Abstract
Terminal regions of the Drosophila embryo are patterned by the localized activation of the Mitogen Activated Protein Kinase (MAPK) pathway [1]. This depends on the MAPK-mediated downregulation of Capicua (Cic), a repressor of the terminal gap genes [2, 3]. We established that downregulation of Cic is antagonized by the anterior patterning morphogen Bicoid (Bcd). We demonstrate that this effect does not depend on transcriptional activity of Bcd and provide evidence suggesting that Bcd, a direct substrate of MAPK, decreases the availability of MAPK for its other substrates, such as Cic. Based on the quantitative analysis of MAPK signaling in multiple mutants, we propose that MAPK substrate competition coordinates the actions of the anterior and terminal patterning systems. In addition, we identify Hunchback (Hb) as a novel target of MAPK phosphorylation that can account for the previously described genetic interaction between the posterior and terminal systems [4]. Thus, a common enzyme-substrate competition mechanism can integrate the actions of the anterior, posterior, and terminal patterning signals. Substrate competition can be a general signal integration strategy in networks where enzymes, such as MAPK, interact with their multiple regulators and targets [5-10].
Anteroposterior (AP) patterning of the Drosophila embryo depends on three inductive signals [11]: The head and thorax are specified by the anterior gradient of Bicoid (Bcd), a homeobox transcription factor; abdomen formation is directed by the reciprocal gradient of Nanos (Nos), a translational repressor; and the non-segmented termini are patterned by the localized activation of the Extracellular Signal Regulated/Mitogen Activated Protein Kinase (ERK/MAPK) pathway [6, 12]. MAPK signaling is induced by a uniformly expressed receptor tyrosine kinase Torso (Tor), which is activated by its ligand produced at the poles of the embryo [1].
Activated Tor promotes the expression of tailless (tll) and huckebein (hkb), zygotic gap genes required for the development of the terminal structures. This depends on MAPK-mediated phosphorylation of the transcriptional repressors Capicua (Cic) and Groucho (Gro), both of which are initially distributed uniformly throughout the embryo [1-3, 13]. Phosphorylation of Cic and Gro relieves their repression of tll and hkb and enables expression of these genes at both poles of the embryo. At the anterior pole, MAPK also phosphorylates Bcd [14, 15]. Thus, MAPK is activated in a localized pattern and phosphorylates substrates that can be either uniformly (Cic and Gro) or non-uniformly (Bcd) distributed along the AP axis of the embryo (Fig. 1A-C).
Figure 1. Spatial asymmetries of MAPK phosphorylation.
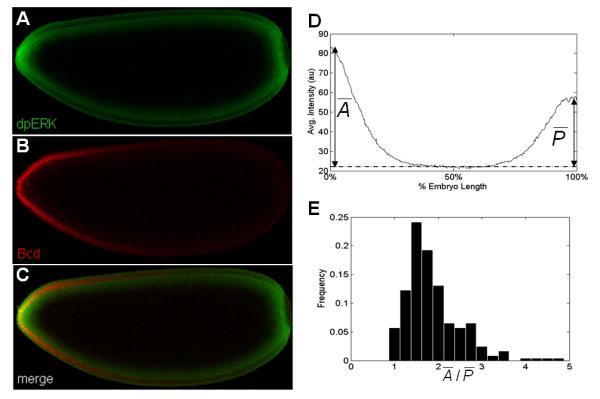
(A-C) Co-immunostaining of the anteroposterior (AP) gradient of phosphorylated MAPK, dpERK (green) and Bcd (Red). In all figures, embryos are oriented with anterior to the left and dorsal side up.
(D) An averaged AP gradients of dpERK at early cell cycle 14 obtained from 40 embryos. The dashed line indicates the reference level of dpERK. Quantification of the gradients reveals a clear AP asymmetry: The anterior levels are higher than the posterior levels.
