Abstract
The recruitment and trafficking of leukocytes are essential aspects of the inflammatory process. Although chemokines are thought to be the main regulators of cell trafficking, extracellular cyclophilins have been shown recently to have potent chemoattracting properties for human leukocytes. Cyclophilins are secreted by a variety of cell types and are detected at high levels in tissues with ongoing inflammation. CD147 has been identified as the main signaling receptor for cyclophilin A (CypA) on human leukocytes. It is interesting that the expression of CD147 is elevated on leukocytes from inflamed tissue, suggesting a correlation among the presence of extracellular cyclophilins, CD147 expression, and inflammatory responses. Thus, cyclophilin-CD147 interactions may contribute directly to the recruitment of leukocytes into inflamed tissues. In the current studies, we show that activated human T lymphocytes express elevated levels of CD147, compared with resting T cells and that these activated T cells migrate more readily to CypA than resting cells. Furthermore, we show that unlike resting CD4+ T cells, the cyclophilin-mediated migration of activated T cells does not require interaction with heparan sulfate receptors but instead, is dependent on CD147 interaction alone. Such findings suggest that cyclophilin-CD147 interactions will be most potent when leukocytes are in an activated state, for example, during inflammatory responses. Thus, targeting cyclophilin-CD147 interactions may provide a novel approach for alleviating tissue inflammation.
Keywords: chemokines, inflammation
INTRODUCTION
Cyclophilins are ubiquitously distributed intracellular proteins, first recognized as the host cell receptors for the potent immunosuppressive drug Cyclosporine A. Although most studies have focused previously on the intracellular activities of cyclophilins, accumulating evidence suggests a role for these proteins as mediators of intercellular communication [1, 2]. Indeed, several groups, including ours, have observed that secreted cyclophilin A (CypA) is a potent leukocyte chemoattractant in vitro [3–5]. CypA has also been shown to elicit inflammatory responses, characterized by a rapid influx of leukocytes, when injected in vivo [4]. Our group previously identified CD147, a Type I transmembrane protein, as the receptor for extracellular cyclophilins and demonstrated that the presence of CD147 is essential for cyclophilin-mediated events to occur [3]. However, the relevance of these findings to physiological or pathological conditions remains unclear.
Indirect evidence that extracellular cyclophilins might be a contributing factor during disease pathology is provided by several human inflammatory conditions in which the presence of increased levels of CypA and CypB has been observed. These include rheumatoid arthritis [6, 7], vascular smooth muscle cell disease [8], and severe sepsis [9]. For example, the level of CypA in synovial fluid isolated from rheumatoid arthritis patients has been reported to correlate directly with the number of neutrophils present within synovial spaces, as well as with disease severity [6]. Furthermore, recruited and circulating leukocytes have an up-regulated expression of CD147 [10–12] in arthritic patients. Such findings strongly implicate a role for extracellular cyclophilins, via interaction with CD147, on the recruitment of circulating leukocytes to sites of inflammation and suggest that cyclophilin-CD147 interactions might contribute directly to the pathogenesis of inflammatory diseases. In support of this, we reported recently that treatment with anti-CD147 mAb could reduce leukocyte influx significantly to inflamed lungs in mouse models of acute lung injury [13] and allergic asthma [14].
In vitro studies, looking at the capacity of extracellular cyclophilins to promote integrin-mediated adhesion of T lymphocytes to extracellular matrix proteins, have demonstrated significant differences in responsiveness to cyclophilins, correlating with the differentiation status of the T cells [15]. These findings suggest that extracellular cyclophilins might interact preferentially with specific populations of T cells and therefore, induce the recruitment of these subsets preferentially during inflammatory responses. Unlike naive lymphocytes, memory and activated T cells in blood can migrate to peripheral, nonlymphoid organs, and this migration is enhanced greatly by inflammatory conditions in tissue (reviewed in ref. [16]). Furthermore, activated T lymphocytes have been shown to contribute directly to the progression of tissue inflammation by release of proinflammatory cytokines, which activate bystander leukocytes, or by direct cytolytic activity [17, 18]. Such findings led us to investigate whether extracellular cyclophilins might have the capacity to interact more readily with activated populations of lymphocytes and whether such activity might be CD147-mediated. Thus, in the current study, we have examined the capacity of CypA to induce the migration of human CD4+ T cells and establish how the responses relate to CD147 expression. Our findings demonstrate that the extent of cyclophilin-induced migration correlates with the activation status of a CD4+ T cell and is associated with increased expression of CD147 receptors.
