Abstract
Thirteen homologous proteins comprise the long-chain acyl-CoA synthetase (ACSL), fatty acid transport protein (FATP), and bubblegum (ACSBG) subfamilies that activate long-chain and very-long-chain fatty acids to form acyl-CoAs. Gain- and loss-of-function studies show marked differences in the ability of these enzymes to channel fatty acids into different pathways of complex lipid synthesis. Further, the ability of the ACSLs and FATPs to enhance cellular FA uptake does not always require these proteins to be present on the plasma membrane; instead, FA uptake can be increased by enhancing its conversion to acyl-CoA and its metabolism in downstream pathways. Since altered fatty acid metabolism is a hallmark of numerous metabolic diseases and pathological conditions, the ACSL, FATP and ACSBG isoforms are likely to play important roles in disease etiology.
A family of acyl-CoA synthetases activates intracellular long-chain fatty acids
Before a fatty acid (FA) from an exogenous or endogenous source can enter any metabolic pathway with the exception of eicosanoid metabolism, it must first be activated to form an acyl-CoA. This activation step is catalyzed by acyl-CoA synthetase (ACS) via a two-step reaction: 1) the formation of an intermediate fatty acyl-AMP with the release of pyrophosphate, and 2) the formation of a fatty acyl-CoA with the release of AMP.
The ACS family is comprised of at least 25 members [1]. Although overlap exists, ACS family members are broadly classified by their substrate specificities for FAs of varying chain length. This review will focus on the three subfamilies of ACS enzymes that activate long-chain and/or very-long-chain saturated and unsaturated FAs, long-chain ACSs (ACSL), very-long-chain ACSs (FATP), and bubblegum (ACSBG) isoforms [2].
The ACSL, FATP and ACSBG families include multiple isoforms, each encoded by a separate gene (Tables 1, 2). Additionally, splice variants of ACSL isoforms have been identified in both humans and rodents [3]. ACS enzymes share significant amino acid sequence similarity, particularly in two highly conserved regions, a putative ATP-AMP signature motif for ATP binding and a motif for FA binding [4]. Despite these similarities, purified ACSL and FATP isoforms and their splice variants show distinct enzyme kinetics, differences in sensitivity to inhibitors (Table 1, 2) and differences in their substrate preferences. For example, ACSL4 has a marked preference for C20:4 [5], whereas ACSL1 prefers saturated and monounsaturated FA that are 16–18 carbons in length [6]. Very-long-chain ACS isoforms, also designated as FATP (FA transport proteins), generally prefer 16–18 carbon FA but can also activate FA as long as 26 carbons [7, 8]. It should be noted that FAs may not only be the sole substrate for some isoforms. For example, FATP5 preferentially activates bile acids and FATP2 activates both long-chain FA and 3α, 7α, 12α-trihydroxy-5β-cholestanoate [9]. The ACSBG isoforms prefer long-chain FA [10, 11].
Table 1.
Long-chain acyl-CoA synthetases
| Isoforms | Tissue expression with highest mRNA abundance | Intracellular locations1 | Inhibitors | Accession Numbers (Human)2 |
|---|---|---|---|---|
| ACSL13 | Liver, adipose tissue, heart [6, 48] | ER, nuclear fraction, plasma membrane (3T3-L1 adipocytes) [12]; GLUT4 vesicle (rat adipocytes) [13]; mitochondria (PtK2 epithelial cells) [15]; ER, MAM, cytosol (rat liver) [79]; lipid droplet fraction (3T3-L1 adipocytes) [80] | Triacsin C [35, 81] | NP_001986 |
| ACSL3 | Brain, gonads [48, 82] | Lipid droplet fraction (3T3-L1 adipocytes [80]; HuH7 cells [83]; CHO cells [84]; epithelial A431 cells [85]; HepG2 cells [86] | Triacsin C [35] |
NP_004448 NP_976251.1 |
| ACSL4 | Adrenal gland, liver [5, 48] | MAM, peroxisomes (rat liver) [79]; lipid droplet (3T3-L1 adipocytes [80]; HuH7 cells [83], CHO cells [84], HepG2 hepatoma cells [86] | Triacsin C, rosiglitazone, troglitazone, pioglitazone, N-ethylmaleimide [35, 81] |
NP_004449 NP_075266 |
| ACSL5 | Small intestine, liver, brown adipose tissue [48, 87] | ER, mitochondria (rat liver) [25, 79]; ER, mitochondria (McArdle-RH 7777 hepatoma cells) [25]; lipid droplets (rat liver) [88] | — |
NP_057318 NP_976313 NP_976314 |
| ACSL6 | Brain, gonads [48, 89] | — | — |
NP_056071 BAA74860 |
ER, endoplasmic reticulum; MAM, mitochondrial-associated membrane
Multiple numbers indicate splice variants
See [3] for other aliases
Table 2.
Fatty acid transport proteins/Very-long-chain acyl-CoA synthetases/ACSBG
| Isoforms | Tissue expression in highest mRNA abundance | Intracellular locations | Inhibitors | Accession Numbers (Human) |
|---|---|---|---|---|
| FATP11 (Slc27a1) | Heart, WAT, skeletal muscle [21] | Plasma membrane (3T3-L1 adipocytes) [12, 16], mouse adipocytes [20]; plasma membrane, Golgi (human primary muscle cells) [18] | --- | NP_940982 |
| FATP22 (Slc27a2) | Kidney cortex, liver [90] | Microsomes, peroxisomes (mouse hepatocytes) [90] | — | NP_003636 |
| FATP3 (Slc27a3) | Broad distribution, highest in lung, adrenal, gonads [91] | Mitochondria, MAM (MA-10 Leydig cells, mouse Neuro2a cells) [91] ER (overexpressed in COS-7) [91] |
— | NP_077306 |
| FATP4 (Slc27a4) | Small intestine [92]; brain, liver, kidney [93] | Apical plasma membrane (mouse enterocyte) [26]; plasma membrane (mouse adipocytes) [20]; ER (multiple cell lines) [15]; mitochondria, nuclei, MAM, peroxisomes (skin fibroblasts) [1] | n-dodecyl-D-maltopyranoside, triacsin C (C16:0 esterification), troglitazone (C16:0 esterification) 4-aryl-dihydropyrimidinones [8, 27, 94] | NP_005085 |
| FATP5 (Slc27a5) | Liver (exclusively) [21, 95] | Basal plasma membrane (mouse hepatocytes) [21] | — | NP_036386 |
| FATP6 (Slc27a6) | Heart (exclusively) [22] | Sarcolemma (monkey cardiomyocytes) [22] | — | NP_054750 |
| ACSBG1 | Brain, adrenal, gonads, spleen [2, 10, 96–98] | Cytoplasm (COS-1 cells, [2], mouse Leydig tumor cells; mitochondria (Neuro2a cells, mouse brain) [10]; microsomes (COS7 cells) [98] | — | NP_055977 |
| ACSBG2 | Testis [11, 99] | Cytoplasm (COS-1 cells, mouse TM4 Sertoli cells) [99]; microsomes (mouse testis) [99]; mitochondria, microsomes (COS-7 cells) [11] | — | NP_112186 |
FATP has been termed ACSVL and VLCS
FATP2 has been termed VLCS and VLACS
FATP5 has been termed ACSB, BACS, VLCS-H2 and VLACSR
The intracellular locations of the ACSL and FATP isoforms differ depending upon the cell type (Table 1). Not only can several ACSL isoforms be present in different subcellular locations within a single cell, but a single ACSL isoform may vary in its subcellular location. For example, ACSL1 has been reported to be located on the plasma membrane [12] and in GLUT4 vesicles [13] in adipocytes, and on the ER in hepatocytes [14] and mitochondria in epithelial cells [15]. Cell-specific differences in location could arise by differential splicing or protein-protein interactions, both of which may contribute to tissue-specific functions. Such functions include facilitating FA uptake into cells, channeling FAs towards specific synthetic and degradative pathways, and regulating the use of FAs and acyl-CoAs as ligands for transcription factors and as modifiers of cellular physiology.