(E) Histogram of the ratios of anterior to posterior peak levels of MAPK phosphorylation from 244 wild type embryos. Individual peak values of dpERK at the termini are determined as described in Suppl. Fig. 1. In wild type embryos, this ratio has mean of 1.87 and standard deviation of 0.64, which indicates that the anterior levels of dpERK are significantly higher than the posterior levels (t-test, p<10−5)
The spatial pattern of MAPK activation can be visualized using an antibody that recognizes the double phosphorylated form of ERK (dpERK) [16, 17]. We found that this pattern is strongly asymmetric: the anterior levels of dpERK are significantly higher than the posterior levels (Fig. 1; see also Supplemental Figure 1). Since the early Drosophila embryo is highly polarized, multiple factors can potentially contribute to the observed asymmetry. These include the differences in the anterior and posterior levels of the extracellular ligand that activates Tor [18] and/or in the intracellular localization of maternal determinants.
We hypothesized that the higher levels of dpERK at the anterior pole reflect the presence of an anteriorly localized maternal factor. One candidate for such a factor is Bcd, which is localized to the anterior of the embryo and is one of the phosphorylation targets of MAPK [14]. To test whether Bcd can affect the AP asymmetry of the dpERK pattern, we examined this pattern in embryos with different levels of Bcd. We found that the anterior levels of dpERK are significantly decreased in embryos from bcd null or heterozygous mothers, and increased in embryos with two extra copies of bcd (Fig. 2A, B). Importantly, the posterior levels of dpERK are unaffected in these embryos, which reflects the anterior localization of Bcd. Thus, changing the level of Bcd, a substrate of MAPK, influences the level of MAPK phosphorylation.
Figure 2. The anterior level of dpERK responds to changes in the level of Bcd.
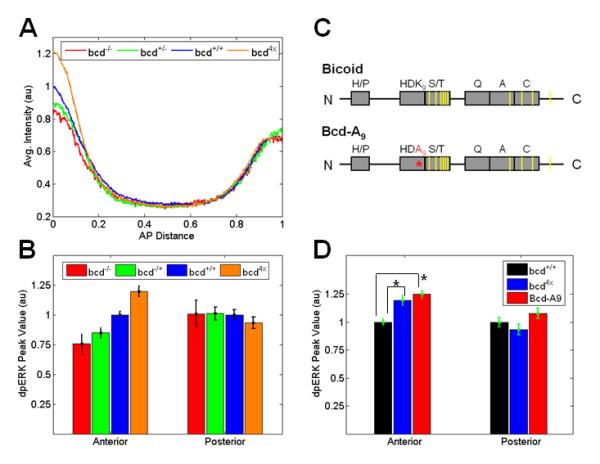
(A) The AP gradient of dpERK in progeny of females with different bcd copy number. Each line indicates an average gradient of dpERK for 20-25 individual embryos of the same genotype. Note that the anterior level of dpERK changes as the amount of maternal bcd present is altered, while posterior levels are not affected.
(B) Changes in the anterior and posterior levels of dpERK as a function of maternal bcd copy number. Each bar represents an average of MAPK phosphorylation for 20-25 individual embryos of the same genotype with standard error (SE) indicated. The data are normalized such that the values of wild type (bcd+/+, embryos marked with Histone-GFP) are set at 1. Only the anterior level shows an increasing trend as a function of bcd copy number (generalized linear model: pAnterior = 2.9 × 10−8, pPosterior = 0.30).
(C) Schematics of Bcd and a Bcd variant (Bcd-A9) used in the experiments. Gray boxes show following domains in the Bcd protein: the PRD repeat (H/P), the DNA binding domain (HD), the S/T rich domain, a glutamine rich domain (Q), an alanine rich domain (A), and an acidic domain (C). Putative MAPK phosphorylation sites (based on [14]) are indicated by yellow bars. Bcd-A9 encodes a Bcd variant with a single lysine-to-alanine amino acid substitution at position 50 of the homeodomain (K9 to A9, shown in red).
