 | 4.89 | |
| Right OFC | 4.29 | <.001 | 40 | 46 | −12 | 4.42 | |
| ID Switching–Nonswitching | |||||||
| Main effect | Left DLPFC | 4.32 | .00102 | −42 | 24 | 24 | 6.7 |
| Right DLPFC | 4.28 | .001137 | 40 | 34 | 16 | 5.45 | |
| Left VLPFC | 3.81 | .004169 | −34 | 22 | −6 | 5.77 | |
| Right VLPFC | 3.17 | .022419 | 36 | 22 | −6 | 5.36 | |
| Left PPC | 8.05 | <.001 | −36 | −52 | 42 | 8.94 | |
| Right PPC | 6.57 | <.001 | 36 | −48 | 44 | 8.26 | |
| Left OFC | 3.19 | .021306 | −40 | 46 | −12 | 2.46 | |
| Right OFC | 3.98 | .002585 | 30 | 48 | −12 | 3.87 | |
| Age effect | Left VLPFC | 2.48 | .009* | ||||
| Right VLPFC | 2.06 | .024* | |||||
| True–False Feedback | |||||||
| Main effect | Medial OFC | 5.19 | <.001 | −2 | 46 | −2 | 8.37 |
| Left ACC | 4.28 | .001157 | −16 | 38 | 42 | 3.14 | |
| Right ACC | 2.26 | .186917 | 2 | 34 | 20 | 2.91 | |
| False–True Feedback | |||||||
| Main effect | Left DLPFC | 2.69 | .072924 | −44 | 24 | 26 | 4.12 |
| Right DLPFC | 3.04 | .03115 | 42 | 26 | 30 | 5.87 | |
| Right PPC | 4.17 | .001544 | 36 | −52 | 46 | 5.53 | |
Uncorrected.
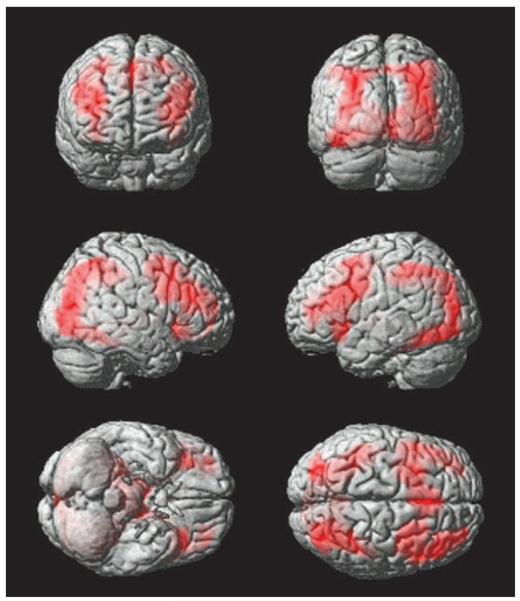
Figure 4.
Brain regions activated when working out which object was the target. This figure displays the whole-brain analysis collapsed across age groups for the solution search phase of the task versus the period of time when the target identity is known, with FDR correction at p < .05 for the whole brain mass. A common fronto-parietal network is recruited during solution search.
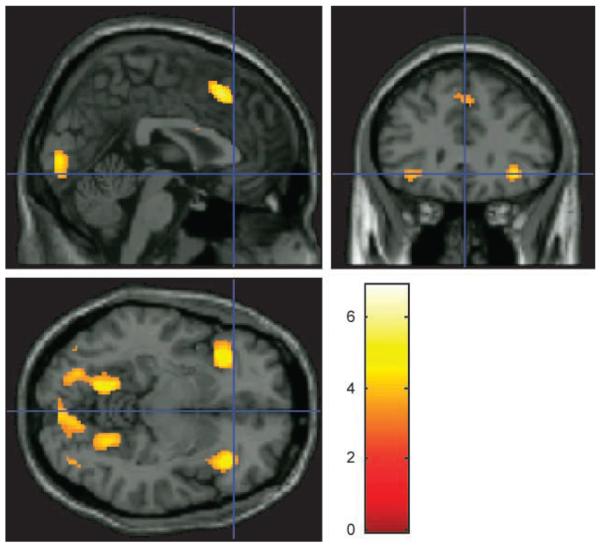
Age-group differences were observed bilaterally in the VLPFC, and the PPC ROIs at the corrected threshold for this contrast, with greater activity in the younger participants (Table 1). The DLPFC ROIs followed the same trend, but were only significantly affected by age at the uncorrected threshold. The whole-brain analysis confirmed this finding with significant age differences observed bilaterally in the VLPFC (FDR corrected for the whole brain mass at p = .05). Significant age differences were also observed in the pre-SMA and in posterior brain visual areas (Figure 5).