Role of acyl-CoA synthetases in modulating FA uptake
The FATP family was discovered in 1994 when Schaffer and Lodish searched for adipocyte proteins that would increase cellular uptake of the fluorescent FA analog Bodipy-C12 [16]. They cloned two proteins–one was the previously cloned ACSL1 [6], and they named the other FATP (subsequently FATP1). An additional 5 FATP isoforms have since been cloned, and considerable discussion has taken place regarding whether the FATP family members transport FA directly or, instead, indirectly facilitate FA transport.
Both in vitro and in vivo studies in a variety of cell types and tissues have shown that manipulating the expression of FATP1 affects FA uptake [17–19]. Interestingly, insulin causes FATP1 and FATP4 to translocate from the ER to the plasma membrane, a process that could enable insulin to enhance FA uptake [20]. The location of FATP5 on the basal plasma membrane of hepatocytes [21] and FATP6 on the plasma membrane of monkey cardiac myocytes [22] suggest that a plasma membrane location is required to mediate FA uptake, since hepatocytes from mice lacking FATP5 have 50% lower rates of FA uptake and overexpressing FATP6 in HEK293 cells enhances FA uptake [21, 22].
However, in some instances FA uptake appears to depend more on inherent ACS activity than on a plasma membrane location, and some members of the FATP and ACSL families increase FA uptake despite their location on internal cell membranes. For example, overexpressing ACSL and FATP isoforms increases FA uptake despite the fact that ACSL1 [15, 23], ACSL4 [24], ACSL5 [25], and FATP4 [15] are located only on intracellular organelles in the cells examined. (FATP4 is present on the apical side of enterocytes but it was not located to a specific membrane [26]). Like the ACSL family members, most FATP isoforms have either been directly or indirectly shown to possess ACS activity. Purified FATP1 and FATP4 activate a wide range of FAs and show no preference for very-long-chain FAs [7, 27]. Additionally, when overexpressed in a genetically modified yeast strain with low ACS activity, all FATPs with the exception of FATP5 are able to increase ACS activity using different long-chain and very-long-chain substrates [28]. The same study shows that rates of FA uptake and ACS activity are often dissociated. Yet, others show that ACS activity is required for FATP-mediated FA uptake, so that when FATP1 contains a mutation that abolishes ACS activity, overexpression severely suppresses FA uptake [29]. Similarly, overexpressing normal FATP4 enhances FA uptake in COS cells, but overexpressing a FATP4 mutant that lacks ACS activity abolishes these effects [15]. Clearly, the role of ACS activity of ACSL and FATP isoforms in facilitating FA transport needs further evaluation.
Because FAs must first be converted to acyl-CoAs before they enter most metabolic pathways, their activation by ACSLs and FATPs traps them within the cell as acyl-CoAs, and thereby diminishes intracellular FA pools so that less FA is available for efflux [30]. Thus, intracellular acyl-CoA metabolism may enhance FA influx regardless of the location of the ACSL or FATP protein.
Role of acyl-CoA synthetases in FA channeling
The existence of 13 ACSL, FATP and ACSBG isoforms that all activate long-chain FA has suggested that each has an independent role in channeling FA within cells. Unique roles for ACS isoforms were first identified in studies of Saccharomyces cerevisiae mutants that lacked the ACS isoforms Faa1 and Faa4, and were unable to use exogenously provided fatty acids [31]. Similarly, complementation studies of ACS-deficient E. coli showed that each of the 5 rat ACSL isoforms differs in its ability to channel FA into specific pathways like phospholipid synthesis and β-oxidation [32]. The ACSL and FATP isoforms also differ in their ability to complement FA uptake and activation in mutated yeast strains that lack ACS activity [28, 33].
In cultured mammalian cells, gain-of-function and loss-of-function studies also strongly suggest that the different ACSL isoforms channel FA into specific metabolic pathways, consistent with differences in their subcellular locations and substrate preferences. In rat hepatocytes and human fibroblasts, for example, triacsin C, a competitive inhibitor of ACSL1, ACSL3, and ACSL4 [34, 35], preferentially decreases [1-14C]oleic acid incorporation into TAG relative to other glycerolipids and oxidation products [36, 37]. These indirect studies suggested that ACSLs direct the metabolic fate of FAs, but did not identify unique roles for individual ACSL isoforms.
Overexpression studies in mammalian cells have shown the effects of specific ACSL isoforms more directly. Adenovirus-mediated overexpression of ACSL5, which doubles total ACS activity in McArdle-RH7777 rat hepatoma cells, partitions oleic acid almost exclusively into intracellular TAG without increasing the amount of TAG secreted into the medium [25]. Because overexpressing ACSL5 does not increase the incorporation of [14C]acetic acid into any lipid class, it appears that ACSL5 uses only exogenous FA and does not activate FA synthesized de novo within the cell. Overexpressing ACSL1 in NIH-3T3 fibroblasts or PC12 neurons, also increases oleic acid incorporation into TAG [38, 39], and in ACSL1 heart-specific transgenic mice, heart TAG content increases approximately 12-fold and the choline glycerophospholipid content increases 50% [17], again suggesting an anabolic role for ACSL, although ACS activity itself was not measured in these hearts. In contrast to its role in TAG synthesis, overexpressing ACSL1 in rat primary hepatocytes resulted in a 3.7-fold increase in ACS activity but did not enhance incorporation of oleic acid into TAG [14]; instead, ACSL1 increased oleate incorporation into phospholipid and diacylglycerol while decreasing incorporation into cholesterol esters. Pulse-chase experiments further revealed that overexpressed ACSL1 decreased the turnover of intracellular TAG and phospholipids, possibly through altering the reacylation of lysophospholipids. Unlike ACSL5, overexpressed ACSL1 increases the incorporation of de novo synthesized FA into glycerolipids [14]. In somewhat contrasting experiments in HepG2 cells, adenovirus-mediated overexpression of ACSL1 resulted in a 20-fold increase in ACS activity, increases in cell acyl-CoA content, and enhanced oleic acid partitioning into cellular TAG [40]. However in this study, the lack of carnitine in the medium may have limited CPT-1 activity and FA β-oxidation, while the marked increase in ACS activity may have overwhelmed other pathways. Adenoviral-mediated overexpression of ACSL1 in rodent liver in vivo also increased hepatic TAG, but did not affect FA clearance from the blood; cholesterol and phospholipid metabolism were not measured [40]. Taken as a whole, these overexpression studies suggest that, not only do ACSL1 and ACSL5 channel FA towards different lipid pathways, but that the direction of channeling may vary in different cell types. A caveat to this interpretation is that in several of the studies cited above, overexpression was ascertained only by changes in mRNA without measuring ACS activity directly. A very large increase in activity could potentially give results that are not relevant to the physiological function.