(D) Embryos expressing two wild type bcd and two copies of the bcd-A9 transgene show increased phosphorylation of MAPK at the anterior poles, similar to embryos with four copies of wild type bcd (bcd4x). Mean ± SE of 20-30 embryos for each genotype; (*) denotes a statistically significant difference from the wild type (t-test, p<0.01).
Previous studies had shown that overexpressing MAPK substrates in a heterologous cell culture system leads to a higher level of MAPK phosphorylation [19]. Furthermore, experiments with purified components revealed that MAPK substrates can directly compete with the MAPK phosphatases for binding to the common docking domain of MAPK [19-23]. Thus, the level of MAPK substrates can increase the level of MAPK phosphorylation by outcompeting the MAPK phosphatase. Correspondingly, a similar effect can be responsible for the observed dose-dependent control of MAPK phosphorylation levels by Bcd: The total concentration of MAPK substrates at the anterior pole is higher than at the posterior, due to the anterior localization of Bcd, resulting in a stronger interference with MAPK dephosphorylation in this region of the embryo. This can account for the higher level of dpERK at the anterior pole.
The fact that the anterior level of dpERK is sensitive to the dose of Bcd is consistent with the proposed competition model. This, however, does not exclude the possibility of a more indirect transcriptional effect, whereby one or more transcriptional targets of Bcd would affect the levels MAPK phosphorylation. To distinguish between these two alternatives, we used the bcd transgene (Bcd-A9), which encodes a Bcd variant that has an impaired ability to bind its DNA recognition sequence[24] but still contains all the sites required for binding to and phosphorylation by MAPK (Fig. 2C). We first confirmed that the expression of hunchback (hb), a direct target of Bcd, was not affected in embryos with two copies of wild type bcd and two copies of the Bcd-A9 transgene (data not shown). Remarkably, the anterior level of dpERK was significantly increased in these embryos (Fig. 2D). In fact, the anterior level of dpERK was indistinguishable from the anterior level of dpERK in embryos with two extra copies of wild type bcd (bcd4x). Taken together, these results imply that Bcd can affect the level of MAPK phosphorylation independently of its transcriptional activity.
The results presented so far are consistent with the idea that Bcd makes phosphorylated MAPK less available for binding to and dephosphorylation by the MAPK phosphatase. According to the same model, Bcd can make the phosphorylated MAPK less available for its other substrates. To explore this possibility, we examined the distribution of Cic, which is downregulated at the poles as a result of its direct phosphorylation by MAPK (Fig. 3A-C). Statistical analysis revealed that, similar to the wild type pattern of dpERK, the wild type pattern of Cic is asymmetrical, with the levels of Cic significantly higher at the anterior pole (Fig. 3D). Since the distribution of Cic is uniform in the absence of MAPK signaling, the wild type pattern of Cic downregulation suggests that, although the level of MAPK phosphorylation is higher at the anterior pole, its activity directed towards Cic is actually lower.
Figure 3. Asymmetry of MAPK-mediated downregulation of Cic.
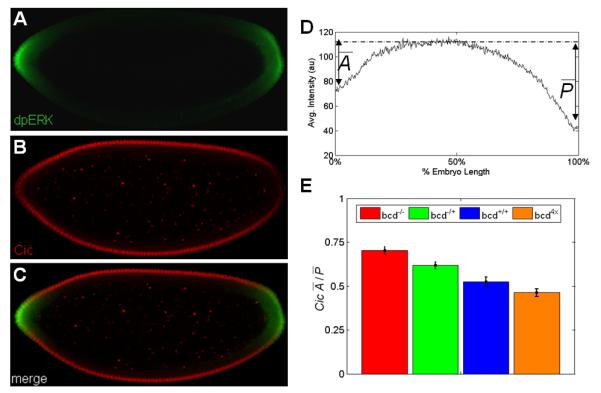
(A-C) Co-immunostaining reveals the spatial patterns of dpERK (green) and Cic (red).
(D) Averaged AP gradient of nuclear Cic at cell cycle 14 from 40 embryos. The dashed line indicates reference level of Cic repression. Similar to MAPK phosphorylation gradient, Cic pattern also exhibits a clear AP asymmetry.
