Abstract
Background
development of encapsulated therapeutics that could be released upon ultrasound exposure has strong implications for enhancing drug effects at the target site. We have developed echogenic liposomes (ELIP) suitable for ultrasound imaging of blood flow and ultrasound-mediated intravascular drug release. Papaverine was chosen as the test drug because its clinical application requires high concentration in the target vascular bed but low concentration in the systemic circulation.
Methods
the procedure for preparation of standard ELIP was modified by including Papaverine hydrochloride in the lipid hydration solution, followed by three freeze-thaw cycles to increase encapsulation of the drug. Sizing and encapsulation pharmacokinetics were performed using a Coulter counter and a phosphodiesterase activity assay. Stability of Papaverine-loaded ELIP (PELIP) was monitored with a clinical diagnostic ultrasound scanner equipped with a linear array transducer at a center frequency of 4.5 MHz by assessing the mean digital intensity within a region of interest over time. The stability of PELIP was compared to those of standard ELIP and Optison™.
Results
relative to standard ELIP, PELIP were larger (median diameter = 1.88 ± 0.10 μm for PELIP vs 1.08 ± 0.15 μm for ELIP) and had lower MGSV (92 ± 24.8 for PELIP compared to 142.3 ± 10.7 for ELIP at lipid concentrations of 50 μg/ml). The maximum loading efficiency and mean encapsulated concentration were 24% ± 7% and 2.1 ± 0.7 mg/ml, respectively. Papaverine retained its phosphodiesterase inhibitory activity when associated with PELIP. Furthermore, a fraction of this activity remained latent until released by dissolution of liposomal membranes with detergent. The stability of both PELIP and standard ELIP were similar, but both are greater than that of Optison™.
Conclusions
our results suggest that PELIP have desirable physical, biochemical, biological, and acoustic characteristics for potential in vivo administration and ultrasound-controlled drug delivery.
Keywords: Liposomes, papaverine, drug encapsulation, ultrasound contrast agents
Introduction
The development of ultrasound-mediated liposome-based drug delivery systems promises the potential to deliver drugs or genes to target sites at efficacious doses, while minimizing systemic side effects. A few liposome-based drug delivery systems are currently in clinical use in oncology (Park et al., 2004) and infectious diseases (Wasan et al., 1995). Recent advances in liposome-based delivery systems have focused on modifying liposomal composition to improve biocompatibility and control the rate and extent of drug release from liposomes at target sites. A number of innovative liposome drug delivery designs have been proposed and many have been reviewed (Allen, 1998).
Our laboratory has developed a novel submicron liposomal formulation that contains both gas and fluid suitable for ultrasound imaging and drug loading (Alkan-Onyuksel et al., 1996). Other gas-containing liposomes or microbubbles require additional gas entrapment. Our standard echogenic liposomes (ELIP) are unique in that gas entrapment occurs upon rehydration after a critical step in the process, namely lyophilization in the presence of mannitol. Our gas-containing liposomes are efficient scatterers of ultrasound and are suitable for visualization by diagnostic ultrasound. By modifying the surface components and attachment of suitable ligands, our liposomes can be targeted to specific tissues. Such echogenic immunoliposomes have been successfully used to highlight left ventricular thrombus (Hamilton et al., 2002) and inflammation in peripheral arteries (Hamilton et al., 2004) for ultrasound imaging in vivo. Other liposome-based drug delivery systems depend on passive or active targeting mechanisms for drug delivery. We hypothesized that the combined encapsulation of air and drug in liposomes may provide an attractive mechanism for visualization of the drug delivery process and retention of pharmacologic activities for potential ultrasound-triggered targeted drug delivery.