MATERIALS AND METHODS
Isolation of human leukocytes
Blood was obtained from healthy human donors by venipuncture and the PBMCs enriched by centrifugation over lymphocyte-separating medium (LSM; Mediatech Inc., Herndon, VA, USA). Isolation of CD4+ T cells was conducted by MACS separation using a negative depletion kit (Miltenyi Biotech, Aurora, CA, USA). Neutrophils were enriched from blood by centrifugation over Histopaque 1077 (Sigma-Aldrich, St. Louis, MO, USA), followed by differential sedimentation in 3% dextran solution. For isolation of PBLs, RBCs were removed by water lysis of whole blood.
Flow cytometric analysis
PBMCs or PBLs were costained with TriColor (TC)-conjugated anti-CD4 or APC-conjugated anti-CD14 and FITC anti-human CD147 (Research Diagnostics, Flanders, NJ, USA) or FITC-IgG1 isotype control mAb. For studies looking at activation markers, PE-labeled anti-CD25 or PE-labeled anti-HLA-DR was included. Staining was conducted on ice for 30 min, followed by fixation in 1% paraformaldehyde. Flow cytometric analysis was done using a FACSCalibur instrument and CellQuest software (Becton Dickinson, San Jose, CA, USA).
In vitro activation of T lymphocytes
PBMCs were suspended in tissue-culture medium (Clicks medium containing 5% FCS) at 3 × 106 per ml in the presence of 1 μg/ml PHA (Sigma-Aldrich) or 1 ng/ml Staphylococcal enterotoxin (SEA; Sigma-Aldrich). The cultures were incubated at 37°C for 24 – 48 h. Cells recovered from PHA or SEA cultures were centrifuged over LSM to remove dead cells and debris and then used immediately for flow cytometric staining or were enriched for CD4+ T cells by MACS separation for chemotaxis assays or Western blot analysis. For studies comparing activated versus nonactivated CD4+ T cells, a fresh blood draw from the same donor was used as the source of nonactivated cells on the day of the assay. For studies in which heparan sulfate receptors were removed, populations of PHA-activated and nonactivated PBMCs were treated for 3 h at 37°C with Heparinase I (Sigma-Aldrich) at two units per 106 cells prior to CD4+ T cell purification.
Chemotaxis assays
All chemotaxis assays were conducted using purified CD4+ T cells. The assays were set up using 48-well-modified Boyden chambers (Neuro Probe Inc., Gaithersbug, MD, USA) with the two compartments separated by a 5-μm polycarbonate membrane (Neuro Probe Inc.). CD4+ T cells (104 cells/well) in RPMI-1640 culture medium, supplemented with 1% BSA, were added to the upper compartments, and medium containing different dilutions of recombinant human CypA (Calbiochem, La Jolla, CA, USA) or recombinant human RANTES (Endogen, Woburn, MA, USA) at 1 ng/ml or medium alone was added to the lower compartments. For blocking experiments, anti-human CD147 mAb (Ancell, Bayport, MN, USA) or IgG1 isotype control mAb were added at an optimal concentration of 10 μg/ml to the upper and lower compartments. The chambers were incubated for 1 h at 37°C, after which the membrane was removed, the nonmigrated cells scraped off, and the membranes then stained with Wright-Giemsa (Camco, Fort Lauderdale, FL, USA) to discriminate bound cells. A chemotactic index was calculated for each test well by dividing the number of cells counted for that well by the number of cells counted in media wells. In some studies, equivalent numbers of activated and nonactivated CD4+ T cells were compared side-by-side, or activated CD4+ T cells were diluted 1:1, 1:2, 1:4, and 1:8 with nonactivated CD4+ T cells to establish a serial dilution.