(E) The dose of bcd affects the asymmetry of the spatial pattern of Cic downregulation in the early embryo. Each bar represents an average asymmetry of nuclear Cic gradient for 40-90 embryos of the same genotype. As the amount of maternal bcd mRNA is lowered, the anterior and posterior levels of Cic become more symmetric (generalized linear model: p = 2.5×10−12).
We emphasize that this observation argues against the possibility that the AP asymmetry of the wild type MAPK phosphorylation pattern is generated only by the asymmetry in the extracellular activation of Tor. If this were true, then higher levels of MAPK phosphorylation at the anterior pole would lead to a higher level of Cic downregulation, which is contrary to what we observe. Based on this, we argue that the AP asymmetry of the wild type MAPK signaling pattern is generated mainly by the intracellular asymmetries of the early embryo. Upon quantifying the spatial pattern of Cic downregulation in embryos with varying levels of Bcd, we established that this asymmetry is increased in embryos with an extra copy of bcd and reduced in embryos with progressively lowered levels of bcd (Fig. 3E). These effects are consistent with the model, where anteriorly localized Bcd acts as a competitive inhibitor of MAPK-mediated Cic downregulation.
If Bcd is the only source of the AP asymmetries of MAPK phosphorylation and Cic downregulation, then both of these patterns should become symmetric in bcd null embryos. Surprisingly, however, we found that both MAPK phosphorylation and Cic downregulation exhibit a significant amount of AP asymmetry even in the bcd mutant embryos (Fig. 4A, B). It is possible that this residual asymmetry reflects the presence of an additional MAPK substrate, which is nonuniformly distributed in bcd null embryos and contributes to the AP asymmetry of MAPK signaling in this genetic background.
Figure 4. Hb is a new substrate of MAPK in the embryo.
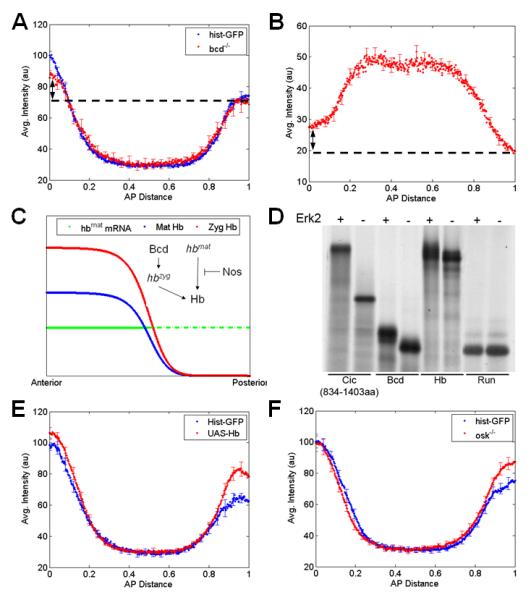
(A) Comparison of the dpERK gradients in wild type (histone-GFP) embryos and embryos from bcd null mothers. Average dpERK gradients for each genotype (N~15) are plotted with SE indicated. Although the anterior levels of dpERK in bcd null embryos are lower than that in wild type, the gradient is still asymmetric along the AP axis (arrow).
(B) Averaged AP gradient of nuclear Cic at cell cycle 14 obtained from 27 embryos laid by bcd null flies. Similar to the dpERK gradient, the Cic pattern is still asymmetric along the AP axis (arrow).
(C) Simplified description of Hb regulation in a wild type embryo. Maternal hb mRNA is deposited uniformly throughout the embryo (green), but its translation is repressed in posterior half by Nos (dashed green), resulting in anterior gradient of maternal Hb protein (blue). At the anterior half of the embryo, Bcd activates zygotic hb transcription which further adds to the pre-existing Hb gradient (red). Note that even in the absence of Bcd, Hb protein is still present in anterior gradient (blue).