Certain clinical scenarios require different approaches in liposomal design. Relevant to the present study is the management of symptomatic and medically refractory cerebral vasospasm following aneurysmal subarachnoid hemorrhage. This condition is often treated by direct intraarterial infusion of vasodilators, such as papaverine, vera-pamil or nicardipine via an indwelling catheter positioned in the internal carotid or vertebral artery. Among all vasodilators, most experience involves the use of papaverine (Kassell et al., 1992; Smith et al., 2000; Minami et al., 2001; Vajkoczy et al., 2001; Liu et al., 2002; Oskouian et al., 2002; Srinivasan et al., 2002). Papaverine is a benzyliso-quinolone alkaloid and potent smooth muscle relaxant. The vasodilatory mechanism of papaverine is uncertain but may involve inhibition of phosphodiesterase activity in smooth muscle cells (Scroop et al., 2004). Immediate angiographic relief of vasospasm has been reported to range from 57 to 90% (Fandino et al., 1998; Milburn et al., 1998; Firlik et al., 1999). The main drawback of endovascular administration of papaverine is its temporary relief of vasospasm and the need for repeated endovascular procedures (Liu et al., 2004). Additional treatments require repetition of invasive catheterization procedures with incremental accumulation of procedure-related risks (Andaluz et al., 2002). Adverse side effects of systemic vasodilator administration include hypotension and exacerbation of ischemic neurological injury. Encapsulation of papaverine in acoustically activated liposomes may overcome some of the disadvantages of endovascular papaverine administration, such as transient efficacy and the need for invasive cere-brovascular instrumentation. Our conceptual model for ultrasound-controlled delivery of papaverine with acoustically activated liposomes involves systemic intravenous administration of the liposomal preparation and selective papaverine release into the carotid or vertebral artery circulation using a transcutaneous ultrasound beam centered on the cervical internal carotid or vertebral arteries. The administration procedure would be relatively noninvasive and could be repeated multiple times at the bedside in the intensive care unit. Furthermore, since the encapsulated microbubbles provide ultrasonic contrast, the process of liposome delivery, activation and drug release could be imaged in real time. Response to treatment could also be assessed in real time at the bedside with transcranial Doppler ultrasound measurements of cerebral arterial blood flow velocity. Consequently, papaverine-loaded ELIP may provide a unique controlled drug delivery system for the delivery of Papaverine to the cerebral circulation. For this study, we hypothesized that both papaverine and air can be encapsulated within our standard ELIP and the resultant preparation will have desirable physical, chemical, biological, and acoustic characteristics for in vivo administration.
Methods
Preparation of Echogenic Liposomes Containing Papaverine Hydrochloride
Papaverine-containing echogenic liposomes (PELIP) were prepared using a modification of our established methods for preparing standard ELIP (Huang et al., 2004). The lipid composition of the standard ELIP formulation was modified by omitting dipalmi-toyphosphatidylglycerol (DPPG) in PELIP to prevent electrostatic interactions between DPPG and papaverine. Chloroform solutions of lipids (30 mg total) containing egg yolk phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL), dipalmitoyphosphatidylethano-lamine (DPPE; Avanti Polar Lipids, Alabaster, AL), and cholesterol (Sigma Chemical Co., St. Louis, MO) in the molar ratio of 69:8:15 were mixed in a round-bottomed flask. The chloroform was removed by evaporation under argon in a 50°C water bath to form a thin film of lipid on the flask wall. The resulting lipid film was placed under high vacuum (< 0.1 mmHg) for 2–8 h to complete removal of the solvent. The dry lipid film was hydrated with unbuffered papaverine hydrochloride solution to yield a concentration of 10 mg lipid/ml. The hydrated mixture was subjected to three freeze-thaw cycles, followed by sonication in a water bath until the solution became translucent. Equal volumes of 0.32 M mannitol and hydrated lipid-Papaverine were mixed and frozen for 4 h at −70°C. The frozen samples were immediately lyophilized under a vacuum of 50 mTorr with a condensation chamber temperature of −50°C for 24 h. The samples were stored in a refrigerator at 2–5°C. Lyophilized PELIP preparations were reconstituted in water prior to use.
Estimation of Papaverine Concentration
The concentration of papaverine hydrochloride was estimated by UV spectrophotometry. A spectrophotometric analysis of Papaverine hydrochloride revealed a peak absorbance at 250 nm and a corresponding extinction coefficient of 47,050. Thus, concentration of papaverine hydrochloride [PH] was calculated as follows:
where A250 is the absorbance of the solution at 250 nm, DF is the dilution factor, and 375.9 is the molecular weight of papaverine hydrochloride. This equation remains valid and linear up to a papaverine concentration of 20 μg/ml, and all samples were diluted within the concentration range for estimation of papaverine concentration.
Estimation of Encapsulation Efficiency
Separation of encapsulated papaverine hydrochloride in PELIP from free papaverine hydrochloride was carried out by the minicolumn centrifugation method (Fry et al., 1978). Briefly, Sephadex G-50 (hydrogen form, 50–100 mesh; Sigma Chemical Co., St Louis, MO) was allowed to swell in 0.9% NaCl solution overnight and stored at 4°C until required for use. The minicolumn was prepared by removing the plunger of a 1 ml disposable plastic syringe and plugging the barrel with glass wool. The barrel was filled with hydrated Sepha-dex gel and placed in a 13 × 10 mm centrifuge tube. The tube was spun at 1000g for 3 min to remove excess saline solution, and the height of the bed was level with the 0.9 ml mark. One hundred microliters of liposome suspension were loaded into to the top of the gel bed, and the column was spun at 1000g for 3 min. Papaverine-loaded ELIP were eluted from the minicolumn and free papaverine hydrochloride was retained inside the gel.