Figure 5.
Whole-brain coordinates during solution search when contrasted between age groups. This figure displays the whole-brain analysis contrasted between groups for the solution search phase of the task minus the period of time when the target identity is known, with FDR correction at p < .05 for the whole brain mass. Bilateral regions of the VLPFC, the pre-SMA, and posterior brain visual areas were activated at the corrected threshold.
Switches in the focus of attention between object types (extradimensional) were then compared with switches within object type (intradimensional). The resulting statistical maps were examined at the group level, both collapsed across the age groups, and contrasting between the age groups, using an independent-samples t test. The VLPFC and ACC ROIs were significantly activated bilaterally during extradimensional switching at the corrected threshold (Table 1). There were no significant areas of activation at the whole-brain corrected threshold for this contrast, however, due to the strong prior prediction of VLPFC activation during extradimensional switching in this task (Hampshire & Owen, 2006), we re-examined the whole-brain maps at the more liberal threshold of p = .001, uncorrected. The results concurred well with those from the focused ROI analysis, with significant activity bilaterally in the VLPFC and in ACC during extradimensional switching (Figure 6). Younger adults also displayed greater activity than the older group in both the VLPFC and ACC ROIs, however, this effect was only significant uncorrected for multiple comparisons. There were no other significant age-related activation differences in the whole brain in this contrast.
Figure 6.
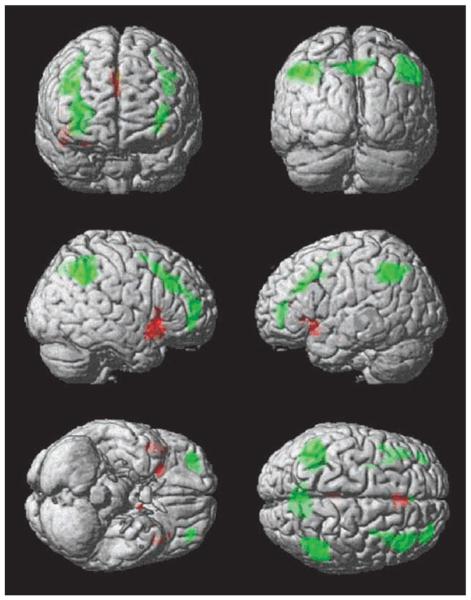
Whole-brain analyses for extradimensional switching and reversal. This figure illustrates the whole-brain analysis for extradimensional versus intradimensional switches of attention (red) and reversal of reward contingency versus stimulus-set change (green). Results are presented at p < .001, uncorrected for display purposes. The bilateral VLPFC and the pre-SMA were associated with the extradimensional shift component of the task, whereas a swathe of the cortex extending from the lateral OFC up to the premotor cortex, and the PPC were associated with the reversal component of the task.
Comparing the ID switching events to the late nonswitch events generated activation peaks in the DLPFC and in the PPC ROIs bilaterally at the corrected threshold. In contrast to previous findings (Hampshire & Owen, 2006), the lateral OFC and the VLPFC ROIs were also significantly activated in this contrast bilaterally. This difference was presumably due to the increased power brought to the analysis by the increased number of participants. These results were confirmed in the whole-brain analysis with FDR correction at p = .05 (Table 1). Age-group differences were again observed in the VLPFC ROIs, with decreased activity in older compared with younger participants, however, this difference was only significant at the uncorrected threshold.