(D) Radiolabelled Hb migrates with slower mobility on SDS-PAGE following incubation in the presence (+), but not in the absence (−), of activated ERK2, indicating that Hb is phosphorylated by MAPK. Similar mobility shift is observed for known targets of MAPK such as Cic and Bcd, but not for Runt. Note that Hb contains several consensus MAPK phosphorylation sites.
(E) Ubiquitous expression of hb using a maternal GAL4 driver leads to increase in MAPK phosphorylation both at the anterior and posterior poles of the embryo (t-test: pAnterior = 0.03, pPosterior = 0.01); average dpERK gradients for each genotype (N~25-30) plotted with SE indicated.
(F) Quantifying the dpERK gradient in embryos mutant for osk shows an increase in MAPK phosphorylation at the posterior region. In osk null embryos, maternal hb mRNA is translated at the posterior of the embryo. Average dpERK gradients for each genotype (N~25-30) are plotted with SE indicated.
One candidate for this residual asymmetry is Hunchback (Hb), a transcription factor that plays a key role in the embryonic AP patterning [25, 26]. The distribution of Hb protein depends on both maternal and zygotic inputs [25, 27]. Briefly, maternal hb transcript is deposited uniformly, but its translation is repressed in the posterior region of the embryo by Nos, resulting in an anterior gradient of maternal Hb protein. In the anterior half of the embryo, hb is also zygotically activated by Bcd, providing an additional gradient of zygotic Hb protein (Fig. 4C). As a consequence of its dual control by Bcd and Nos, the pattern of Hb protein is not symmetric in bcd null embryos: the levels of Hb are still higher at the anterior pole. This led us to test whether, similar to Bcd and Cic, Hb acts as a hitherto unrecognized MAPK substrate that modulates the patterns of MAPK phosphorylation and Cic downregulation along the AP axis.
Indeed, in a proteomics screen aimed at identifying potential MAPK substrates in the early blastoderm embryo, we found that Hb is phosphorylated by MAPK in vitro. Like Bcd and Cic, but unlike Runt and other segmentation gene products, Hb displays a MAPK-dependent electrophoretic mobility shift on SDS-PAGE (Fig. 4D and results not shown). To test if Hb can affect the level of MAPK phosphorylation in vivo, we examined MAPK phosphorylation in embryos with uniform maternal expression of Hb. We found that Hb expression leads to a statistically significant increase in the posterior dpERK signal, as expected if Hb were acting as a MAPK substrate in the early embryo (Fig. 4E). To summarize, the proposed substrate competition model accounts for the experimentally observed AP asymmetry of the wild type patterns of MAPK phosphorylation and the activity towards its substrates, predicts how this asymmetry responds to multiple genetic perturbations, and identifies Hb as a novel potential target of MAPK phosphorylation.
In addition, the proposed model can account for the previously unexplained genetic interaction between the posterior and terminal systems: It was established that removal of posterior patterning determinants (Nos or Oskar) increases the posterior level of Cic, and consequently reduces the posterior expression domains of its targets tll and hkb [4]. In light of our results, these effects can be interpreted by the fact that, in nos- or osk- embryos, ectopic Hb acts as a competitive substrate of MAPK, reducing its ability to downregulate Cic. This would be analogous to the effect at the anterior pole, where downregulation of Cic is antagonized by Bcd. In partial confirmation of this model, we found that removal of either nos or osk increases the posterior level of dpERK, indicating that increased amounts of Hb, like Bcd, can influence the level of MAPK phosphorylation (Fig. 4F). Thus, a common substrate competition mechanism can provide a basis for the modulation of MAPK signaling by the anterior and posterior systems.
Competitive binding effects have been shown to influence the operation of signaling networks in mathematical models and in vitro [7, 19, 28-33]. Our results support the existence of these effects in vivo and demonstrate how MAPK signaling can be regulated by the levels of MAPK substrates. Competitive interactions can provide a general control strategy in signaling networks where enzymes, such as MAPK, interact with their multiple regulators and targets [5-9].