RESULTS
CD147 is up-regulated on activated human CD4+ T cells
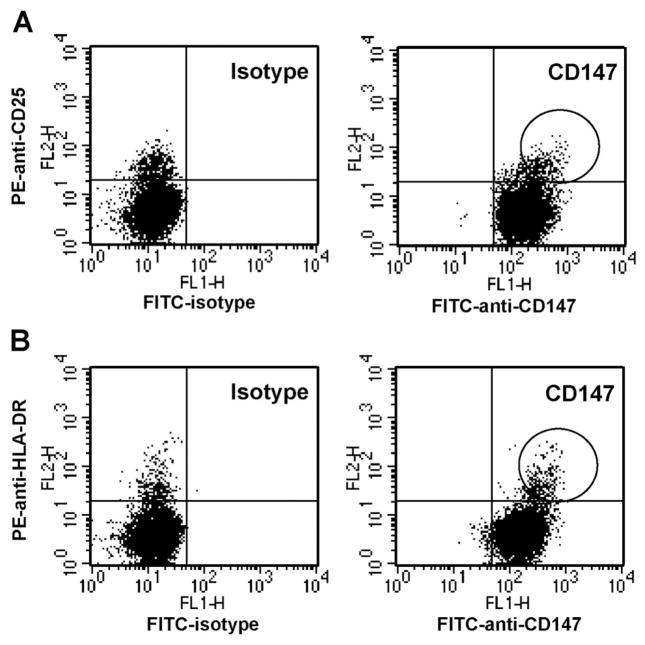
In initial studies, we established the baseline expression of CD147 on circulating, nonactivated versus activated CD4+ T cells. For these studies, human PBMCs were costained with anti-CD4 and anti-CD25 or anti-HLA-DR (markers associated with the activation status of lymphocytes) plus anti-human CD147 or isotype control mAb. Figure 1 shows the distribution of isotype and CD147 expression versus CD25 (Fig. 1A) or HLA-DR (Fig. 1B), after gating on CD4+ lymphoid cells. Although CD147 was expressed at a high level on all CD4+ T cells, we observed that CD25+ and HLA-DR+ cells expressed the highest levels of CD147 (CD147bright). Among four different donors, the average CD147 mean fluorescence intensity (MFI) of CD4+ T cells with an activated status was 82.9 ± 1.8 MFI units, compared with resting cells, which was 53.6 ± 1.2 units. This difference was highly significant (P<0.0001). The observation that activated CD4+ T cells express higher levels of CD147 than resting cells was confirmed by generating populations of CD4+ T cells enriched for activated cells by in vitro activation using PHA. Figure 2 shows the expression of CD147 versus CD25 or HLA-DR on CD4+ T cells after 48 h of PHA activation. As observed with peripheral CD4+ T cells, the CD147bright subset of T cells cosegregated with elevated expression of CD25 and HLA-DR, confirming that activated CD4+ T cells express higher levels of CD147 than nonactivated cells.
Fig. 1.
CD147 expression is up-regulated on circulating, activated CD4+ T cells. PBMCs obtained from human peripheral blood were stained with TC-conjugated anti-CD4 and FITC-conjugated anti-CD147 mAb or isotype control mAb plus PE-conjugated anti-CD25 or anti-HLA-DR. Dot plots show expression of CD147 or isotype versus CD25 expression (A) or HLA-DR (B) after gating on lymphoid CD4+ cells. Circled regions denote populations of cells showing CD147bright expression.
Fig. 2.
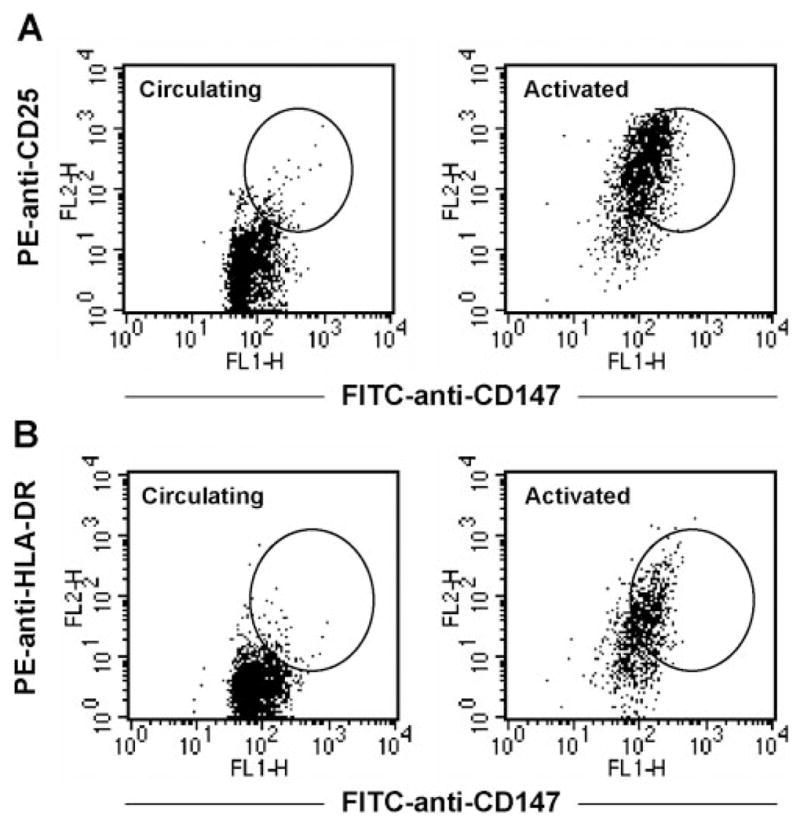
CD147 expression is up-regulated on in vitro-activated CD4+ T cells. PBMCs obtained from human peripheral blood were stimulated in vitro with PHA for 48 h. These activated cells, as well as circulating PBMCs from the same donor, were stained with TC-conjugated anti-CD4 and FITC-conjugated anti-CD147 plus PE-conjugated anti-CD25 or PE-conjugated anti-HLA-DR. Dot plots show expression of CD147 versus CD25 (A) and CD147 versus HLA-DR (B) on circulating and PHA-activated CD4+ T cells. Circled regions denote populations of cells showing CD147bright expression.