The next contrast compared switches in attentional focus due to reward contingency change with those due to stimulus set change at the group level, both collapsed across the age groups, and contrasting between the age groups, using an independent-samples t test, to examine the reversal component of attentional switching. Significant activity was observed in the lateral OFC and in the PPC ROIs bilaterally at the corrected threshold (Table 1). The whole-brain analysis confirmed the results of the ROI analysis, with significant areas of activation in the PPC and the lateral OFC bilaterally (FDR corrected for the whole brain mass at p = .05). Interestingly, the brain maps appeared to reveal a whole swathe of activity running bilaterally between the lateral OFC, to the premotor cortex along the anterior surface of Brodmann's area 10, and the superior surface of the lateral prefrontal cortex (Figure 6). There were no significant activation differences associated with age group in any of the ROIs or in the unconstrained whole-brain analysis, suggesting that the network underlying reversal learning is relatively retained with age.
To examine those areas involved in abstract reward processing, events involving negative feedback were contrasted with those involving positive feedback at the group level, both collapsed across the age groups, and contrasting between the age groups, using an independent-samples t test. Strong activation was observed in the MOFC ROI when contrasting true minus false feedback events. In addition, activity was observed in the left ACC ROI during reception of positive feedback (Table 1). The reverse contrast generated strong activation bilaterally in the DLPFC and in the right PPC ROIs (Table 1). The whole-brain analysis confirmed the results of the ROI analysis, with strong activation in the MOFC, spreading up the medial wall to ACC during the reception of positive feedback, and bilateral DLPFC and right-sided PPC activation during the reception of negative feedback. In addition, there was strong bilateral temporal cortex activity during positive feedback, and regions of activation during negative feedback in the pre-SMA, and bilaterally in the VLPFC. There were no significant activation differences between the younger and the older groups in either the ROI analysis or the unconstrained whole-brain analysis for this contrast.
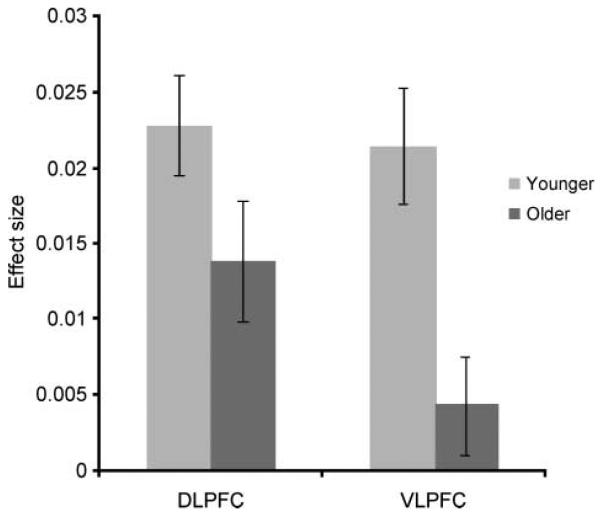
The imaging results appeared to indicate a particular susceptibility to the effects of aging in the more ventral regions of the lateral prefrontal cortex, with relatively retained functionality in the anatomically adjacent DLPFC. To examine this apparent VLPFC/DLPFC dissociation more closely, the data from the contrast of solution search versus knowing the target were extracted from DLPFC and VLPFC ROIs for each individual. These data were then averaged across hemisphere and examined in a one-way ANOVA, in which the factor was ROI (VLPFC vs. DLPFC), with age included as a between-subject factor. An interaction of Age × ROI was evident [F(1, 30) = 4.15, p = .05], indicating that activity in the VLPFC and DLPFC ROIs was differentially affected by age group (Figure 7). There were also significant main effects of ROI [F(1, 30) = 7.54, p = .01] and age group [F(1, 30) = 7.70, p < .01].
Figure 7.