Experimental Procedures
Drosophila strains and whole-mount immunostaining
The following stocks were used in this study: Histone-GFP (a gift from E. Wieschaus), bcdE1, bcd4x, osk6, nosBN, Cic-HA; cic1[3], Cic-HA; cic1 bcdE1, UAS-hb (a gift from N. Dostatni), and Bcd-A9 [24]. All flies were raised and embryos were collected at 25°C. Antibody stainings were performed as described in [17]. Monoclonal mouse anti-dpERK (1:100, Sigma) and polyclonal rabbit anti-HA (1:500, Roche) were used as primary antibodies. Alexa Flour and Oregon Green (1:500, Invitrogen) were as secondary antibodies.
Microscopy and image processing
Imaging was done on a Zeiss LSM510 confocal microscope, with a Zeiss 20x (NA 0.6) A-plan objective. High resolution images (512×512 pixels, 12bits depth) were obtained from the focal plane in the mid-horizontal cross section of the embryo. Images of individual embryos were automatically extracted from raw confocal images and re-oriented for gradient quantification as described elsewhere [17].
In vitro MAPK/ERK2 phosphorylation assays
Proteins were synthesized and labeled using the Quick TNT-coupled rabbit reticulocyte lysate system (Promega), in the presence of [35S]-methionine. Labeled proteins were then incubated with (or without) 0.2 micrograms of active ERK2, in a total volume of 50 microliter of kinase reaction buffer (20 mM HEPES, 0.1 mM benzamidine, 25 mM beta-glycerophosphate, 0.1 mM DTT, 1 mM Na3VO4, 10 mM MgCl2 and 0.1 mM ATP) for 30 minutes at 30°C. Reactions were stopped by adding SDS sample buffer x3 (0.25M Tris pH 6.8, 6% SDS, 30% Glycerol, B-mercapto-ethanol and a few grains of Bromo-phenol-blue). The phosphorylation state of the proteins was subsequently analyzed by SDS-PAGE and autoradiography. To activate ERK2, a HIS-tagged ERK2 fusion protein was expressed in Escherichia coli, purified on nickel beads (Qiagen) and activated using active MEK1 (Upstate).
Statistical Analysis
A paired t-test was used to compare the mean levels of both anterior and posterior dpERK between wild type and mutant embryos of interest. For this analysis, dpERK gradients were extracted from 20-30 wild type and mutant embryos, and the dpERK levels were determined as described in Suppl. Fig. 1. To examine the correlation between the amount of Bcd and level of MAPK phosphorylation, a generalized linear model (GLM) was employed with copies of bcd as the independent variable and either the anterior or posterior dpERK levels as the response variable. Similarly, GLM was also used to show the correlation between the bcd copy number and the Cic asymmetry where Cic asymmetry is defined as the ratio of anterior to posterior repressions of Cic as described in Suppl. Fig. 1.
Supplementary Material
Supplemental Figure 1: Quantification of MAPK Phosphorylation and Cic downregulation
(A) The anterior (left) and posterior (right) dpERK gradients are fitted with a Gaussian curve independently and the peak levels of these fitted Gaussians are used as anterior and posterior levels of MAPK phosphorylation respectively.
(B) The anterior (left) and posterior (right) troughs of Cic gradients are fitted with inverted Gaussians and the differences between minima of these curves and the base line are used as anterior and posterior Cic repressions respectively.