Activated T cells show enhanced migration to extracellular cyclophilins
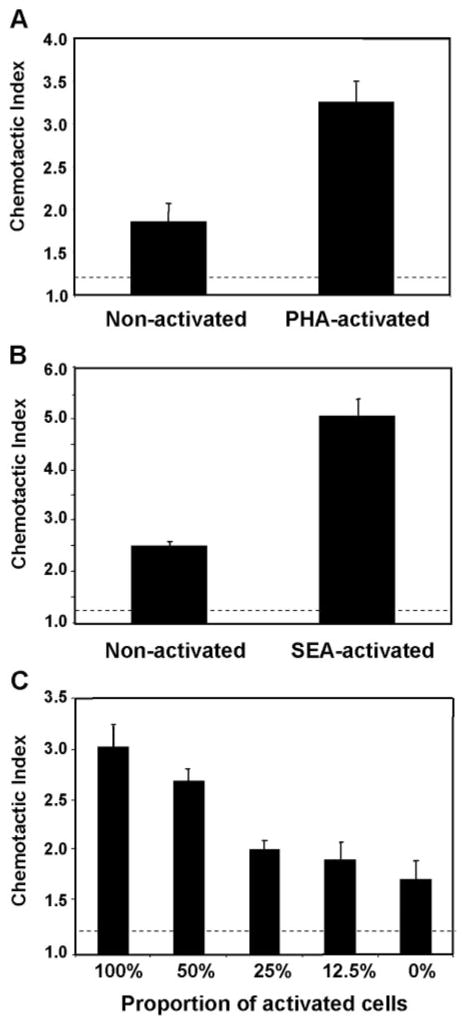
Our next question was to address the correlation between CD147 expression and cyclophilin-mediated responses. Thus, we compared the capacity of recombinant CypA to induce migration in populations of resting CD4+ T cells versus populations with a high frequency of activated CD4+ T cells. For these studies, human PBMCs were stimulated in vitro with PHA or SEA to generate populations containing a high frequency of activated T cells. After activation, the CD4+ T cells were purified and compared with CD4+ T cells obtained from a fresh blood draw from the same donor, consisting of mostly (>90%) resting cells. The two pools of CD4+ T cells were set up in Boyden chambers in the presence of a previously optimized dose of recombinant CypA or medium alone. Based on published studies [15] and previous experience, a chemotactic index of >1.2 (shown as dashed line in figure) was considered significant. Figure 3, A (PHA-activated) and B (SEA-activated), shows that resting and activated populations of CD4+ T cells migrated in response to CypA. However the activated population responded with a significantly greater chemotactic index than resting cells. Furthermore, this increase in response could be diluted out by reducing the ratio of activated:resting CD4+ T cells within the activated pool (Fig. 3C). Taken together, these findings suggest that activated T cells migrate more readily in response to extracellular CypA than their resting counterparts and suggest that their interaction with CypA may be more sensitive. It is interesting that previous studies have reported that neutrophils and monocytes also demonstrate high-potency migration in response to CypA [3, 11]. As shown in Figure 4, the elevated level of CD147 expression on activated CD4+ T cells is comparable with that on neutrophils and monocytes, suggesting a likely association between potent CypA-induced responses and elevated CD147 expression.
Fig. 3.
Activated CD4+ T cells migrate more readily to CypA than nonactivated cells. The capacity of recombinant CypA to induce migration of nonactivated versus activated CD4+ T cells was examined using 48-well Boyden chemotaxis chambers. Populations of activated cells were generated by stimulating human PBMCs with PHA or SEA, followed by MACS separation to isolate CD4+ T cells. These activated CD4+ T cells were compared with circulating CD4+ T cells isolated from the same donor. The migratory response of PHA-activated (A) or SEA-activated (B) CD4+ T cells versus nonactivated (circulating) CD4+ T cells to 100 ng/ml CypA is shown. (C) Serial dilutions of activated CD4+ T cells mixed with nonactivated CD4+ T cells were stimulated in the presence of 100 ng/ml CypA. Bar graphs in all panels show mean (±SE) chemotactic index (number of cells migrating in response to chemotactic agent/number of cells migrating to medium alone) for each group, and n = 4 wells per group. Dashed lines delineate significant chemotaxis (>1.2).
Fig. 4.