Post hoc analysis of the differential effects of age on activation related to solution search within the DLPFC and the VLPFC. This figure illustrates the average effect size when contrasting the period of time when the target identity was being derived with the period of time when the target was known for the DLPFC and the VLPFC, separately for the older and younger age groups, and collapsed across hemisphere. ANOVA indicated a significant interaction of ROI with age [F(1, 30) = 4.15, p = .051], favoring a greater decrease in activity in the VLPFC with increasing age.
One possible explanation for this result is that the DLPFC in our sample was affected by age at a later stage, and it is notable, therefore, that many studies have focused on participants in only the older age range of 50 through 80 years (e.g., Robbins et al., 1998). A post hoc correlation analysis was therefore carried out to investigate whether the DLPFC or VLPFC ROIs showed a significant effect of age when just the older subgroup (46–77 years) was examined. The data were extracted for the contrast of solution search versus knowing the target from the VLPFC and the DLPFC ROIs for each individual. These data were averaged across hemisphere, and analyzed in SPSS using a series of simple linear regression models in which the dependent variable was effect size, and the independent variable age. In the DLPFC, the regression analysis revealed an inverse correlation with age (standardized beta = −0.769, t = −4.499, p < .001), (ANOVA F = 20.24, p < .001). The VLPFC regression model also revealed an inverse correlation with age (standardized beta = −0.629, t = −3.026, p <.01),(F =9.155, p < .01). The results indicated, therefore, that within the older age group, activity associated with problem solving was negatively correlated with age in both the VLPFC and the DLPFC ROIs.
DISCUSSION
This study has provided a new perspective into the nature of age-related executive dysfunction. Our novel approach, which focused on the individual participant's responses as opposed to experimenter controlled manipulations, has allowed the time course of the individual's chosen problem-solving strategy to be scrutinized with greater precision than has previously been possible. This approach has revealed an age-related difference in top–down executive control in the normal aging population, which behaviorally, is characterized by the use of an inefficient strategy when breaking down a routine/familiar problem. Our task was also specifically designed to both localize those regions of the brain involved in top–down executive control, and to fractionate those brain regions according to their sensitivity to variations in a number of distinct cognitive factors (Hampshire & Owen, 2006). The results reveal that, although the whole fronto-parietal network is recruited during the search for targets, the observed age-related differences in problem-solving strategy are accompanied by reduced activity in the VLPFC and the PPC. Activity in the DLPFC was also affected by age, but only within the older subgroup. Similar age-related decreases in VLPFC activity were observed in the finer contrasts, which examined the self-organized intradimensional and extradimensional switches in the attentional focus. This observation suggests that the VLPFC may play a key role in an individual's ability to optimally sequence the subcomponents of an efficient problem-solving strategy.
The Effects of Aging during Abstract Positive Reward Processing and the Maintenance of Target Identity
The older participants displayed no increased tendency to make errors after the target had been correctly identified, indicating that the processing of positive feedback and the maintenance of the target identity in working memory were not responsible for the observed increase in the general number of errors made in the older group. This observation also rules out the possibility that poor performance in the older group was caused by a general impairment in motor control or visual perception. Either of these low-level impairments would be expected to increase the number of errors, even when the target was correctly identified, due to an increased probability of mistakenly pressing the incorrect key. In the imaging analysis, the MOFC and bilateral temporal cortex regions were associated with the phase of the task when the individual was responding in a routine manner to the location of the correctly identified target object. In concordance with the behavioral results, there were no significant age-related differences in activation in these brain regions.
The Effects of Aging during Abstract Negative Reward Processing and Inhibitory Control
The ability to exert inhibitory control on the basis of negative feedback is known to be a key factor during performance of the WCST and its analogs (e.g., Buchsbaum, Greer, Chang, & Berman, 2005; Konishi et al., 1999). It has previously been reported that inhibitory control is detrimentally affected in the normal aging population, for example, when inhibiting a routine motor response to a rapid and frequent “go” signal on the basis of an infrequent “no-go” signal during go/no-go tasks (see Bedard et al., 2002). An important aspect of our task design, therefore, was that it enabled us to precisely characterize the individuals' choices when it became necessary, at the point of reversal, to inhibit their response to the previously rewarded target object.