The concentration of encapsulated papaverine hydrochloride in PELIP was measured by diluting PELIP samples in 2.5% (v/v) Zwittergent (EMD Biosciences, La Jolla, CA) at 0.5% (v/v) to release encapsulated papaverine hydrochloride from liposomes. Zwittergent does not have any significant absorbance at 250 nm and does not interfere with the measurement of papaverine concentration. Encapsulation efficiency of papaverine hydrochloride in PELIP was calculated as follows:
where Cencapsulated is the concentration of papaverine in the eluant after minicolumn centrifugation and Ctotal is the total concentration of papaverine in the reconstituted PELIP before minicolumn centrifugation.
Size Distribution of Papaverine-Containing Echogenic Liposomes
Papaverine-containing ELIP was diluted 1000 fold in 0.9% saline solution. The size distribution of PELIP and standard ELIP was measured using a Multisizer 3 Coulter Counter (Beckman-Coulter, Inc., Miami, FL) equipped with a 20 μm aperture tube. Two PELIP preparations were analyzed and the size distribution of each specimen was measured 3 times.
Measurement of Phosphodiesterase Inhibitory Activities of Papaverine and Papaverine-Containing Echogenic Liposomes
Phosphodiesterase (PDE) activities were assayed using the PDE-Glo™ phosphodiesterase assay kit (Promega Corp., Madison, WI). The concentrations of papaverine in free solution or Zwittergent-treated PELIP were determined by absorbance at 250 nm as described above. In a 96-well plate, 12.5 μl of 0.67 mU of PDE from bovine brain and 12.5 μl of the indicated concentrations of papaverine or PELIP were preincubated for 5 min, followed by incubating with 0.0625 μM cAMP substrate in each well for 15 mins.
The reaction was terminated with a buffer containing 100mM 3-isobutyl-1-methylxanthine (IBMX). Luminescence was developed with a detection buffer and Kinase Glo solution in the assay kit, and subsequently read with a Tecan Safire2™ plate reader (Mannedorf/Zurich, Switzerland). Means and standard deviations were derived from 4 samples at each papaverine concentration.
Measurement of Echogenicity
Imaging of samples was performed with a 20-MHz high-frequency intravascular ultrasound image catheter (SciMed, Inc., Sunnyvale, CA) as described previously (Huang et al., 2001). Briefly, lyophilized samples were reconstituted in water and then serially diluted in 0.9% saline to lipid concentrations between 200 μg/ml and 6.25 μg/ml. Instrument settings for gain, zoom, compression, and rejection levels were optimized at the initiation of the experiment and held constant for all samples. Images were digitalized and videodensitometric analysis was performed using ImagePro Plus software V4.1 (Media Cybernetics, Silver Spring, MD). The annular area between the vial wall and the imaging catheter was manually outlined (excluding the area of the “strut” artifact of the IVUS imaging catheter) and designated as the area of interest.
Stability of PELIP Compared to Standard ELIP or Optison®
Ultrasound contrast agent (UCA) stability was monitored with a Philips HDI 5000 diagnostic ultrasound scanner using an L12-5 linear array transducer (Philips Medical Systems, Bothell, WA, USA). This transducer was designed to perform superficial vascular diagnostic studies clinically and enables the use of a small sample volume for each experiment. The apparatus consisted of the transducer mounted to a three-axis positioner over an anechoic chamber. A 25.0 ml sample of contrast agent suspension filled the chamber for echogenicity analysis (Smith et al., 2007).
Each sample volume was exposed to diagnostic harmonic B-mode imaging at a center frequency of 4.5 MHz. The Mechanical Index (MI) was manually fixed throughout the duration of each experiment. The focus position was set at 1.0 cm from the transducer face. The 2D overall gain, 2D pulse repetition frequency (PRF), time gain compensation (TGC) curve, inactive persistence, most linear gray scale map, image depth, and focal settings were fixed through the duration of every experiment by saving a customized preset in each mode. The diagnostic ultrasound scanner continuously acquired image frames at a specified frame rate (FR) related to the PRF (or image depth) chosen. For the harmonic B-mode setting, the FR was equal to 7 Hz at an image depth of 2.3 cm (PRF = 5.1 kHz).