Similar to these ACSL overexpression studies, altering the expression of FATP isoforms also affects FA channeling. For example, overexpressing FATP1 in HEK293 cells alters partitioning of both exogenously added oleic acid and FA synthesized de novo from acetate into cellular lipids and increases cellular TAG content while decreasing cholesterol and sphingomyelin content [41]. In skeletal muscle, adenovirus-mediated overexpression of FATP1 increases the partitioning of oleate or palmitate into TAG and away from β-oxidation [18], and overexpression in mouse heart increases FA uptake and TAG content and causes a lipotoxic cardiomyopathy [42]. Conversely, in muscle from FATP1 null mice fed a high fat diet, the content of TAG, DAG and acyl-CoA is lower than in wildtype controls (Table 3) [19]. Knockdown of FATP1 in 3T3-L1 adipocytes reduces FA uptake without changes in lipolysis, but knockdown of FATP4 does the reverse; it does not affect FA influx, but instead, increases basal lipolysis [43]. In isolated enterocytes, antisense knockdown of FATP4 diminishes the uptake of oleate 50% [26]. Like the ACSL isoforms, these differences in FA metabolism suggest the possibility that interactions with downstream enzymes may mediate the fates of acyl-CoAs synthesized by FATP1 and FATP4. To date, however, no studies have examined such potential interactions. The ACSBG isoforms have not been studied with respect to FA uptake into cells.
Table 3.
ACSL and FATP knockout mice
| Isoforms | Mouse phenotype | FA uptake and labeling studies | Lipid abnormalities | Ref. |
|---|---|---|---|---|
| ACSL4 | Female heterozygote: decreased fertility, enlarged uteri with cysts | Uterus: 50% increases in PGE2, 6-keto PGF1α, PGF2α | [100] | |
| FATP1 | Normal phenotype; protection from high fat-induced insulin resistance; no diet-induced obesity; smaller adipocytes; | Skeletal muscle: no change in FA uptake; Adipocytes: decreased insulin-stimulated FA uptake | Muscle: no increase in TAG, acyl-CoA on high fat diet; Liver: contains more TAG with both chow and high fat feeding | [19, 58] |
| FATP2 | Normal phenotype and histology | Decreased peroxisomal acyl-CoA synthetase activity with 24:0; decreased β-oxidation of 24:0; no accumulation of very-long-chain FA | [101] | |
| FATP4 | Lethal restrictive dermopathy; smallmouth and nose, low set ears, flexion contractures | Squamous epithelium: absent lipid droplets | [46] | |
| FATP4 | embryonic lethality at 9.5 d of gestation | Heterozygote enterocytes: 40% reduced FA uptake; no difference in diet fat absorption | — | [47] |
| FATP4 | Lethal restrictive dermopathy with hyperkeratosis, flexion contractures, facial dysmorphia | Decreased 24:0 activation | Liver, brain, lung, intestine: no changes in lipids; Liver: 15–30% decreases in molar content of PC, PE, cholesterol esters; 81% increase in ceramides with increased 16–24 carbon FA and decreased C26:0 and C26:0-OH FA | [8, 45] |
| FATP5 | No weight gain on high fat diet because of decreased intake and increased energy expenditure | Hepatocytes: 40% decrease in uptake of C12-Bodipy; Intestine: normal diet FA uptake | Liver: 60% less TAG with relatively less saturated and polyunsaturated FA; 37% less free FA; 60% increase in PS content; no change in FA composition of phospholipids; increased fatty acid synthase mRNA; impaired VLDL secretion and ketogenesis (probably due to decreased liver TAG; decreased bile acid conjugation | [21, 102] |
FA, fatty acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; TAG, triacylglycerol
The different substrate preferences of the individual ACSL and FATP isoforms suggest that altering the activity of a single isoform might change the FA composition of intracellular glycerolipids. For example, ACSL6 preferentially activates very-long-chain polyunsaturated FAs, and when ACSL6 is overexpressed in PC12 neurons, it enhances the uptake and metabolism of docosahexaenoic acid relative to oleic acid [44]. In the several mouse models that lack FATP4 (Table 3), mice are variably characterized by altered epidermal FA composition accompanied by a neonatally lethal restrictive dermopathy [45], altered skin development, impaired hair growth and abnormal lipid metabolism [46], and early embryonic lethality [47]. The decreased amount of very-long-chain FAs in tissues from FATP4 knockouts supports data from ACS activity assays showing that FATP4 prefers very-long-chain FAs over long chain FAs [45]. Supporting this interpretation are data from fibroblasts from FATP4 null mice in which the rate of 24:0 degradation and incorporation into phospholipids, TAG and cholesterol esters were diminished [1]. In contrast, compared to wild type controls, livers from FATP5 null mice contain 60% less TAG, a greater decrease in saturated and polyunsaturated FAs in TAG relative to monounsaturated FA, and 37% lower unesterified FA [21]. Although no studies have tested whether the different ACSL and FATP isoforms alter the FA composition of phospholipids in specific cellular organelles, the FA preferences of these enzymes suggest that they could mediate the composition of distinct lipid pools.
Differential regulation of acyl-CoA synthetases
Specific functional roles for individual ACSL isoforms are suggested by their tissue-specific responses to nutritional changes and to unique transcriptional regulation. For example, when rats are fasted for 48 h, hepatic Acsl1 and Acsl4 mRNA abundance increases, but Acsl3 and Acsl5 mRNA abundance decreases [48]. When rats are refed a high sucrose diet, the reverse occurs: hepatic Acsl1 and Acsl4 mRNA decreases, and Acsl5 mRNA abundance increases [48]. These responses may reflect regulation by different transcription factors. PPARα, which is upregulated in liver during fasting, increases Acsl1 gene expression via a PPRE in the Acsl1 promoter [49]. In contrast, the increase of hepatic ACSL5 mRNA by refeeding is consistent with its upregulation by insulin via SREBP-1c [50]. Insulin also up-regulates Acsl6 in heart [51]. Although the controls are, as yet, unknown, ACSLs are regulated differently in different tissues. Thus, the fasting- induced changes in hepatic Acsl3 and Acsl5 mRNA levels are not observed in adipose tissue or gastrocnemius muscle [48]. High-fat feeding greatly increases Acsl1 mRNA in rat liver [6], but has no effect on Acsl1 mRNA in rat heart [51]. Treatment of rats with PPARγ agonists has no effect on Acsl1 mRNA in heart, but decreases it in liver, and increases it 7-fold in epididymal and omental adipose tissue and 3-fold in skeletal muscle [52]. These examples of tissue-specific regulation suggest that the ACSL isoforms each contribute activated FAs that have different metabolic fates in each tissue.