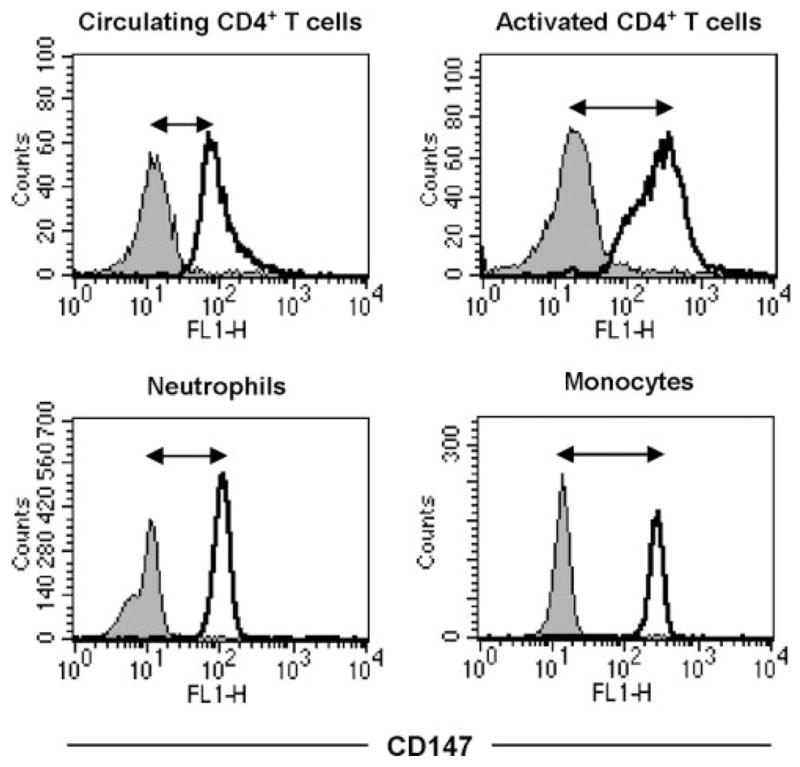
CD147 expression on activated CD4+ T cells is comparable with expression on neutrophils and monocytes. PBMCs obtained from human peripheral blood were stimulated in vitro with PHA for 24 h. The next day, a fresh blood draw was used to obtain PBLs, which served as a source of nonactivated T cells, neutrophils, and monocytes. Cells were stained with FITC-conjugated anti-CD147 or isotype control mAb plus TC-conjugated anti-CD4 or APC-conjugated CD14 to gate on neutrophils (CD14-negative) and monocytes (CD14-positive). Histograms show expression of CD147 on individual populations of leukocytes after gating on their representative markers.
Cyclophilin-mediated migration of resting and activated T cells is CD147-dependent
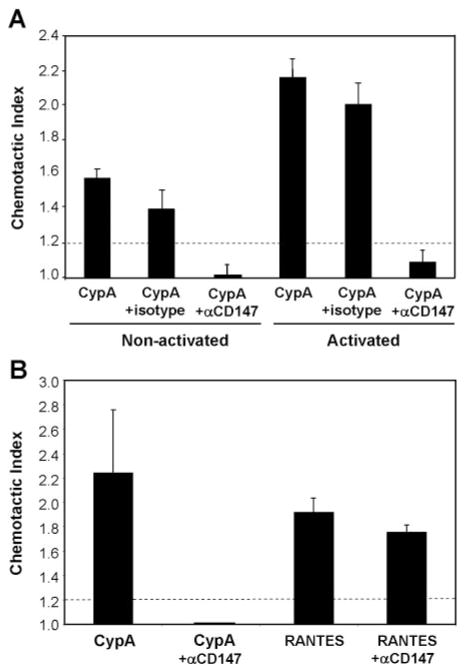
To address the importance of CD147 expression on the observed migration of resting and activated T cells to CypA, we conducted experiments in which anti-CD147 mAb (or IgG1 isotype mAb) was included in the chemotaxis assay. As shown in Figure 5A, the presence of anti-CD147 mAb inhibited the CypA-mediated chemotactic responses of resting CD4+ T cells, as well as activated CD4+ T cells, by >90%. It is important that anti-CD147 mAb had no impact on the chemotactic responses of the same cells to RANTES, confirming the specificity of cyclophilin-CD147 interactions in activated CD4+ T cells (Fig. 5B). Further evidence that these interactions might be more potent in activated compared with resting CD4+ T cells was obtained in experiments looking at the requirement for cointeraction with cell surface heparan sulfate receptors. Previous studies have shown that extracellular cyclophilins must bind initially to cell surface heparan sulfate proteoglycans before they can interact with CD147 signaling receptors. Indeed, preincubation of human neutrophils [3] or resting T lymphocytes [15] with heparinase was shown to inhibit cyclophilin-mediated signaling events by >90%. It is striking that in our current studies, we observed that although heparinase treatment of nonactivated (resting) CD4+ T cells resulted in a >95% inhibition in CypA-induced migration, the same treatment had no impact on the migration of activated CD4+ T cells (Fig. 6A). Primary T cells, including activated CD4+ T cells, have been shown previously to express very low levels of heparans, relative to other leukocyte subsets [19, 20], so it is unlikely that the heparinase treatment was ineffective at removing all cell surface heparan sulfates from the activated T cells. Indeed, as shown in Figure 6B, the same heparinase regimen abrogated the capacity of neutrophils, known to require heparan cointeraction [3], to respond to CypA. Further-more, activated CD4+ T cells treated with a fivefold greater dose of heparinase still demonstrated a potent migration to CypA (data not shown).