For each sample, 0.5% bovine serum albumin (BSA) or phosphate buffered saline (PBS) was pipetted into the anechoic chamber for alignment. Standard ELIP or PELIP were suspended in 0.5% BSA, and Optison® microspheres were diluted in PBS at room temperature (21–22 °C). All liposome suspensions were prepared by reconstituting 6 mg of lyophilized liposomes in 0.6 ml of filtered, deionized water (17.8 Ω·cm) saturated with air (100% oxygen saturation at 21–22 °C; Oakton® Dissolved Oxygen 100 Series meter, Oakton Instruments, Vernon Hills, IL, USA). The reconstituted liposomes were pipetted into BSA to yield a lipid concentration of 0.05 mg/ml. This liposome concentration was employed in animal studies by Hamilton et al. (Hamilton et al., 2002). The number density at this particular lipid concentration was 8.3 × 107 liposomes/ml based on flow cytometry data (Coussios et al., 2004). All Optison® suspensions were prepared by drawing fresh Optison® (using a secondary vent to prevent agent rupture) from the vial with a syringe and gently diluted with PBS to a final volumetric ratio of 0.1 ml/1 L. The Optison® microspheres were pipetted into PBS to yield a concentration of 5–8 × 104 microspheres/ml. All samples (ELIP or Optison® suspensions) were stirred gently by hand and left to settle in the anechoic chamber for approximately 20–30 seconds prior to insonification to allow bulk fluid motion to cease. No stirring of the sample was performed thereafter. Each experiment was repeated at least five times with a fresh UCA suspension to assess variability.
To determine the stability of PELIP, standard ELIP, and Optison® in suspension, echogenicity data were gathered continuously for 300 seconds (Smith et al., 2007). Images were freeze-captured every 5 seconds for 120 seconds for both Optison® and liposome suspensions, and then every 10 seconds thereafter for the next 180 seconds. The exposure output (MI) for the stability studies was chosen to be below the acoustically driven diffusion threshold for Optison® suspensions insonified with B-mode pulses at a center frequency of 3.5 MHz (MI = 0.04, Pr = 0.12 MPa; Porter et al., 2006). For every harmonic B-mode image, a 20 mm2 ROI (region of interest) was analyzed to determine a mean gray scale value (MGSV) using NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA). The MGSV was then converted to a mean digital intensity (MDI) value and plotted against time (Smith et al., 2007).
Ion Exchange Chromatography
The water-soluble form of Papaverine hydrochloride was expected to reside inside the aqueous core of liposomes, but the detection of phosphodiesterase inhibitory activities of intact PELIP prompted us to evaluate the nature of the association of Papaverine in echogenic liposomes. The site of Papaverine incorporation in PELIP was examined using an ion-exchange column containing DOWEX-50W hydrogen form (50–100 mesh). The column was filled with DOWEX-50W resin to a height of 2.5 ml. Prior to the loading of samples, the column was washed extensively with 2M HCl, followed by water. The concentration of Papaverine and the echogenicity of PELIP in the elu-ant were measured by UV spectroscopy and intravascular ultrasound imaging, respectively, as described above.
Statistics
Data were analyzed using Sigma Stat, version 2.03 statistical software (SPSS Inc, Chicago, IL). The results are expressed as the mean ± SD. Differences between groups were analyzed using Student’s t-test. A p value < 0.05 was considered statistically significant.
Results
Encapsulation Efficiency of Papaverine Hydrochloride in Echogenic Liposomes
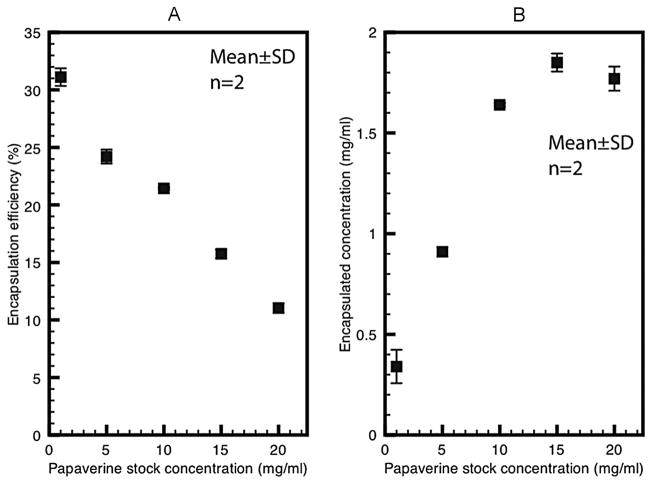
The minicolumn centrifugation method was rapid and efficient in separating free from encapsulated Papaverine hydrochloride without dilution. Increasing amounts of papaver-ine were loaded onto the spin column to determine the loading capacity of the spin column. With a 0.9 ml gel bed in each minicolumn, this technique removed 99.8% to 89.1% of free papaverine hydrochloride, with the highest extraction efficiency obtained when the column was loaded with 2.1 mg to 4.2 mg of papaverine, which was the usual amount in subsequent analysis. The encapsulation efficiency of papaverine hydrochloride in PELIP was inversely related to the concentration of papaverine hydrochloride stock solution used to hydrate the initial lipid film (Figure 1A). The net amount of encapsulated drug in echogenic liposomes was directly related to the concentration of drug used to hydrate the initial lipid film, which reached a maximum at a papaverine stock solution concentration of 10 mg/ml and did not increase further beyond that concentration (Figure 1B). Maximum concentrations of encapsulated drug were achieved with papaverine stock solution concentrations between 10 mg/ml and 20 mg/ml. For the rest of the experiments, all PELIP preparations were prepared using a papaverine hydrochloride stock solution of 10 mg/ml. The average encapsulation efficiency of papaverine in PELIP was 29%.
