Abstract
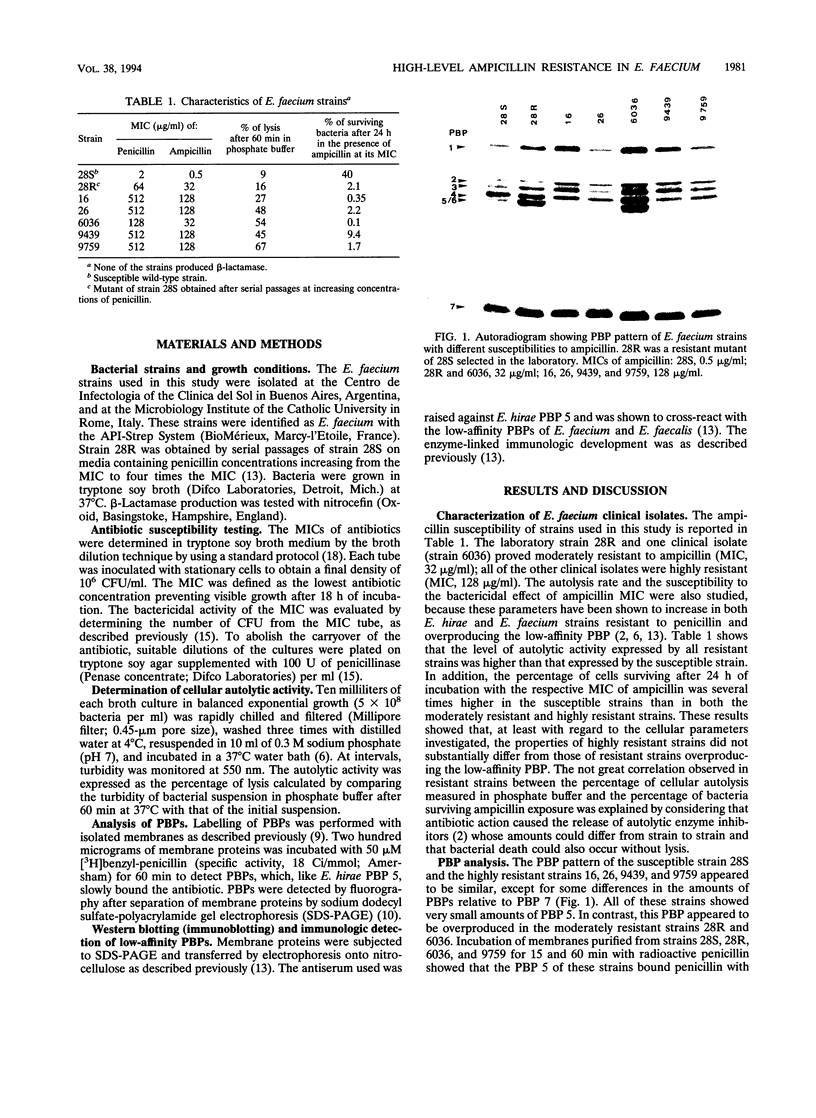
Five ampicillin-resistant clinical isolates of Enterococcus faecium were analyzed for a correlation between overproduction of the low-affinity penicillin-binding protein (PBP 5) and the level of ampicillin resistance. Comparison was made with one susceptible clinical isolate and its ampicillin-resistant derivative obtained in the laboratory by selection with increasing concentrations of penicillin. Overproduction of the low-affinity PBP relative to the susceptible isolate was noted in moderately resistant strains (MIC, 32 micrograms/ml) but not in highly resistant strains (MIC, 128 micrograms/ml). Polyclonal antibodies specifically reacting with the low-affinity PBP of Enterococcus hirae, Enterococcus faecalis, and Enterococcus faecium (M. Ligozzi, M. Aldegheri, S. C. Predari, and R. Fontana, FEMS Microbiol. Lett. 83:335-340, 1991) were used to determine the amount of this PBP in the E. faecium isolates. In all strains, the antibody preparation reacted with a membrane protein of the same molecular mass as PBP 5. The amount of this protein was very small in the susceptible strain but large in all of the resistant strains. These results suggest that the highly resistant strains also overproduced the low-affinity PBP, which, compared with PBP 5 of moderately resistant strains, appeared to be modified in its penicillin-binding capability.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canepari P., Lleò M. M., Cornaglia G., Fontana R., Satta G. In Streptococcus faecium penicillin-binding protein 5 alone is sufficient for growth at sub-maximal but not at maximal rate. J Gen Microbiol. 1986 Mar;132(3):625–631. doi: 10.1099/00221287-132-3-625. [DOI] [PubMed] [Google Scholar]
- Canepari P., Lleò M. M., Fontana R., Satta G. Streptococcus faecium mutants that are temperature sensitive for cell growth and show alterations in penicillin-binding proteins. J Bacteriol. 1987 Jun;169(6):2432–2439. doi: 10.1128/jb.169.6.2432-2439.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Canepari P., Satta G., Coyette J. Identification of the lethal target of benzylpenicillin in Streptococcus faecalis by in vivo penicillin binding studies. Nature. 1980 Sep 4;287(5777):70–72. doi: 10.1038/287070a0. [DOI] [PubMed] [Google Scholar]
- Fontana R., Canepari P., Satta G., Coyette J. Streptococcus faecium ATCC 9790 penicillin-binding proteins and penicillin sensitivity are heavily influenced by growth conditions: proposal for an indirect mechanism of growth inhibition by beta-lactams. J Bacteriol. 1983 May;154(2):916–923. doi: 10.1128/jb.154.2.916-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Cerini R., Longoni P., Grossato A., Canepari P. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol. 1983 Sep;155(3):1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Grossato A., Rossi L., Cheng Y. R., Satta G. Transition from resistance to hypersusceptibility to beta-lactam antibiotics associated with loss of a low-affinity penicillin-binding protein in a Streptococcus faecium mutant highly resistant to penicillin. Antimicrob Agents Chemother. 1985 Nov;28(5):678–683. doi: 10.1128/aac.28.