Here, the older group displayed no disproportionate age-related increase in either the number of errors made when a reversal was required or in the response time when they switched their response away from the previous target object. Further evidence for a lack of perseverative behavior is provided by the observation that the older participants did not exhibit any tendency to keep responding directly to the previously rewarded target item when the reward contingency was changed. The lack of perseveration observed here indicates that inhibitory control and the processing of abstract negative feedback were relatively preserved in the older group. In concordance with the behavioral data, activation in the lateral OFC, which occurred at the point of reversal in this task, was not significantly affected by age. These results support the hypothesis that inhibitory control forms a cognitively and anatomically distinct component of top–down executive control (Hampshire & Owen, 2006; Miyake et al., 2000; Dias et al., 1996), which is relatively preserved in the aging brain compared with some other components of executive control (Robbins et al., 1998).
It seems likely that the apparent divergence between the results presented here, and those from previous studies reporting age-related deficits in inhibitory control, is due to differences in the specific type of inhibitory control that is required by the task. For example, the go/no-go task, although simple in design, confounds the participant's ability to inhibit a response with their ability to maintain their focus of attention to a rapid and repetitive task. It is interesting to observe, therefore, that performance on the go/no-go task is disrupted in patients with damage to the VLPFC (e.g., Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003), and furthermore, this region is underactivated in the older age group in the current study.
The Effects of Aging during Attentional Set Shifting
In line with our previous findings (Hampshire & Owen, 2006), the VLPFC was significantly more activated when the individual chose to make an extradimensional versus an intradimensional attentional shift. This result concurs well with previous findings in nonhuman primates, which have shown that lesions of the lateral prefrontal cortex impair the ability to perform an extradimensional shift (e.g., Dias et al., 1996). It is surprising, therefore, given that we observed significantly lowered activity bilaterally in the VLPFC during the solution search phase of the task, that the older participants displayed no specific differences in behavior during extradimensional shifting in this task. The older group made neither a disproportionately higher number of errors when an extradimensional switch was required, nor were they disproportionately slower at the point of an extradimensional switch. This lack of a specific difference in switching attention across object dimensions is in direct contrast to previous findings (Gunning-Dixon & Raz, 2003; Robbins et al., 1998). It should be noted, however, that the precise relationship between the VLPFC and extradimensional set shifting is far from clear. This region is known to be involved in a wide range of other tasks that may involve little or no extradimensional shifting component, such as the maintenance of items in working memory (Owen et al., 1999), the recognition of target objects (Hampshire, Duncan, & Owen, 2007), changes in attended items (Hon, Epstein, Owen, & Duncan, 2006), and the deliberate committal of information to long-term memory (Dove, Manly, Epstein, & Owen, 2008). In fact, in contrast to our previous findings (Hampshire & Owen, 2006), the VLPFC was also activated by intradimensional switches of attention when compared to nonswitches, presumably due to the increased statistical power afforded by the larger number of participants used in the current study. Moreover, age-related activation decreases were observed in this region during both extra and intradimensional shifts. On this basis, it seems likely that this region, although involved in extradimensional shifting, also plays a more general role in organizing the top–down control of attention during problem solving.