Figure 1.
A. Effects of different papaverine stock solution concentration on the encapsulation efficiency of papaverine in echogenic liposomes. B. Effects of different papaverine stock solution concentration on encapsulation concentration of papaverine in echogenic liposomes.
Size Distribution of Papaverine-Loaded Echogenic Liposomes and Standard Echogenic Liposomes
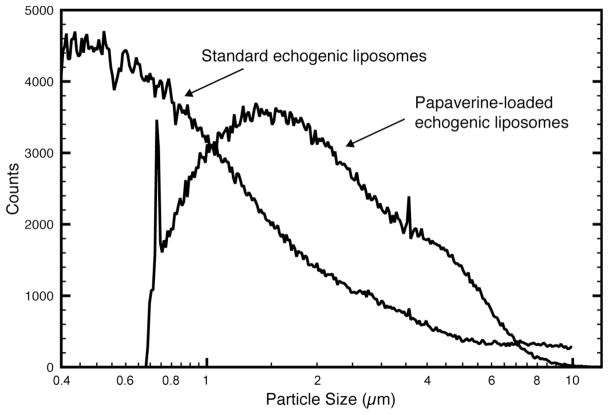
Size distributions of echogenic liposomes were measured with a Beckman-Coulter Multi-sizer 3 counter equipped with a 20 μm aperture tube. Median diameters of PELIP and standard ELIP were 1.88 ± 0.10 μm and 1.08 ± 0.15 μm, respectively (Figure 2). The Beckman-Coulter Multisizer 3 with a 20 μm aperture tube has a detection limit of 0.6 μm, and the sizing of particles close to the detection limit may be unreliable and account for the rapid drop in the number of PELIP particles in the smaller size range.
Figure 2.
Size distribution of standard echogenic liposomes and papaverine-loaded echogenic liposomes as measured by a Beckman-Coulter Multisizer 3 counter using a 20 μm aperture tube.
Measurement of Phosphodiesterase Inhibitory Activities of Papaverine-Containing Echogenic Liposomes
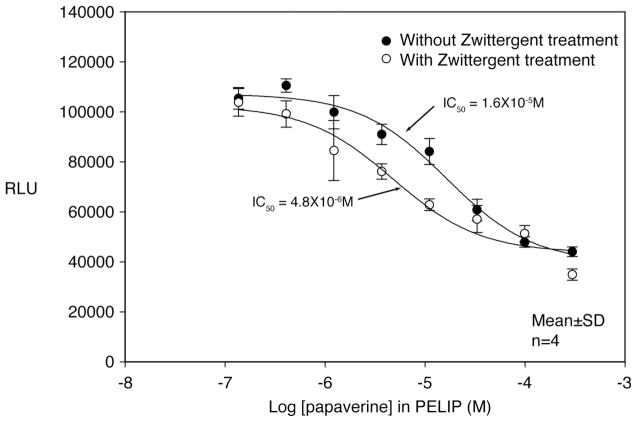
One of the mechanisms of papaverine on smooth muscle relaxation is mediated by inhibition of phosphodiesterase activity. The inhibitory activity of intact papaverine-containing echogenic liposomes was compared with papaverine released by complete dissolution of liposomes with a detergent. The luminescence signal (RLU) in the phosphodiesterase assay is directly proportional to the phosphodiesterase activity. The concentrations at which 50% of the enzymatic activity is inhibited (or IC50 value) of papaverine-containing ELIP and detergent treated papaverine-containing ELIP were 1.6 × 10−5 M and 4.8 × 10−6 M, respectively. This indicates that a fraction of the total papaverine is stabilized by lipo-somes and not released for phosphodiesterase inhibitory activities until it is released by detergent treatment (Figure 3).
Figure 3.
Phosphodiesterase inhibitory activities of papaverine-loaded echogenic liposomes with or without detergent treatment. The PDE-Glo™ phosphodiesterase assay was performed in a 96-well plate using 0.67 mU of PDE from bovine brain, 0.0625 μM cAMP substrate, and the indicated amount of Papaverine. The PDE and papaverine were preincubated together for 5 min. The substrate was added, and the reactions were incubated for an additional 15 min at room temperature. The reaction was terminated with a buffer containing 100mM IBMX. Luminescence was developed with a detection buffer and Kinase Glo solution and subsequently read with a Tecan Safire2™ plate reader (Mannedorf/Zurich, Switzerland). Means and standard deviations were derived from 4 samples at each papaverine concentration.