Acknowledgement
We thank Eric Wieschaus, Trudi Schüpbach, Mark Lemmon, Ruth Steward, Alex Ninfa, Sofia Merajver, Mike Levine, Alexey Veraksa, Marla Tipping, and all members of the Shvartsman lab for helpful discussions and comments on the paper. We thank Eric Wieschaus, Oliver Grimm, Nathalie Dostatni, Steve Small, Steve Hanes, and Julien Dubuis for flies and reagents. We are grateful to Nir Yakoby and Jeremy Zartman for help with molecular characterization of the Bcd-A9 flies, and to Alistair Boettiger for his contribution to the development of image processing tools. SYS is supported by the R01 GM078079 NIH grant. ZP is supported by grants from the Israel Science Foundation (Center of Excellence; 180/09), Israel Cancer Research Fund and the Król Charitable Foundation. GJ is supported by the Spanish Ministry of Science and Education (BFU2005-02673) and ICREA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furriols M, Casanova J. In and out of Torso RTK signalling. Embo Journal. 2003;22:1947–1952. doi: 10.1093/emboj/cdg224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jimenez G, Guichet A, Ephrussi A, Casanova J. Relief of gene repression by Torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes & Development. 2000;14:224–231. [PMC free article] [PubMed] [Google Scholar]
- 3.Astigarraga S, Grossman R, Diaz-Delfin J, Caelles C, Paroush Z, Jimenez G. A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. Embo Journal. 2007;26:668–677. doi: 10.1038/sj.emboj.7601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinnamon E, Gur-Wahnon D, Helman A, St Johnston D, Jimenez G, Paroush Z. Capicua integrates input from two maternal systems in Drosophila terminal patterning. Embo Journal. 2004;23:4571–4582. doi: 10.1038/sj.emboj.7600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popescu SC, Popescu GV, Bachan S, Zhang ZM, Gerstein M, Snyder M, Dinesh-Kumar SP. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes & Development. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith EJ, Akella R, Min X, Zhou T, Humphreys JM. Substrate and docking interactions in serine/threonine protein kinases. Chem Rev. 2007;107:5065–5081. doi: 10.1021/cr068221w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Gerstein M, Snyder M. Getting connected: analysis and principles of biological networks. Genes Dev. 2007;21:1010–1024. doi: 10.1101/gad.1528707. [DOI] [PubMed] [Google Scholar]
- 9.von Kriegsheim A, Baiocchi D, Birtwistle M, Sumpton D, Bienvenut W, Morrice N, Yamada K, Lamond A, Kalna G, Orton R, et al. Cell fate decisions are specified by the dynamic ERK interactome. Nat Cell Biol. 2009;11:1458–1464. doi: 10.1038/ncb1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE. Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. Journal of Biological Chemistry. 2008;283 doi: 10.1074/jbc.M801074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Johnston D, Nusslein-Volhard C. The Origin of Pattern and Polarity in the Drosophila Embryo. Cell. 1992;68:201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu BE, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chemical Reviews. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 13.Cinnamon E, Helman A, Ben-Haroush SR, Orian A, Jiménez G, Paroush Z. Multiple RTK pathways downregulate Groucho-mediated repression in Drosophila embryogenesis. Development. 2008;135:829–837. doi: 10.1242/dev.015206. [DOI] [PubMed] [Google Scholar]
- 14.Janody F, Sturny R, Catala F, Desplan C, Dostatni N. Phosphorylation of bicoid on MAP-kinase sites: contribution to its interaction with the torso pathway. Development. 2000;127(2):279–89. doi: 10.1242/dev.127.2.279. 279-289. [DOI] [PubMed] [Google Scholar]
- 15.Ronchi E, Treisman J, Dostatni N, Struhl G, Desplan C. Down-regulation of the Drosophila morphogen bicoid by the torso receptor-mediated signal transduction cascade. Cell. 1993;74:347–355. doi: 10.1016/0092-8674(93)90425-p. [DOI] [PubMed] [Google Scholar]
- 16.Gabay L, Seger R, Shilo BZ. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development. 1997;124:3535–3541. doi: 10.1242/dev.124.18.3535. [DOI] [PubMed] [Google Scholar]