The Effects of Aging on Efficient Problem-solving Strategy and Top–down Attentional Set
The lowered VLPFC and PPC activation in the older group was concomitant with a general decrease in efficient strategy during the solution search phase of the task. More specifically, when trying to eliminate objects as possible candidates for the current target, older participants tended to repeatedly check those objects that had already been eliminated. They not only made more repetitive responses to the same nontarget object, despite receiving negative feedback, but also had an increased probability of going back and rechecking objects that they had previously eliminated and switched away from. The question remains, therefore, as to what the exact difference is that causes this lack of a coherent problem-solving strategy? One possibility is that the older participants approach the task with no predetermined strategy at all, instead, randomly selecting one of the four objects from trial to trial, unless positively rewarded. The fact that there is no interaction between age and extradimensional shifting, but a significant extradimensional shifting main effect, suggests that the older participants do, in fact, approach the task with at least some degree of strategy. Thus, no strategy at all would effectively nullify the difference between extradimensional and intradimensional shifting, as at the point of target change, the individual would tend to randomly choose the candidate objects with no regard for the recently attended category (Williams-Gray, Hampshire, Barker, & Owen, 2008; Roberts et al., 1994). It seems probable, therefore, that the age-related decrease in efficient problem solving observed here is caused by an inability to optimally sequence the strategic subgoals. This hypothesis concurs well with the previous observation by Robbins et al. (1998) that, although older participants undertaking a complex spatial search task do attempt to use a defined strategy, they do not reap the same level of benefit from that use of strategy. There are two clear hypotheses capable of explaining this loss of coherent strategy. One obvious possibility is that older individuals may find it harder to maintain the object identities in working memory, with this working memory difference reflected in reduced VLPFC activity. It seems unlikely that the age-related difference observed here reflects a simple failure of working memory for objects, however, as the older participants were clearly able to maintain the target identity once it had been identified. That said, it is important to note that the observed problem in self-organizing a strategy in the face of multiple demands could still be explained by a working memory deficit that only becomes apparent at higher load, as when the target is unknown, up to three nontargets may need to be maintained, compared with only one when the target has been identified. However, a recent meta-analysis of studies (Verhaeghen & Cerella, 2002) has reported no consistent age-related working memory deficit. Furthermore, Robbins et al. (1998) also reported remarkably preserved performance even in a 75- to 79-year-old group during basic tests of working memory. An alternative possibility is that, in line with previous findings (Verhaeghen & Cerella, 2002), the age-related difference observed during problem solving is caused by a problem that occurs when the older participants are faced with multiple competing choices, in which there is no clear winner, and an overall logical strategy must be applied. We suggest, therefore, that in this study, the impairment in the older participants reflects a decrease in their ability to select among the various subgoals required to maintain a consistent and efficient strategy for problem solving in the face of strong competition from distractors. This behavioral impairment is related to reduced activity in the VLPFC, which we believe plays a crucial role in maintaining the attentional focus on the task at hand. A likely mechanism for this role, suggested by recent imaging studies (e.g., Hampshire et al., 2007; Hampshire & Owen, 2006; Kastner & Ungerleider, 2001) and monkey electrophysiology data (e.g., Freedman, Riesenhuber, Poggio, & Miller, 2001; Desimone & Duncan, 1995), is that the VLPFC acts by biasing or “tuning” attentional processing between competing representations in modality-specific posterior regions in order to maintain their relevance to current behavioral goals. Such a view is anatomically plausible given the strong bidirectional connections between many posterior cortical association areas and the mid-ventrolateral frontal region, which, in turn, is closely interconnected with the entire lateral prefrontal cortex (Petrides, 1994). Moreover, a frontal module with such properties has been proposed recently (Frank, Seeberger, & O'Reilly, 2004; O'Reilly, Noelle, Braver, & Cohen, 2002; see also, Dehaene, Kerszberg, & Changeux, 1998), although in those computational models, the critical region was defined rather more generally as the “lateral prefrontal cortex.” Flexible tuning of task-relevant variables within the mid-ventrolateral frontal cortex would be consistent with accounts of prefrontal function that emphasize its importance in switching (Hampshire & Owen, 2006; Cools, Clark, Owen, & Robbins, 2002; Nakahara et al., 2002; Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000; Konishi et al., 1999) and the “top–down” modulation of attention (e.g., Dias et al., 1996; Desimone & Duncan, 1995; Owen et al., 1993; Owen, Roberts, Polkey, Sahakian, & Robbins, 1991). Compromising such a function would be expected to affect a wide variety of tasks at a rather general level, a suggestion that is entirely consistent with the reported behavioral profile of the normal aging population (Verhaeghen & Cerella, 2002).