In addition to transcriptional regulation with changes in mRNA abundance, the protein amounts of individual ACSL isoforms, and total ACS activities are discordant under fasting and refeeding condition and during development [48, 53], suggesting that each isoform may be regulated translationally or by post-translational modifications. The disconnect between mRNA, protein and activity should be considered and further characterized in future studies. Indirect evidence also suggests that ACS activity is regulated acutely. For example, in rat adipocytes, norepinephrine and glucagon simultaneously decrease ACS activity and increase lipolysis, whereas insulin rapidly reverses the norepinephrine effect and restores ACS activity to control levels within 5 minutes [54]. The similar dose-response curves for ACS inactivation and lipolysis stimulation [54], suggest that ACSL and hormone-sensitive lipase may be regulated concomitantly in adipocytes. Since ACSL1 is the most abundant ACSL isoform in adipocytes, and insulin does not alter the content of ACSL1 protein [55], the acute regulation by insulin must occur via another mechanism. Perhaps during fasting, glucagon acutely inhibits adipocyte ACSL1 by phosphorylation. Inactivating ACSL1 would prevent re-esterification of newly hydrolyzed FA and would enhance the release of FA into the circulation. Rodent and human ACSL1 contain two highly conserved serine residues that are predicted sites for protein kinase A and Tyr-85 is phosphorylated in ACSL1 from rat liver [56].
Again arguing for independent regulation, ACSL isoforms are not only expressed in a tissue-specific manner, but they are also expressed differently during development. During 3T3-L1 adipocyte differentiation, Acsl1 mRNA abundance increases ~160-fold while other isoforms remain unchanged [23], suggesting that ACSL1 is the major isoform responsible for TAG synthesis in adipocytes. In contrast, during the differentiation of PC12 neuronal cells, Acsl1 and Acsl3 mRNA content remains unchanged whereas that of Acsl4, 5, and 6 increases significantly [23]. Further, in mouse heart, Acsl1 mRNA increases 4-fold postnatally, while Acsl3 mRNA decreases and other ACSL isoforms do not change. These data suggest that ACSL1 is the main isoform responsible for the 14-fold increase in ACS activity as the heart adapts to the use of FA as its primary source of energy in the postnatal period [53]. In summary, the unique developmental patterns of the ACSL isoforms suggest that they play different tissue-specific roles during different stages of life.
Except for FATP1, the regulation of the FATP isoforms has been less well studied. Fatp1 mRNA increases 5- to 7-fold when 3T3-L1 cells differentiate into mature adipocytes [20]. In mouse adipose tissue, fasting increases Fatp1 mRNA, suggesting a role that would be unrelated to FA uptake or TAG synthesis [57]. Characterization of the FATP1 null mouse also suggests that FATP1 is critical for acute insulin-stimulated uptake of exogenous FA [58].
FAs and acyl-CoAs affect multiple cellular process that influence the etiology of metabolic diseases
ACSL and FATP isoforms regulate the intracellular content of long-chain FA and acyl-CoA, both of which are important intracellular signaling molecules. Long-chain acyl-CoAs have the potential to alter multiple cellular processes including signal transduction via PKC isoforms and Ca2+ release, enzymes involved in lipid and energy metabolism like acetyl-CoA carboxylase and glucokinase [59], and ATP-sensitive K+ channels, including those that control insulin release from pancreatic β-cells [60]. FAs and acyl-CoAs regulate numerous transcription factors including the PPAR family, SREBP, ChREBP, LXR, HNF-4α and NF-κβ [61].
Additionally, alterations in specific ACSL and FATP isoforms influence the concentration and content of numerous intracellular lipids, many of which are intimately involved in disease development. For example, apoptosis increases after pancreatic islets are incubated with FAs, and is preceded by an 82% rise in ceramide; both apoptosis and the rise in ceramide can be blocked by triacsin C, an inhibitor of ACSL isoforms 1, 3, and 4 [62]. On the other hand, palmitate may induce apoptosis independent of ceramide production [63], perhaps related to its inhibition of cardiolipin synthesis [64, 65]. Palmitate-mediated apoptosis requires metabolism, as evidenced by the finding that in β-cells, triacsin C blocked apoptosis as well as the palmitate-mediated decrease in the anti-apoptosis factor Bcl-2, indicating that changes in Bcl-2 requires acyl-CoAs or their metabolites [66]. Transgenic mice with overexpressed ACSL1 in heart have increased TAG content in cardiac myocytes, cardiac hypertrophy, severe left-ventricular dysfunction, and apoptosis of cardiac myocytes [17]. These studies exemplify the importance role of the ACSL and FATP-isoforms in regulating fatty acid metabolism and the subsequent metabolic changes that may lead to or prevent a host of metabolic diseases.
Acyl-CoA synthetases in pathological conditions
Increases or decreases in ACSL isoforms under pathological conditions suggest that each is playing an independent role that cannot be compensated for by alterations in other isoforms. For the most part, however, the effects of these alterations on tissue lipids or fatty acid composition have not been investigated. ACSLs have been associated with various forms of cancer, including acute myelogenous leukemia (ACSL6) [67], colorectal adenocarcinomas (ACSL5) [68, 69] and colon adenocarcinomas (ACSL4) [70]. It has been suggested that upregulation of ACSL4, which has a marked preference for arachidonate [5], promotes carcinogenesis by blocking the apoptosis that free arachidonate would otherwise induce [71]. ACS specific activity and Acsl1 mRNA abundance are upregulated in liver and adipose tissues in genetic obese models such as Zucker fatty rat (fa/fa) and ob/ob mice [72, 73]. Although the causality between the change of ACSL isoforms and specific diseases has not been established, these observations indicate that ACSL isoforms are linked to metabolic changes or FA demand under several pathological conditions. For example, in patients with inflammatory bowel disease, the increase of Acsl1 and Acsl4 mRNA in the terminal ileum and colon might provide acyl-CoAs for the synthesis of phospholipids; these can serve as precursors for inflammatory mediators or support membrane integrity of the affected intestine [74].
Despite the presence of other ACSL isoforms in brain, human ACSL4 is associated with depression [75] and mutations in the human Acsl4 gene that decrease 20:4-CoA synthetase activity cause a form of X-linked mental retardation [76–78]. The effect of these mutations suggests that ACSL4 is critical for normal brain function, perhaps related to its preference for long-chain polyunsaturated FAs [35], which are enriched in brain phospholipids.
Conclusion
The differences in tissue expression, cellular location, enzyme kinetics and substrate preferences suggest that individual ACSL, FATP, and ACSBG isoforms play unique roles in FA channeling and lipid metabolism. In addition to controlling the metabolic fate of FAs, the ACSL and FATP isoforms also regulate intracellular pools of FAs and acyl-CoAs, both of which have broad effects on cellular metabolism ranging from altering gene expression to allosterically regulating enzymes. Despite the importance of the ACS family in FA trafficking and the intimate role of lipid metabolism in disease etiology, mechanisms linking the two have been largely neglected.
Future perspectives
Although recent advances have provided insight into the physiological role of ACSLs, FATPs, and ACSBGs, much work remains to elucidate their roles in FA channeling and the development of disease. We anticipate that future studies will show that individual isoforms of these enzymes differ in function depending on tissue-specific expression, expression of splice variants, subcellular location, translational or post-translational modifications, and expression of other proteins that interact with a given isoform. Because it is widely accepted that the composition of FAs largely dictates their regulatory effects within the cell, identifying the ACSL, FATP, and ACSBG isoforms that mediate specific effects of different FAs should provide valuable insights. Additionally, identifying isoforms that influence the formation of distinct pools of other intracellular lipids like DAG and ceramide may identify novel pharmaceutical targets for diseases characterized by altered lipid metabolism and lipotoxicity.