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klare I., Rodloff A. C., Wagner J., Witte W., Hakenbeck R. Overproduction of a penicillin-binding protein is not the only mechanism of penicillin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1992 Apr;36(4):783–787. doi: 10.1128/aac.36.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligozzi M., Aldegheri M., Predari S. C., Fontana R. Detection of penicillin-binding proteins immunologically related to penicillin-binding protein 5 of Enterococcus hirae ATCC 9790 in Enterococcus faecium and Enterococcus faecalis. FEMS Microbiol Lett. 1991 Oct 15;67(3):335–339. doi: 10.1016/0378-1097(91)90498-y. [DOI] [PubMed] [Google Scholar]
- Ligozzi M., Pittaluga F., Fontana R. Identification of a genetic element (psr) which negatively controls expression of Enterococcus hirae penicillin-binding protein 5. J Bacteriol. 1993 Apr;175(7):2046–2051. doi: 10.1128/jb.175.7.2046-2051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleó M. M., Canepari P., Cornaglia G., Fontana R., Satta G. Bacteriostatic and bactericidal activities of beta-lactams against Streptococcus (Enterococcus) faecium are associated with saturation of different penicillin-binding proteins. Antimicrob Agents Chemother. 1987 Oct;31(10):1618–1626. doi: 10.1128/aac.31.10.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopardo H., Casimir L., Hernández C., Rubeglio E. A. Isolation of three strains of beta-lactamase-producing Enterococcus faecalis in Argentina. Eur J Clin Microbiol Infect Dis. 1990 Jun;9(6):402–405. doi: 10.1007/BF01979469. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinehart E., Smith N. E., Wennersten C., Gorss E., Freeman J., Eliopoulos G. M., Moellering R. C., Jr, Goldmann D. A. Rapid dissemination of beta-lactamase-producing, aminoglycoside-resistant Enterococcus faecalis among patients and staff on an infant-toddler surgical ward. N Engl J Med. 1990 Dec 27;323(26):1814–1818. doi: 10.1056/NEJM199012273232606. [DOI] [PubMed] [Google Scholar]
- Williamson R., le Bouguénec C., Gutmann L., Horaud T. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to benzylpenicillin. J Gen Microbiol. 1985 Aug;131(8):1933–1940. doi: 10.1099/00221287-131-8-1933. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Gutmann L., Williamson R. Correlation of penicillin-induced lysis of Enterococcus faecium with saturation of essential penicillin-binding proteins and release of lipoteichoic acid. Antimicrob Agents Chemother. 1990 Oct;34(10):1901–1907. doi: 10.1128/aac.34.10.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Obeid S., Gutmann L., Williamson R. Modification of penicillin-binding proteins of penicillin-resistant mutants of different species of enterococci. J Antimicrob Chemother. 1990 Nov;26(5):613–618. doi: 10.1093/jac/26.5.613. [DOI] [PubMed] [Google Scholar]
- el Kharroubi A., Jacques P., Piras G., Van Beeumen J., Coyette J., Ghuysen J. M. The Enterococcus hirae R40 penicillin-binding protein 5 and the methicillin-resistant Staphylococcus aureus penicillin-binding protein 2' are similar. Biochem J. 1991 Dec 1;280(Pt 2):463–469. [PMC free article] [PubMed] [Google Scholar]