Echogenicity of PELIP, Standard ELIP and Optison®
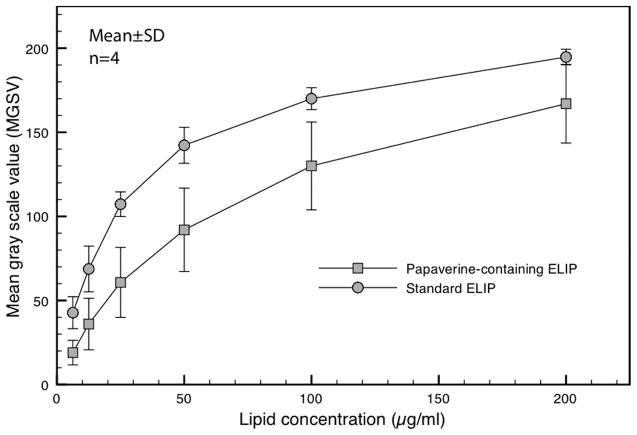
Our modification of the standard ELIP procedure for papaverine loading preserved the acoustic properties of ELIP (Figure 4). At lipid concentrations of 200 μg/ml and 50 μg/ml, the reflectivity of PELIP as measured by the mean gray scale values (MGSV) was 167±23 and 92 ± 25, respectively. MGSV of PELIP was somewhat lower than that of standard ELIP at each lipid concentration (Figure 5).
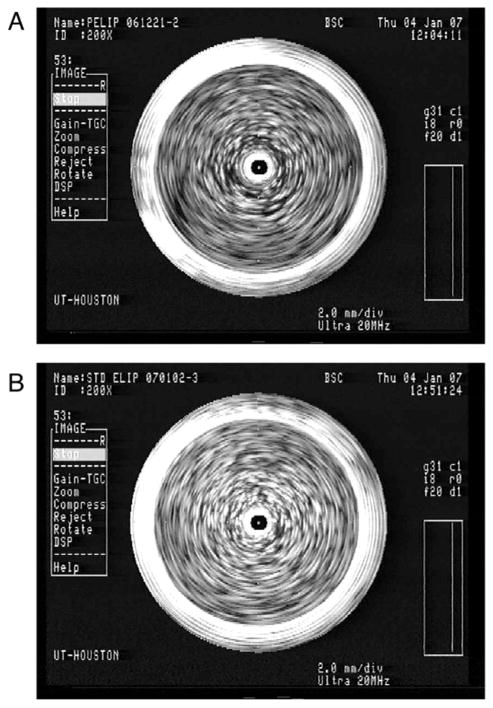
Figure 4.
A. Papaverine-loaded echogenic liposomes (PELIP) and B. Standard ELIP in a vial imaged with a 20-MHz intravascular ultrasound imaging catheter. The bright objects within the vial represent liposomal clusters. The central artifact represents the imaging catheter.
Figure 5.
Echogenicity of PELIP and ELIP expressed as mean gray scale values (MGSV) at different lipid concentrations imaged with a 20-MHz intravascular ultrasound imaging catheter.
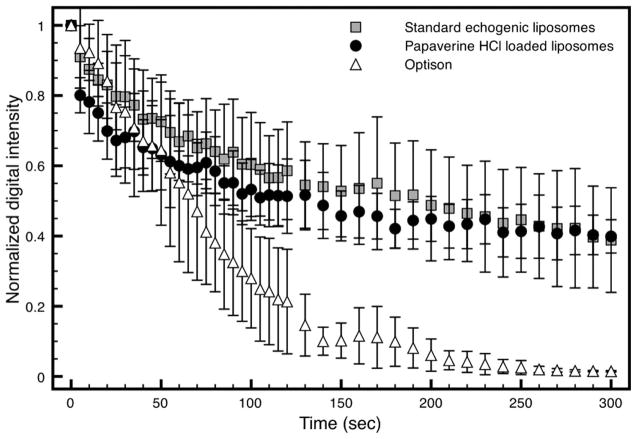
More detailed measurements of the stability of PELIP, standard ELIP and Optison® suspensions against gas diffusion in a static fluid are shown in Figure 6. After 5 min, the Optison® suspensions lost all echogenicity, but both standard ELIP and PELIP suspensions retained approximately 40% of their initial echogenicity. In addition, there is no difference in the echogenicity of standard ELIP and PELIP suspensions exposed continuously to harmonic B-mode imaging (MI = 0.04, Pr = 0.12 MPa). Thus, we conclude that PELIP and standard ELIP were more stable against gas diffusion in static suspensions of physiologic bovine serum albumin than another ultrasound contrast agent, Optison®.