Fig. 5.
CypA-mediated migration of CD4+ T cells is CD147-dependent. (A) Activated and circulating (nonactivated) CD4+ T cells were stimulated with 100 ng/ml CypA, with or without 10 μg/ml anti-CD147 or isotype control mAb. (B) Activated CD4+ T cells were stimulated with 100 ng/ml CypA or 1 ng/ml RANTES, with or without 10 μg/ml anti-CD147. Graphs show mean (±SE) chemotactic index for each group, and n = 4 wells per group. Dashed lines delineate significant chemotaxis (>1.2). (A and B) Studies were performed using cells from different donors.
Fig. 6.
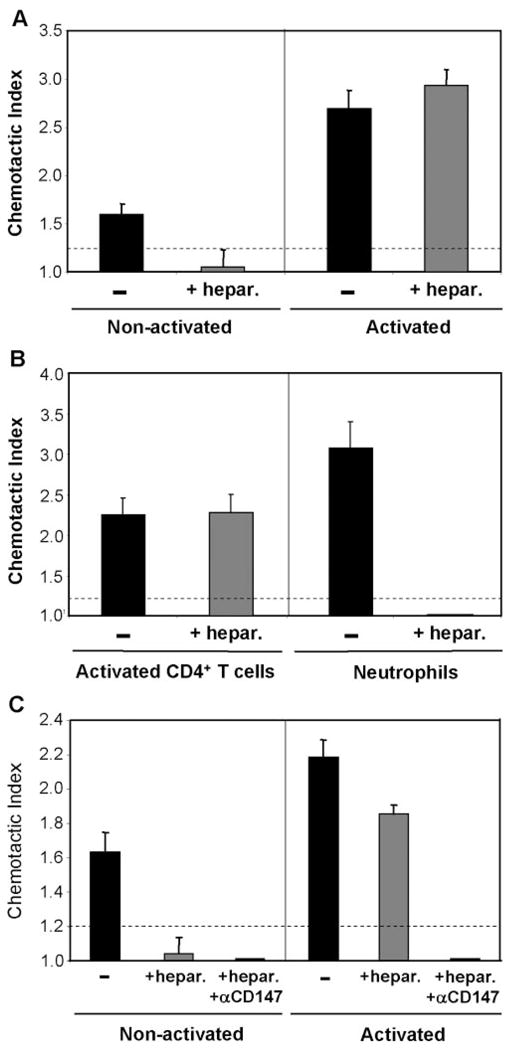
CypA-mediated migration of activated CD4+ T cells does not require cell surface heparans. Groups of nonactivated and activated PBMCs were treated with Heparinase I prior to CD4+ T cell isolation. Each group was then tested for cyclophilin-mediated chemotaxis using 100 ng/ml CypA, with or without 10 μg/ml anti-CD147 mAb. (A) Bar graphs show the CypA-mediated chemotaxis of untreated versus heparinase-treated, nonactivated and activated CD4+ T cells. (B) Bar graphs show the CypA-mediated chemotaxis of activated CD4+ T cells and neutrophils, with and without heparinase treatment. (C) Same groups as A, with additional groups of heparinase-treated cells, which included anti-CD147 mAb during chemotaxis. All graphs show mean (±SE) chemotactic index for each groups, and n = 4 – 6 wells per group. Dashed lines delineate significant chemotaxis (>1.2). Studies were performed using cells from different donors.
To establish whether the cyclophilin-induced migration of activated CD4+ T cells treated with heparinase was still dependent on interaction with CD147 receptors, chemotaxis assays were conducted with heparinase-treated CD4+ T cells in the presence of anti-CD147 mAb. As shown in Figure 6C, anti-CD147 antibody inhibited the migration of heparinase-treated, activated CD4+ T cells by >90%, demonstrating a continued dependence on CD147 for migration to extracellular cyclophilins. Taken together, these findings suggest that the interactions, which regulate cyclophilin-mediated migration in T cells, may be less stringent in activated compared with resting cells and that CD147 alone may be sufficient to induce the binding and signaling events required for activated T cell migration/recruitment to extracellular cyclophilins.