These results suggest, therefore, that previous findings reporting a specific deficit at the stage of extradimensional shifting in older individuals are probably not due to the need to shift between object or task dimensions per se (Gunning-Dixon & Raz, 2003; Robbins et al., 1998), but rather, one of the other factors with which this cognitive demand is typically confounded. During the problem-solving phase of the CANTAB extradimensional shifting task (Robbins et al., 1998) novelty, combined with poor strategy and a partial reward contingency when selecting exemplars from the irrelevant stimulus dimension, may make it particularly hard to identify the fact that an extradimensional switch is required at all. Likewise, in the case of the WCST (Gunning-Dixon & Raz, 2003), a general lack of ability when eliminating nontarget exemplars would be expected to cause an increase in the perseveration measure, but it would also be expected to cause more errors in general (Hartman, Bolton, & Fehnel, 2001).
Age-related Differences in the Posterior Parietal Cortex
Age-related differences in activity were also observed in the PPC when the target object was being derived. There was, however, no evidence of age-related activation differences in the finer contrasts between the different event types that constitute the search for the target item. This lack of specificity makes the role of the PPC in the age-related behavioral differences difficult to define. One possibility is that the activation differences in this region, along with those observed in the lower visual areas, are secondary consequences of decreased top–down modulation (Desimone & Duncan, 1995) due to age-related changes in frontal activity. However, one should not rule out the possibility that the PPC plays a more central role in both top–down executive control, and the age-related behavioral differences observed here.
Evidence for Differential Rates of Decline in the Lateral Prefrontal Cortex
In line with previous imaging findings using this task (e.g., Williams-Gray et al., 2008; Hampshire & Owen, 2006), the DLPFC was activated to a similar extent throughout the solution search phase of the task, with activity in this region decreasing only once the target had been correctly identified. In this context, it is interesting to note that, although the older participants were inefficient in their elimination of nontarget items, there was no significant effect of age on the DLPFC in the main group analysis. However, a supplementary analysis, which examined just the older subgroup (46–77 years), did reveal a large negative correlation between activation and age in this region. This finding tentatively indicates that age-related decreases in activation in the DLPFC and VLPFC may occur at different rates, with the VLPFC affected earlier than the DLPFC, suggesting an intriguing possibility for future research. If correct, this possibility suggests that, in any given study, the observed loci of age-related activation change in the lateral prefrontal cortex could be dependent upon the exact age groups compared, potentially explaining some of the controversy in the current literature. For example, the majority of our participants were in their late 50s to early 60s, and the primary activation differences observed were in the VLPFC. However, previous studies of aging typically focus on older participants, and may therefore tend to elicit differences in the DLPFC (e.g., Robbins et al., 1998). In addition, it seems sensible to suggest that with increased aging, as more regions of the brain become significantly compromised, the range of cognitive processes that are affected is likely to diversify.
Summary
In summary, using a novel attentional switching task that examines individuals chosen problem-solving strategies, we have demonstrated that a decrease in the ability to optimally structure the subcomponents of complex goal-directed behavior could be a key factor in age-related decline. The neural correlates of this age-related difference in top–down executive control appear to lie within the more ventral subregion of the lateral prefrontal cortex and in the posterior parietal cortex. By contrast, functionality in the dorsolateral prefrontal cortex appeared to follow a slower degenerative time course, with activity in regions of the orbito-frontal cortex remaining relatively stable in the aging brain.
Acknowledgments
We thank the Wellcome Trust for their support in providing funds for this project.