Figure 6.
Loss of echogenicity of PELIP, standard ELIP, and Optison™ suspensions during intermittently scanned harmonic B-mode imaging (fc = 4.5 MHz) at MI = 0.04 (Pr = 0.12 MPa). MI = Mechanical Index, Pr = peak rarefractional pressure, fc = center frequency.
Ion Exchange Chromatography
The sites of papaverine incorporation in PELIP were examined using an ion-exchange column containing DOWEX-50W hydrogen form. The maximum binding capacity of papav-erine by 2.5 ml of DOWEX-50W in the ion exchange column was about 28 mg. Standard ELIP retained their echogenicity after passage through the ion exchange column. Elution of PELIP on the column resulted in a substantial loss of liposomes and the eluant was not echogenic (Table 1).
Table 1.
Echogenicity of PELIP and ELIP expressed as mean gray scale values (MGSV) at 200X dilution before and after passage through a column containing DOWEX-50W hydrogen form strongly acidic cationic exchanger
| MGSV | Pre-DOWEX | Post-DOWEX |
|---|---|---|
| Standard ELIP | 69.9 ± 22.8 | 64.9 ± 15.7 |
| PELIP | 86.7 ± 35.1 | 19.0 ± 2.8* |
p < 0.05 (n = 4).
Discussion
Our results show that papaverine hydrochloride can be loaded into echogenic liposomes without significantly reducing liposomal echogenicity or papaverine bioactivity. The association of papaverine in echogenic liposomes is complex and multicompartmental with both an aqueous core phase and a lipid membrane phase, as demonstrated by an analysis with an ion-exchange column. Such drug-loaded echogenic liposomes can potentially be used for ultrasound-mediated drug release. Our discussion will focus on the unique clinical applications of loading both papaverine and air into liposomes, biochemical considerations, and unique challenges when loading papaverine into liposomes. We will also address the biological activity of papaverine when associated with echogenic liposomes.
Numerous drugs have been loaded into liposomes for site-specific drug delivery, with the objective of maximizing local effects and minimizing systemic side effects. However, only a few studies, including another of ours, have described coencapsulation of both drug and gas into liposomes (Unger et al., 1998; Tiukinhoy et al., 2004; Zhao et al., 2005).
Coencapsulation of both drug and gas into liposomes endows them with a number of unique, clinically important characteristics. First, encapsulated air in liposomes provides ultrasonic contrast, which enables imaging of the process of liposome delivery, activation, and drug release in real time. Second, ultrasound can interact with entrapped air in the lipid membrane and may facilitate the release of a drug from liposomes. Third, local destruction of echogenic liposomes may enhance local drug effects such as thrombolysis (Tachibana et al., 1995; Mizushige et al., 1999). In posthemorrhage cerebral vasospasm, selective release of papaverine from PELIP into the cerebral circulation may be achieved noninvasively by transcutaneous ultrasound beams focused on the internal carotid or vertebral arteries. It is hoped that the use of PELIP will obviate the need for invasive interventions, minimize systemic side effects, and achieve sustained relief of cerebral vasospasm.
A number of technical challenges were noted during the development of PELIP. The chemical characteristics of papaverine hydrochloride had a major influence on the suitability of lipid components for our liposomes. Our standard ELIP contain a small amount of negatively charged phospholipids, DPPG, which prevents aggregation by maintaining electrostatic repulsion among liposomes. However, the presence of positively charged papaverine ion in a standard ELIP dispersion was incompatible with the presence of DPPG as one of the lipid components. The resultant PELIP exhibited both an unacceptable extent of aggregation and poor echogenicity (results not shown). Thus, DPPG was eliminated from the final PELIP formulation. The lack of aggregation may also be due to bound Papaverine hydrochloride, which gives them a positive charge, mitigates against aggregation, and possibly facilitates hydration. In fact, the elimination of negatively charged DPPG in PELIP may provide a better safety profile for this agent. Liposomes containing negatively charged phospholipids such as DPPG were reported to have undesirable clinical characteristics, such as enhanced plasma clearance (Juliano et al., 1980) and complement activation (Szebeni et al., 1999).