DISCUSSION
The long-term objective of our studies is to identify novel factors during inflammatory processes, which might be targeted for therapeutic intervention or prevention. Given that extracellular cyclophilins are present at high levels during inflammatory responses and that extracellular cyclophilins are potent chemoattractants for many proinflammatory leukocytes, including neutrophils, eosinophils, and monocytes, we have proposed that cyclophilins might contribute directly to inflammatory responses by recruiting leukocytes into inflamed tissues. In the current study, we examined the capacity of extracellular cyclophilins to induce the migration of another subset of leukocytes that contributes significantly to the pathology of many inflammatory diseases—activated CD4+ T cells. Our findings have demonstrated the following observations: Activated CD4+ T cells have a higher expression of CD147, the main signaling receptor for CypA, than nonactivated CD4+ T cells; activated CD4+ T cells migrate more readily to CypA than nonactivated CD4+ T cells; and unlike resting T cells, the CypA-mediated migration of activated CD4+ T cells does not require interaction with cell surface heparans and is dependent on CD147 alone. The elevated expression of CD147 on activated CD4+ T cells fits with the findings of increased CD147 expression on leukocytes present within inflammatory sites, including fibro-blast-like cells, granulocytes, and macrophages in the synovial joints of rheumatoid arthritis patients [10, 11, 21]. Whether the increased expression of CD147 observed in these synovial cells was present prior to their entry into tissues or was induced subsequent to their entry is unknown. However, elevated levels of CD47 have been reported on circulating neutrophils [22] as well as monocytes [11] in rheumatoid arthritis patients. Our current findings with CD4+ T cells suggest that a high expression of CD147 will likely favor the recruitment of cells into tissues with elevated cyclophilins. Indeed, as shown in our current studies, the highest levels of CD147 expression are found on leukocytes often associated with tissue inflammation, including neutrophils, monocytes, and activated T cells.
Our findings that the CypA-mediated chemotaxis of activated CD4+ T cells does not require an initial interaction with heparan sulfate proteoglycans are intriguing. To date, several leukocyte subsets, including neutrophils, resting memory CD4+ T cells, and naïve CD4+ T cells, have been reported to lose their capacity to respond to cyclophilins following treatment with Heparinase I, II, or III [3, 15]. A potential contributing factor for heparan-independent responses to CypA in activated T cells is that CD147 molecules can cluster upon cell activation. For example, previous studies have shown that low-affinity mAb specific for CD147 were able to bind to PHA-activated but not resting T cells [23]. The investigators attributed this finding to clustered-upon-activation CD147 molecules, which facilitate a more stable, bivalent interaction of the low-affinity mAb. Clustering may enable a more potent and/or stable interaction between CD147 and CypA, thereby reducing the dependence on heparans for optimal CD147-mediated signaling. Studies are underway currently in our laboratory to establish whether such clustering is required for the changes in responsiveness of activated T cells or whether an elevated density of monomeric CD147 molecules is sufficient for enhanced responsiveness.
Although our data provide strong evidence that activated CD4+ T cells are recruited more readily by CypA than resting T cells, such findings need to be put in the context of an in vivo inflammatory response, where multiple cytokine- and chemokine-mediated signals will be ongoing. To address the contribution of extracellular cyclophilins, relative to other chemoattractants in vivo, we conducted studies using several different animal models of inflammatory disease in which elevated levels of extracellular cyclophilins are induced [13, 14]. We observed that blocking cyclophilin-CD147 interactions using in vivo anti-CD147 mAb treatment significantly reduces the recruitment of proinflammatory leukocytes into inflamed tissues. It is interesting that in the case of asthma-mediated lung inflammation, the anti-CD147 treatment was most effective at reducing the influx of activated CD4+ T cells (>50% reduction), agreeing with our current findings that cyclophilin-CD147 interactions are likely to contribute more significantly to activated rather than resting T cell migration. In conclusion, we propose that targeting extracellular cyclophilins and/or their interaction with CD147 might be considered a novel, therapeutic approach for the alleviation of ongoing inflammatory responses as a result of the higher affinity of cyclophilins for proinflammatory leukocytes, which express high levels of CD147.
Acknowledgments
These studies were funded by National Institutes of Health grants AI-067254 (S. L. C.) and AI-060720 (M. I. B.) and American Heart Association Predoctoral Fellowship 0615392U (J. M. D.). The authors thank Larisa Dubrovsky, Tatiana Pushkarsky, and Alan Watson for technical help.