REFERENCES
- Aine CJ, Woodruff CC, Knoefel JE, Adair JC, Hudson D, Qualls C, et al. Aging: Compensation or maturation? Neuroimage. 2006;32:1891–1904. doi: 10.1016/j.neuroimage.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Knight RT. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia. 2002;40:349–356. doi: 10.1016/s0028-3932(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Bedard A, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Developmental Neuropsychology. 2002;2:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract]; Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2–6.2002. [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting Task and Component Processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack R, Brett M, Osswald K. An evaluation of the use of magnetic field maps to undistort echo-planar images. Neuroimage. 2003;18:127–142. doi: 10.1006/nimg.2002.1281. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Kerszberg M, Changeux J. A neuronal model of a global workspace in effortful cognitive tasks. Neurobiology. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of attentional and affective shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dove A, Manly T, Epstein RA, Owen AM. The engagement of mid-ventrolateral prefrontal cortex and posterior brain regions in intentional cognitive activity. Human Brain Mapping. 2008;29:107–119. doi: 10.1002/hbm.20378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: An event-related fMRI study. Brain Research, Cognitive Brain Research. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nature Reviews Neuroscience. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:10. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger L, O'Reilly RC. By carrot or by stick: Cognitive reinforcement learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Duncan J, Owen AM. Selective tuning of the BOLD response during simple target detection dissociates human frontoparietal sub-regions. Journal of Neuroscience. 2007;27:6219–6223. doi: 10.1523/JNEUROSCI.0851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event related fMRI. Cerebral Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hartman M, Bolton E, Fehnel S. Accounting for age differences on the Wisconsin Card Sorting Test: Decreased working memory, not inflexibility. Psychology and Aging. 2001;16:385–399. [PubMed] [Google Scholar]
- Hon N, Epstein RA, Owen AM, Duncan J. Frontoparietal activity with minimal decision and control. Journal of Neuroscience. 2006;26:9805–9809. doi: 10.1523/JNEUROSCI.3165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003:302. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function and social decision-making: A dorsolateral prefrontal theory of cognitive aging. Psychology and Aging. 2002;17:598–609. [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Reviews of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: Distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma R, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. Journal of Neuroscience. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- Nordahl WC, Ranganath C, Yonelinas AP, DeCarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. Journal of Cognitive Neuroscience. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. Plenum Press; New York: 1980. [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Noelle DC, Braver TS, Cohen JD. Prefrontal cortex in dynamic categorization tasks: Representational organization and neuromodulatory control. Cerebral Cortex. 2002;12:246–257. doi: 10.1093/cercor/12.3.246. [DOI] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SPMJ, Carpenter TA, et al. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. European Journal of Neuroscience. 1999;11:567–574. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:99–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences, U.S.A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Vol. 9. Elsevier; Amsterdam: 1994. pp. 59–82. [Google Scholar]
- Petrides M. Impairments on non-spatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. Journal of Neuroscience. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Ciranni MA. Contributions of the prefrontal cortex and basal ganglia to set-shifting. Journal of Cognitive Neuroscience. 2002;14:472–483. doi: 10.1162/089892902317361985. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, et al. One-year age changes in MRI brain volumes in older adults. Cerebral Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Rettmann ME, Kraut MA, Prince JL, Resnick SM. Cross-sectional and longitudinal analyses of anatomical sulcal changes associated with aging. Cerebral Cortex. 2006;16:1584–1594. doi: 10.1093/cercor/bhj095. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. Journal of the International Neuropsychological Society. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. Journal of Neuroscience. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set-shifting and reversal learning in humans. Journal of Cognitive Neuroscience. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Picard JD, Sakakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat D, Buckner R, Snyder A, Greve D, Desikan R, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Archives of Neurology. 2001;58:1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Argita EJS, van Boxtel MPJ, Evans AC, Jolles J, et al. Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labelling of activations in spm using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;15:273–283. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uylings HB, De Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease. Brain and Cognition. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella N. Aging, executive control, and attention: A review of meta-analyses. Neuroscience and Biobehavioral Reviews. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang G-J, Fowler JS, Moberg PJ, Ding Y-S, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. American Journal of Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Williams-Gray C, Hampshire A, Barker AR, Owen AM. Attentional control in Parkinson's disease is modulated by the COMT val158met polymorphism. Brain. 2008;131:397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]