Executive summary
Acyl-CoA synthetases in FA activation
ACSL, FATP, and ACSBG activate long-chain and very-long-chain FA by a two-step reaction
ACSL, FATP, and ACSBG families include multiple isoforms that have distinct enzyme kinetics, different but overlapping FA preferences, and unique subcellular locations
The role of acyl-CoA synthetases in FA uptake
ACSL and FATP isoforms are major regulators of both FA transport and metabolism
ACSL and FATP isoforms located on intracellular membranes increase FA uptake, suggesting that ACS activity and downstream metabolism enhance FA uptake and that location on the plasma membrane is not required.
ACSL and FATP appear to enhance FA uptake by activating FAs, a process that traps FAs as acyl-CoAs within the cell and prevents efflux.
Little is known about the specific role of the ACSBG isoforms.
The role of acyl-CoA synthetases in FA channeling
In rat hepatoma cells, ACSL5 increases FA uptake and partitions it to TAG
ACSL1 appears to be involved in TAG accumulation in adipose tissue, but, in liver, it contributes to the synthesis of phospholipid and cholesterol. Thus, the functions of individual isoforms may be tissue-specific
Similar to ACSL1, most studies support a role for FATP1 in TAG synthesis in adipose tissue
FATP4 is essential for normal skin development and its presence is required for neonatal survival.
Differential regulation of acyl-CoA synthetases
ACSL isoforms display tissue-specific response to nutritional changes. Potential mechanisms include transcriptional, translational and post-translational regulations
ACSL isoforms have different developmental patterns, suggesting specific roles during adipocyte and heart development
FATP1 expression responds to cell differentiation and nutritional changes
Acyl-CoA synthetases in pathological conditions
Alterations in ACSL and FATP isoforms influence the content of intracellular lipids
ACSL isoforms may be involved in certain cancers and in disorders like obesity, diabetes and inflammatory bowel disease
Mutations in the human Acsl4 gene cause a form of X-linked mental retardation
Unanswered questions
What are the roles of splice variants of ACSL4 and ACSL6?
Is ACSL1 inhibited in adipocytes during lipolysis to prevent reesterification and promote FA efflux?
How are FATPs regulated acutely, transcriptionally, and during development?
Is channeling mediated by protein-protein interactions?
What is the role of ACSBG in FA metabolism?
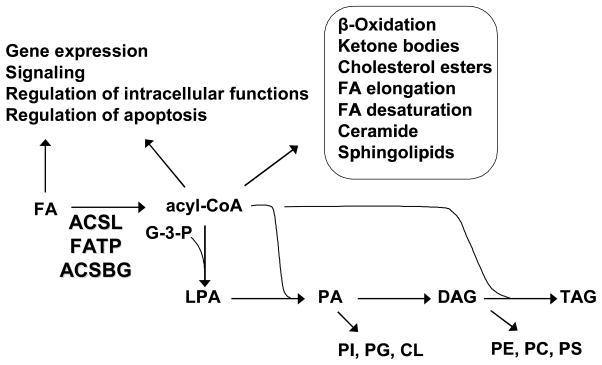
Fig. 1.
Pathways initiated by ACSL, FATP, and ACSBG isoforms. In addition to initiating the synthesis of triacylglycerol (TAG) and all the glycerophospholipids, acyl-CoAs are required for the synthesis of cholesterol esters, ceramide and sphingolipids, for fatty acid (FA) degradation pathways and for FA modification pathways of elongation and desaturation. Intermediates in the glycerolipid synthetic pathway, lysophosphatidic acid (LPA), phosphatidic acid (PA), and diacylglycerol (DAG) are intracellular signals. Both FA and acyl-CoAs are also intracellular signals and regulators of cellular physiology as well as purported ligands for PPAR and HNF4α transcription factors. G-3-P, glycerol-3-phosphate; PI, phosphatidylinositol; PG, phosphatidylglycerol; CL, cardiolipin; PE phosphatidylethanolamine; PC, phosphatidylcholine; PS, phosphatidylserine.
Acknowledgments
This work was supported by grants DK59935 (RAC), P30 DK56350, P30 DK034987 and DK68993 (DGM) from the National Institutes of Health.
References
- 1.Jia Z, Moulson CL, Pei Z, Miner JH, Watkins PA. FATP4 is the principal very long-chain fatty acyl-CoA synthetase in skin fibroblasts. J Biol Chem. 2007 doi: 10.1074/jbc.M700568200. [DOI] [PubMed] [Google Scholar]
- 2•.Steinberg SJ, Morgenthaler J, Heinzer AK, Smith KD, Watkins PA. Very long-chain acyl-CoA synthetases: Human “bubblegum” represents a new family of proteins capable of activating very long-chain fatty acids. J Biol Chem. 2000;275:35162–35169. doi: 10.1074/jbc.M006403200. Identifies amino acid motifs that differentiate the ACS subfamilies. [DOI] [PubMed] [Google Scholar]
- 3•.Mashek DG, Bornfeldt KE, Coleman RA, et al. Revised nomenclature for the mammalian long chain acyl-CoA synthetase gene family. J Lipid Res. 2004;45:1958–1961. doi: 10.1194/jlr.E400002-JLR200. This paper clarifies the ACS nomenclature. [DOI] [PubMed] [Google Scholar]
- 4.Black PN, DiRusso CC. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol Mol Biol Rev. 2003;67:454–472. doi: 10.1128/MMBR.67.3.454-472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang M-J, Fujino T, Sasano H, et al. A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc Natl Acad Sci. 1997;94:2880–2884. doi: 10.1073/pnas.94.7.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki H, Kawarabayasi Y, Kondo J, et al. Structure and regulation of rat long-chain acyl-CoA synthetase. J Biol Chem. 1990;265:8681–8685. [PubMed] [Google Scholar]
- 7.Coe NR, Smith AJ, Frohnert BI, Watkins PA, Bernlohr DA. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J Biol Chem. 1999;274:36300–36304. doi: 10.1074/jbc.274.51.36300. [DOI] [PubMed] [Google Scholar]
- 8•.Hall AM, Wiczer BM, Herrmann T, Stremmel W, Bernlohr DA. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J Biol Chem. 2005;280:11948–11954. doi: 10.1074/jbc.M412629200. Characterization of one of three independent lines of FATP4 knockout mice. [DOI] [PubMed] [Google Scholar]
- 9.Mihalik SJ, Steinberg SJ, Pei Z, et al. Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J Biol Chem. 2002;277:24771–24779. doi: 10.1074/jbc.M203295200. [DOI] [PubMed] [Google Scholar]
- 10.Pei Z, Oey NA, Zuidervaart MM, et al. The acyl-CoA synthetase “bubblegum” (lipidosin): further characterization and role in neuronal fatty acid beta-oxidation. J Biol Chem. 2003;278:47070–47078. doi: 10.1074/jbc.M310075200. [DOI] [PubMed] [Google Scholar]
- 11.Fraisl P, Tanaka H, Forss-Petter S, Lassmann H, Nishimune Y, Berger J. A novel mammalian bubblegum-related acyl-CoA synthetase restricted to testes and possibly involved in spermatogenesis. Arch Biochem Biophys. 2006;451:23–33. doi: 10.1016/j.abb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Gargiulo CE, Stuhlsatz-Krouper SM, Schaffer JE. Localization of adipocyte long-chain fatty acyl-CA synthetase at the plasma membrane. J Lipid Res. 1999;40:881–892. [PubMed] [Google Scholar]
- 13.Sleeman MW, Donegan NP, Heller-Harrison R, Lane WS, Czech MP. Association of acyl-CoA synthetase-1 with GLUT4-containing vesicles. J Biol Chem. 1998;273:3132–3135. doi: 10.1074/jbc.273.6.3132. [DOI] [PubMed] [Google Scholar]