- 17.Coppey M, Boettiger AN, Berezhkovskii AM, Shvartsman SY. Nuclear trapping shapes the terminal gradient in the Drosophila embryo. Curr Biol. 2008;18:915–919. doi: 10.1016/j.cub.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furriols M, Ventura G, Casanova J. Two distinct but convergent groups of cells trigger Torso receptor tyrosine kinase activation by independently expressing torso-like. Proc Natl Acad Sci. 2007;104:11660–11665. doi: 10.1073/pnas.0700991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardwell AJ, Abdollahi M, Bardwell L. Docking sites on mitogen-activated protein kinase (MAPK) kinases, MAPK phosphatases and the Elk-1 transcription factor compete for MAPK binding and are crucial for enzymic activity. Biochem J. 2003;370(Pt 3):1077–1085. doi: 10.1042/BJ20021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JL, Zhou B, Zheng CF, Zhang ZY. A bipartite mechanism for ERK2 recognition by its cognate regulators and substrates. Journal of Biological Chemistry. 2003;278:29901–29912. doi: 10.1074/jbc.M303909200. [DOI] [PubMed] [Google Scholar]
- 21.Zhou B, Wu L, Shen K, Zhang JL, Lawrence DS, Zhang ZY. Multiple regions of MAP kinase phosphatase 3 are involved in its recognition and activation by ERK2. Journal of Biological Chemistry. 2001;276:6506–6515. doi: 10.1074/jbc.M009753200. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B, Zhang JL, Liu SJ, Reddy S, Wang F, Zhang ZY. Mapping ERK2-MKP3 binding interfaces by hydrogen/deuterium exchange mass spectrometry. Journal of Biological Chemistry. 2006;281:38834–38844. doi: 10.1074/jbc.M608916200. [DOI] [PubMed] [Google Scholar]
- 23.Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nature Cell Biology. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 24.Hanes SD, Riddihough G, Ish-Horowicz D, Brent R. Specific DNA recognition and intersite spacing are critical for action of the bicoid morphogen. Moll Cell Biol. 1994;14:3364–3375. doi: 10.1128/mcb.14.5.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Struhl G. Differing strategies for organizing anterior and posterior body pattern in Drosophila embryos. Nature. 1989;338:741–744. doi: 10.1038/338741a0. [DOI] [PubMed] [Google Scholar]
- 26.Yu D, Small S. Precise registration of gene expression boundaries by a repressive morphogen in Drosophila. Current Biology. 2008;18:868–876. doi: 10.1016/j.cub.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tautz D. Regulation of the Drosophila segmentation gene hunchback by 2 maternal morphogenetic centers. Nature. 1988;332:281–284. doi: 10.1038/332281a0. [DOI] [PubMed] [Google Scholar]
- 28.Qiao L, Nachbar RB, Kevrekidis IG, Shvartsman SY. Bistability and oscillations in the Huang-Ferrell model of MAPK signaling. PLoS Comput Biol. 2007;3:1819–1826. doi: 10.1371/journal.pcbi.0030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura AC, Sepulchre JA, Merajver SD. A hidden feedback in signaling cascades is revealed. PLoS Comput Biol. 2008;4:e1000041. doi: 10.1371/journal.pcbi.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legewie S, Schoeberl B, Blüthgen N, Herzel H. Competing docking interactions can bring about bistability in the MAPK cascade. Biophys J. 2007;93:2279–2288. doi: 10.1529/biophysj.107.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markevich NI, Hoek JB, Kholodenko BN. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. Journal of Cell Biology. 2004;164:353–359. doi: 10.1083/jcb.200308060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusari AB, Molina DM, Sabbagh W, Lau CS, Bardwell L. A conserved protein interaction network involving the yeast MAP kinases Fus3 and Kss1. Journal of Cell Biology. 2004;164:267–277. doi: 10.1083/jcb.200310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SY, Ferrell JE. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell. 2007;128:1133–1145. doi: 10.1016/j.cell.2007.01.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Quantification of MAPK Phosphorylation and Cic downregulation
(A) The anterior (left) and posterior (right) dpERK gradients are fitted with a Gaussian curve independently and the peak levels of these fitted Gaussians are used as anterior and posterior levels of MAPK phosphorylation respectively.
(B) The anterior (left) and posterior (right) troughs of Cic gradients are fitted with inverted Gaussians and the differences between minima of these curves and the base line are used as anterior and posterior Cic repressions respectively.