References
- 1.Bukrinsky MI. Cyclophilins: unexpected messengers in intercellular communications. Trends Immunol. 2002;23:323–325. doi: 10.1016/s1471-4906(02)02237-8. [DOI] [PubMed] [Google Scholar]
- 2.Yurchenko V, Constant S, Bukrinsky M. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006;117:301–309. doi: 10.1111/j.1365-2567.2005.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yurchenko V, Zybarth G, O’Connor M, Dai WW, Franchin G, Hao T, Guo H, Hung HC, Toole B, Gallay P, Sherry B, Bukrinsky M. Active-site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 4.Sherry B, Yartlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci USA. 1992;89:3511–3515. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 1992;267:11968–11971. [PubMed] [Google Scholar]
- 6.Billich A, Winkler G, Aschauer H, Rot A, Peichl P. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J Exp Med. 1997;185:975–980. doi: 10.1084/jem.185.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Ceuninck F, Allain F, Caliez A, Spik G, Vanhoutte P. High binding capacity of cyclophilin B to chondrocyte heparan sulfate proteoglycans and its release from the cell surface by matrix metalloproteinases: possible role as a proinflammatory mediator in arthritis. Arthritis Rheum. 2003;48:2197–2206. doi: 10.1002/art.11099. [DOI] [PubMed] [Google Scholar]
- 8.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 9.Tegeder I, Schumacher A, John S, Geiger H, Geisslinger G, Bankg H, Brune K. Elevated serum cyclophilin levels in patients with severe sepsis. J Clin Immunol. 1997;17:380–386. doi: 10.1023/a:1027364207544. [DOI] [PubMed] [Google Scholar]
- 10.Konttinen YT, Li TF, Mandelin J, Liljestrom M, Sorsa T, Santavirta S, Virtanen I. Increased expression of exracellular matrix metalloproteinase inducer in rheumatoid synovium. Arthritis Rheum. 2000;43:275–280. doi: 10.1002/1529-0131(200002)43:2<275::AID-ANR6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Zhu P, Ding J, Zhou J, Dong W-J, Fan C-M, Chen Z-N. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther. 2005;7:R1023–R1033. doi: 10.1186/ar1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu P, Lu N, Shi Z-G, Zhou J, Wu Z-B, Yang Y, Ding J, Chen Z. CD147 overexpression on synoviocytes in rheumatoid arthritis enhances matrix metalloproteinase production and invasiveness of synoviocytes. Arthritis Res Ther. 2006;8:R44. doi: 10.1186/ar1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora K, Gwinn W, Bower M, Watson A, Okwumabua I, Mac-Donald H, Bukrinsky M, Constant S. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005;175:517–522. doi: 10.4049/jimmunol.175.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwinn WM, Damsker J, Falahati R, Okwumabua I, Kelly-Welch A, Keegan A, Vanpouille C, Lee J, Dent LA, Leitenberg D, Bukrinsky M, Constant S. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol. 2006;177:4870–4879. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allain F, Vanpouille C, Carpentier M, Slomianny MC, Durieux S, Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc Natl Acad Sci USA. 2002;99:2714–2719. doi: 10.1073/pnas.052284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay CR. Follicular homing T helper (Th) cells and the Th1/Th2 paradigm. J Exp Med. 2000;192:F31–F34. doi: 10.1084/jem.192.11.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmon M, Hill-Gaston J. The role of T-lymphocytes in rheumatoid arthritis. Br Med Bull. 1995;51:332–345. doi: 10.1093/oxfordjournals.bmb.a072964. [DOI] [PubMed] [Google Scholar]
- 18.Grabie N, Delfs M, Westrich J, Love V, Stavrakis G, Ahmad F, Seidman C, Seidman J, Lichtman A. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Invest. 2003;111:671–680. doi: 10.1172/JCI16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saphire AC, Bobardt M, Zhang Z, David G, Gallay P. Syndecans serve as attachment receptors for human immunodeficiency virus Type 1 on macrophages. J Virol. 2001;75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanpouille C, Denys A, Carpentier M, Pakula R, Mazurier J, Allain F. Octasaccharide is the minimal length unit required for efficient binding of cyclophilin B to heparin and cell surface heparan sulphate. Biochem J. 2004;382:733–740. doi: 10.1042/BJ20031453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita T, Nakase T, Kaneko M, Shi K, Takahi K, Ochi T, Yoshikawa H. Expression of extracellular matrix metalloproteinase inducer and enhancement of the production of matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2002;46:373–378. doi: 10.1002/art.10050. [DOI] [PubMed] [Google Scholar]
- 22.Kasinrerk W, Fiebiger E, Stefanova I, Baumruker T, Knapp W, Stockinger H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol. 1992;149:847–854. [PubMed] [Google Scholar]
- 23.Koch C, Staffler G, Huttinger R, Hilgert I, Prager E, Cerny J, Steinlein P, Majdic O, Horejsi V, Sockinger H. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int Immunol. 1999;11:777–786. doi: 10.1093/intimm/11.5.777. [DOI] [PubMed] [Google Scholar]