- 14•.Li LO, Mashek DG, Jie A, Doughman SD, Newgard CB, Coleman RA. Overexpression of rat long chain acyl-CoA synthetase 1 alters fatty acid metabolism in rat primary hepatocytes. J Biol Chem. 2006;281:37246–37255. doi: 10.1074/jbc.M604427200. Shows channeling dependent on overexpression of ACSL1. [DOI] [PubMed] [Google Scholar]
- 15.Milger K, Herrmann T, Becker C, et al. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J Cell Sci. 2006;119:4678–4688. doi: 10.1242/jcs.03280. [DOI] [PubMed] [Google Scholar]
- 16•.Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. Article that discovered the first member of the FATP family. [DOI] [PubMed] [Google Scholar]
- 17.Chiu H-C, Kovacs A, Ford DA, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Martinez C, Marotta M, Moore-Carrasco R, et al. Impact on fatty acid metabolism and differential localization of FATP1 and FAT/CD36 proteins delivered in cultured human muscle cells. Am J Physiol Cell Physiol. 2005;288:C1264–1272. doi: 10.1152/ajpcell.00271.2004. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Gimeno RE, Higashimor T, et al. Inactivation of fatty acid transport protein 1 prevents fat-induced insulin resistance in skeletal muscle. J Clin Invest. 2004;113:756–763. doi: 10.1172/JCI18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Stahl A, Evans JG, Pattel S, Hirsch DJ, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell. 2002;2:477–488. doi: 10.1016/s1534-5807(02)00143-0. Suggests that insulin stimulates FATP1. [DOI] [PubMed] [Google Scholar]
- 21.Doege H, Baillie RA, Ortegon AM, et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Gimeno RE, Ortegon AM, Patel S, et al. Characterization of a heart-specific fatty acid transport protein. J Biol Chem. 2003;278:16039–16044. doi: 10.1074/jbc.M211412200. [DOI] [PubMed] [Google Scholar]
- 23.Marszalek JR, Kitidis C, Dararutana A, Lodish HF. Acyl CoA synthetase 2 (ACS2) over-expression enhances fatty acid internalization and neurite outgrowth. J Biol Chem. 2004;279:23882–23891. doi: 10.1074/jbc.M313460200. [DOI] [PubMed] [Google Scholar]
- 24.Heimli H, Hollung K, Drevon CA. Eicosapentaenoic acid-induced apoptosis depends on acyl CoA-synthetase. Lipids. 2003;38:263–268. doi: 10.1007/s11745-003-1059-z. [DOI] [PubMed] [Google Scholar]
- 25.Mashek DG, McKenzie MA, Van Horn CG, Coleman RA. Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle -RH7777 cells. J Biol Chem. 2006;281:945–950. doi: 10.1074/jbc.M507646200. [DOI] [PubMed] [Google Scholar]
- 26.Stahl A, Hirsch DJ, Gimeno RE, et al. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 27•.Hall AM, Smith AJ, Bernlohr DA. Characterization of the acyl CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem. 2003;278:43008–43013. doi: 10.1074/jbc.M306575200. First article to show that FATP isoforms possess ACS activity. [DOI] [PubMed] [Google Scholar]
- 28•.DiRusso CC, Li H, Darwis D, Watkins PA, Berger J, Black PN. Comparative biochemical studies of the murine fatty acid transport proteins (FATP) expressed in yeast. J Biol Chem. 2005;280:16829–16837. doi: 10.1074/jbc.M409598200. In this report, FATPs show differences in their ability to complement yeast acyl-CoA synthetase activity. [DOI] [PubMed] [Google Scholar]
- 29.Richards MR, Harp JD, Ory DS, Schaffer JE. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J Lipid Res. 2006;47:665–672. doi: 10.1194/jlr.M500514-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol. 2006;17:274–278. doi: 10.1097/01.mol.0000226119.20307.2b. [DOI] [PubMed] [Google Scholar]
- 31.Johnson DR, Knoll LJ, Levin DE, Gordon JI. Saccharomyces cerevisiae contains four fatty acid activation (FAA) genes: an assessment of their role in regulating protein N-myristoylation and cellular lipid metabolism. J Cell Biol. 1994;127:751–762. doi: 10.1083/jcb.127.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caviglia JM, Li LO, Wang S, DiRusso CC, Coleman RA, Lewin TM. Rat long chain acyl-CoA synthetase 5, but not 1, 2, 3, or 4, complements Escherichia coli fadD. J Biol Chem. 2004;279:11163–11169. doi: 10.1074/jbc.M311392200. [DOI] [PubMed] [Google Scholar]
- 33.Tong F, Black PN, Coleman RA, DiRusso CC. Fatty acid transport by vectorial acylation in mammals: roles played by different isoforms of rat long-chain acyl-CoA synthetases. Arch Biochem Biophys. 2006;447:46–52. doi: 10.1016/j.abb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Tomoda H, Igarashi K, Omura S. Inhibition of acyl-CoA synthetase by triacsins. Biochim Biophys Acta. 1987;921:595–598. [PubMed] [Google Scholar]
- 35.Van Horn CG, Caviglia JM, Li LO, Wang S, Granger DA, Coleman RA. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: Identification of a novel variant of isoform 6. Biochemistry. 2005;44:1635–1642. doi: 10.1021/bi047721l. [DOI] [PubMed] [Google Scholar]
- 36.Muoio DM, Lewin TM, Wiedmer P, Coleman RA. Acyl-CoAs are functionally channeled in liver: potential role of acyl-CoA synthetase. Am J Physiol Endocrinol Metab. 2000;279:E1366–1373. doi: 10.1152/ajpendo.2000.279.6.E1366. [DOI] [PubMed] [Google Scholar]
- 37.Igal RA, Wang P, Coleman RA. Triacsin C blocks de novo synthesis of glycerolipids and cholesterol esters but not recycling of fatty acid into phospholipid: evidence for functionally separate pools of acyl-CoA. Biochem J. 1997;324:529–534. doi: 10.1042/bj3240529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marszalek JR, Kitidis C, Dararutana A, Lodish HF. Acyl-CoA synthetase 2 overexpression enhances fatty acid internalization and neurite outgrowth. J Biol Chem. 2004;279:23882–91. doi: 10.1074/jbc.M313460200. [DOI] [PubMed] [Google Scholar]
- 39.Souza SC, Muliro KV, Liscum L, et al. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. 2002;277:8267–72. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- 40.Parkes HA, Preston E, Wilks D, et al. Overexpression of acyl-CoA synthetase-1 increases lipid deposition in hepatic (HepG2) cells and rodent liver in vivo. Am J Physiol Endocrinol Metab. 2006;291:E737–744. doi: 10.1152/ajpendo.00112.2006. [DOI] [PubMed] [Google Scholar]
- 41.Hatch GM, Smith AJ, Xu FY, Hall AM, Bernlohr DA. FATP1 channels exogenous FA into 1,2,3-triacyl-sn-glycerol and down-regulates sphingomyelin and cholesterol metabolism in growing 293 cells. J Lipid Res. 2002;43:1380–1389. doi: 10.1194/jlr.m200130-jlr200. [DOI] [PubMed] [Google Scholar]
- 42.Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 43.Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DA. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J Lipid Res. 2007;48:609–620. doi: 10.1194/jlr.M600441-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Marszalek JR, Kitidis C, Dirusso CC, Lodish HF. Long-chain acyl-CoA synthetase 6 preferentially promotes DHA metabolism. J Biol Chem. 2005;280:10817–10826. doi: 10.1074/jbc.M411750200. [DOI] [PubMed] [Google Scholar]
- 45.Herrmann T, van der Hoeven F, Grone HJ, et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol. 2003;161:1105–1115. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moulson CL, Martin DR, Lugus JJ, Schaffer JE, Lind AC, Miner JH. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc Natl Acad Sci USA. 2003;100:5274–5279. doi: 10.1073/pnas.0431186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gimeno RE, Hirsch DJ, Punreddy S, et al. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J Biol Chem. 2003;278:49512–49516. doi: 10.1074/jbc.M309759200. [DOI] [PubMed] [Google Scholar]
- 48.Mashek DG, Li LO, Coleman RA. Rat long chain acyl-CoA synthetase mRNA, protein and activity vary in tissue distribution and in response to diet. J Lipid Res. 2006;47:2004–2010. doi: 10.1194/jlr.M600150-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Schoonjans K, Watanabe M, Suzuki H, et al. Induction of the acyl-Coenzyme A synthetase gene by fibrates and fatty acids is medicated by a peroxisome proliferator response element in the C promoter. J Biol Chem. 1995;270:19269–19276. doi: 10.1074/jbc.270.33.19269. [DOI] [PubMed] [Google Scholar]
- 50.Achouri Y, Hegarty BD, Allanic D, et al. Long chain fatty acyl-CoA synthetase 5 expression is induced by insulin and glucose: involvement of sterol regulatory element-binding protein-1c. Biochimie. 2005;87:1149–1155. doi: 10.1016/j.biochi.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Durgan DJ, Smith JK, Hotze MA, et al. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am J Physiol Heart Circ Physiol. 2006;290:H2480–2497. doi: 10.1152/ajpheart.01344.2005. [DOI] [PubMed] [Google Scholar]
- 52.Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J Biol Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- 53.de Jong H, Neal AC, Coleman RA, Lewin TM. Ontogeny of mRNA expression and activity of long-chain acyl-CoA synthetase (ACSL) isoforms in Mus musculus heart. Biochim Biophys Acta. 2007;1771:75–82. doi: 10.1016/j.bbalip.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall M, Saggerson ED. Reversible inactivation by noradrenaline of long-chain fatty acyl-CoA synthetase in rat adipocytes. Biochem J. 1985;226:275–282. doi: 10.1042/bj2260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang YL, Guo W, Zang Y, et al. Acyl Coenzyme A synthetase regulation: putative role in long-chain acyl Coenzyme A partitioning. Obes Res. 2004;12:1781–1788. doi: 10.1038/oby.2004.221. [DOI] [PubMed] [Google Scholar]
- 56.Distler AM, Kerner J, Hoppel CL. Post-translational modifications of rat liver mitochondrial outer membrane proteins identified by mass spectrometry. Biochim Biophys Acta. 2007;1774:628–636. doi: 10.1016/j.bbapap.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Man MZ, Hui TY, Schaffer JE, Lodish HF, Bernlohr DA. Regulation of the murine adipocyte fatty acid transporter gene by insulin. Mol Endocrinol. 1996;10:1021–1028. doi: 10.1210/mend.10.8.8843418. [DOI] [PubMed] [Google Scholar]
- 58•.Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol. 2006;26:3455–3467. doi: 10.1128/MCB.26.9.3455-3467.2006. Shows insulin sensitivity of FATP1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faergeman NJ, Knudsen J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J. 1997;323:1–12. doi: 10.1042/bj3230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larsson O, Deeney JT, Branstom R, Berggren PO, Corkey BE. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. J Biol Chem. 1996;271:10623–10626. doi: 10.1074/jbc.271.18.10623. [DOI] [PubMed] [Google Scholar]
- 61.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr. 2005;25:317–340. doi: 10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- 62.Shimabukuro M, Zhou Y-T, Levi M, Unger RH. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 64.Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278:31861–31870. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- 65•.Ostrander DB, Sparagna GC, Amoscato AA, McMillin JB, Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J Biol Chem. 2001;276:38061–38067. doi: 10.1074/jbc.M107067200. This is the first suggested mechanism for palmitate-induction of apoptosis. [DOI] [PubMed] [Google Scholar]
- 66.Shimabukuro M, Wang MY, Zhou YT, Newgard CB, Unger RH. Protection against lipoapoptosis of beta cells through leptin-dependent maintenance of Bcl-2 expression. Proc Natl Acad Sci USA. 1998;95:9558–9561. doi: 10.1073/pnas.95.16.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yagasaki F, Jinnai I, Yoshida S, et al. Fusion of TEL/ETV6 to a novel ACS2 in myelodysplastic syndrome and acute myelogenous leukemia with t(5;12)(q31;p13) Genes, Chromosomes & Cancer. 1999;26:192–202. doi: 10.1002/(sici)1098-2264(199911)26:3<192::aid-gcc2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 68.Gassler N, Herr I, Schneider A, et al. Impaired expression of acyl-CoA synthetase 5 in sporadic colorectal adenocarcinomas. J Pathol. 2005;207:295–300. doi: 10.1002/path.1831. [DOI] [PubMed] [Google Scholar]
- 69.Yeh CS, Wang JY, Cheng TL, Juan CH, Wu CH, Lin SR. Fatty acid metabolism pathway play an important role in carcinogenesis of human colorectal cancers by Microarray-Bioinformatics analysis. Cancer Lett. 2006;233:297–308. doi: 10.1016/j.canlet.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 70•.Cao Y, Dave KB, Doan TP, Prescott SM. Fatty acid CoA ligase 4 is up-regulated in colon adenocarcinoma. Cancer Res. 2001;61:8429–8434. This paper shows upregulation of ACS in cancer. [PubMed] [Google Scholar]
- 71•.Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. PNAS. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. Shows the importance of intracellular FA and acyl-CoA pools in regulating apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimomura I, Tokunaga K, Jiao S, et al. Marked enhancement of acyl-CoA synthetase activity and RNA, paralleled to lipoprotein lipase mRNA, in adipose tissues of Zucker obese rats (fa/fa) Biochim Biophys Acta. 1992;1124:112–118. doi: 10.1016/0005-2760(92)90086-b. [DOI] [PubMed] [Google Scholar]
- 73.Memon RA, Fuller J, Moser AH, Smith PJ, Grunfeld C, Feingold KR. Regulation of putative fatty acid transporters and acyl-CoA synthetase in liver and adipose tissue in ob/ob mice. Diabetes. 1999;48:121–127. doi: 10.2337/diabetes.48.1.121. [DOI] [PubMed] [Google Scholar]
- 74.Heimerl S, Moehle C, Zahn A, et al. Alterations in intestinal fatty acid metabolism in inflammatory bowel disease. Biochim Biophys Acta. 2006;1762:341–350. doi: 10.1016/j.bbadis.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Covault J, Pettinati H, Moak D, Mueller T, Kranzler HR. Association of a long-chain fatty acid-CoA ligase 4 gene polymorphism with depression and with enhanced niacin-induced dermal erythema. Am J Med Genet B Neuropsychiatr Genet. 2004;127:42–47. doi: 10.1002/ajmg.b.20156. [DOI] [PubMed] [Google Scholar]
- 76.Piccini M, Vitelli F, Bruttini M, et al. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics. 1998;47:350–358. doi: 10.1006/geno.1997.5104. [DOI] [PubMed] [Google Scholar]
- 77.Meloni I, Muscettola M, Raynaud M, et al. FACL4, encoding fatty acid-CoA ligase 4, is mutated in nonspecific X-linked mental retardation. Nat Genet. 2002;30:436–440. doi: 10.1038/ng857. [DOI] [PubMed] [Google Scholar]
- 78.Longo I, Frints SG, Fryns JP, et al. A third MRX family (MRX68) is the result of mutation in the long chain fatty acid-CoA ligase 4 (FACL4) gene: proposal of a rapid enzymatic assay for screening mentally retarded patients. J Med Genet. 2003;40:11–17. doi: 10.1136/jmg.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lewin TM, Kim J-H, Granger DA, Vance JE, Coleman RA. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J Biol Chem. 2001;276:24674–24679. doi: 10.1074/jbc.M102036200. [DOI] [PubMed] [Google Scholar]
- 80.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 81.Kim J-H, Lewin TM, Coleman RA. Expression and characterization of recombinant rat acyl-CoA synthetases 1, 4, and 5: Selective inhibition by triacsin C and thiazolidinediones. J Biol Chem. 2001;276:24667–24673. doi: 10.1074/jbc.M010793200. [DOI] [PubMed] [Google Scholar]
- 82.Fujino T, Kang M-J, Suzuki H, Iijima H, Yamamoto T. Molecular characterization and expression of rat acyl-CoA synthetase 3. J Biol Chem. 1996;271:16748–16752. doi: 10.1074/jbc.271.28.16748. [DOI] [PubMed] [Google Scholar]
- 83.Fujimoto Y, Itabe H, Sakai J, et al. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta. 2004;1644:47–59. doi: 10.1016/j.bbamcr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 84.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 85.Umlauf E, Csaszar E, Moertelmaier M, Schuetz GJ, Parton RG, Prohaska R. Association of stomatin with lipid bodies. J Biol Chem. 2004;279:23699–23709. doi: 10.1074/jbc.M310546200. [DOI] [PubMed] [Google Scholar]
- 86.Sato S, Fukasawa M, Yamakawa Y, et al. J Biochem. Vol. 139. Tokyo: 2006. Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein; pp. 921–930. [DOI] [PubMed] [Google Scholar]
- 87.Oikawa E, Iijima H, Suzuki T, et al. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J Biochem. 1998;124:679–685. doi: 10.1093/oxfordjournals.jbchem.a022165. [DOI] [PubMed] [Google Scholar]
- 88.Turró S, Ingelmo-Torres M, Estanyol JM, et al. Identification and characterization of associated with lipid droplet protein 1: A novel membrane-associated protein that resides on hepatic lipid droplets. Traffic. 2006;7:1254–1269. doi: 10.1111/j.1600-0854.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 89.Fujino T, Yamamoto T. Cloning and functional expression of a novel long-chain acyl-CoA synthetase expressed in brain. J Biochem. 1992;111:197–203. doi: 10.1093/oxfordjournals.jbchem.a123737. [DOI] [PubMed] [Google Scholar]
- 90.Heinzer AK, Kemp S, Lu JF, Watkins PA, Smith KD. Mouse very long-chain acyl-CoA synthetase in X-linked adrenoleukodystrophy. J Biol Chem. 2002;277:28765–28773. doi: 10.1074/jbc.M203053200. [DOI] [PubMed] [Google Scholar]
- 91.Pei Z, Fraisl P, Berger J, Jia Z, Forss-Petter S, Watkins PA. Mouse very long-chain acyl-CoA synthetase 3/fatty acid transport protein 3 catalyzes fatty acid activation but not fatty acid transport in MA-10 cells. J Biol Chem. 2004;279:54454–5462. doi: 10.1074/jbc.M410091200. [DOI] [PubMed] [Google Scholar]
- 92.Stahl A, Hirsch DJ, Gimeno RE, et al. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 93.Hirsch DJ, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci USA. 1998;95:8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blackburn C, Guan B, Brown J, et al. Identification and characterization of 4-aryl-3, 4-dihydropyrimidin-2(1H)-ones as inhibitors of the fatty acid transporter FATP4. Bioorg Med Chem Lett. 2006;16:3504–3509. doi: 10.1016/j.bmcl.2006.03.102. [DOI] [PubMed] [Google Scholar]
- 95.Berger J, Truppe C, Neumann H, Forss-Petter S. A novel relative of the very-long-chain acyl-CoA synthetase and fatty acid transporter protein genes with a distinct expression pattern. Biochem Biophys Res Commun. 1998;247:255–260. doi: 10.1006/bbrc.1998.8770. [DOI] [PubMed] [Google Scholar]
- 96.Tang P-Z, Tsai-Morris C-H, Dufau ML. Cloning and characterization of a homonally regulated rat long chain acyl-CoA synthetase. Proc Natl Acad Sci. 2001;98:6581–6586. doi: 10.1073/pnas.121046998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moriya-Sato A, Hida A, Inagawa-Ogashiwa M, et al. Novel acyl-CoA synthetase in adrenoleukodystrophy target tissues. Biochem Biophys Res Commun. 2000;279:62–68. doi: 10.1006/bbrc.2000.3897. [DOI] [PubMed] [Google Scholar]
- 98.Fraisl P, Forss-Petter S, Zigman M, Berger J. Murine bubblegum orthologue is a microsomal very long-chain acyl-CoA synthetase. Biochem J. 2004;377:85–93. doi: 10.1042/BJ20031062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pei Z, Jia Z, Watkins PA. The second member of the human and murine bubblegum family is a testis- and brainstem-specific acyl-CoA synthetase. J Biol Chem. 2006;281:6632–6641. doi: 10.1074/jbc.M511558200. [DOI] [PubMed] [Google Scholar]
- 100.Cho YY, Kang MJ, Sone H, et al. Abnormal uterus with polycysts, accumulation of uterine prostaglandins, and reduced fertility in mice heterozygous for acyl-coa synthetase 4 deficiency. Biochem Biophys Res Commun. 2001;284:993–997. doi: 10.1006/bbrc.2001.5065. [DOI] [PubMed] [Google Scholar]
- 101.Heinzer AK, Watkins PA, Lu JF, et al. A very long-chain acyl-CoA synthetase-deficient mouse and its relevance to X-linked adrenoleukodystrophy. Hum Mol Genet. 2003;12:1145–1154. doi: 10.1093/hmg/ddg126. [DOI] [PubMed] [Google Scholar]
- 102•.Hubbard B, Doege H, Punreddy S, et al. Mice deleted for fatty acid transport protein 5 have defective bile acid conjugation and are protected from obesity. Gastroenterology. 2006;130:1259–1269. doi: 10.1053/j.gastro.2006.02.012. This is the first study of a targeted knockout of FATP5. It shows that the FATP5 null mouse is defective in both bile acid formation and glycerolipid synthesis. [DOI] [PubMed] [Google Scholar]