Encapsulation of papaverine into echogenic liposomes slightly reduced their echogenicity when compared with our standard ELIP. The reason is uncertain, but may be due to decreased air encapsulation secondary to papaverine loading (Alkan-Onyuksel et al., 1996). Another drug-loaded echogenic liposome prepared in our lab, azithromycin-loaded echogenic liposomes, had comparable echogenicity to standard echogenic liposomes (Tiukinhoy et al., 2004). Both our standard ELIP and PELIP maintained robust echogenicity over time and compared favorably to another echo contrast, Optison® (Figure 6). These results suggest that our standard ELIP and PELIP were physically stable and would likely be clinically useful for ultrasound imaging. The median diameter of PELIP was larger than that of our standard ELIP. However, both are still smaller than the 2–8 μm size limit for adequate transpulmonary transit (Meltzer et al., 1980). This suggests that both PELIP and standard ELIP may have clinically useful characteristics.
Papaverine mediates its vasodilatatory action by inhibition of phosphodiesterase activity. The biological activity of papaverine in association with echogenic liposomes was intact, but was lower than that of detergent released papaverine at comparable concentrations. This suggests that some of the biological activity of papaverine is shielded by the lipid membrane and is not accessible until complete dissolution of the lipid membrane by detergent. It is anticipated that the successful release of papaverine from PELIP by ultrasound will exert biological effects in the local environment.
The water-soluble form of papaverine hydrochloride will be expected to reside inside the aqueous core of liposomes, but the detection of phosphodiesterase inhibitory activities of intact PELIP prompted us to evaluate the nature of the association of papaverine in echogenic liposomes. The retention of PELIP in the strongly acidic cationic exchange column suggests the presence of papaverine on the surface of PELIP (Figure 6). Thus, the physical distribution of papaverine in our PELIP is complex and multicompartmental, with both an aqueous core phase and a lipid membrane phase.
Recently, we have also described the efficacy of the release of hydrophilic and lipo-philic drugs from ELIP exposed to color Doppler ultrasound (Kopechek et al., 2008). We chose calcein and papaverine as prototype hydrophilic and lipophilic agents. In both calcein- and papaverine-loaded ELIP, we noted a complete loss of echogenicity after Color Doppler ultrasound exposure (in situ MI of 0.8), which was consistent with the fragmentation of ELIP. The robust release of calcein, a hydrophilic agent, markedly contrasted with the lack of a release observed for papaverine, a lipophilic pharmaceutical. These results suggest that water-soluble drugs might be better candidates for the design and development of ELIP-based, ultrasound-controlled drug delivery systems than lipophilic drugs. The observed efficient release of a hydrophilic agent from ELIP was consistent with the release of other water-soluble compounds, such as rt-PA from ELIP (Smith et al., 2007; Tiukinhoy-Laing et al., 2007). Based on the phosphodiesterase activity assay, surface bound papaverine is biologically active. Despite the lack of a significant release of papaverine from ELIP, it is possible that ultrasound exposure may alter the bioavailability of the drug and expose papaverine to the surface of ELIP. Modifications of lipophilic pharmaceuticals may facilitate more efficient ultrasound-triggered release of drug from ELIP. By suspending paclitaxel, a lipophilic pharmaceutical in soybean oil before incorporating the drug into lipospheres, it was possible to trigger the release of paclitaxel from lipospheres using both pulsed and continuous ultrasound (Unger et al., 1998). In our laboratory we have used cyclodextran to render another lipophilic drug, Rosiglitazone, hydrophilic before loading the drug into ELIP (Huang et al., 2007). We noted the release profile of such formulation was comparable to ELIP loaded with other hydrophilic drugs.
In conclusion, based on our echogenic liposome template, we have developed a lipo-somal formulation that contains both papaverine and air and retains favorable echogenic characteristics. Such drug-loaded echogenic liposomes have a desirable size, lipid composition, surface charge characteristics, and biological activities for clinical use. Ongoing studies are needed to explore other modifications of papaverine to improve its release efficacy from ELIP for the management of posthemorrhagic vasospasm in animal models.
Acknowledgments
The work was supported in part by grant no. HL-074002 from the National Heart, Lung, and Blood Institute, National Institute of Health, Bethesda, Maryland, and a grant from the American Institute of Ultrasound in Medicine.
Footnotes
This study was presented in part at the American College of Cardiology Scientific Sessions, New Orleans, Louisiana, March 24–27, 2007.
References
- Alkan-Onyuksel H, Demos SM, Lanza GM, Vonesh MJ, Klegerman ME, Kane BJ, Kuszak J, McPherson DD. Development of inherently echogenic liposomes as an ultrasonic contrast agent. J Pharm Sci. 1996;85:486–90. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- Allen TM. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs. 1998;56:747–56. doi: 10.2165/00003495-199856050-00001. [DOI] [PubMed] [Google Scholar]
- Andaluz N, Tomsick TA, Tew JM, Jr, van Loveren HR, Yeh HS, Zuccarello M. Indications for endovascular therapy for refractory vasospasm after aneurysmal subarachnoid hemorrhage: Experience at the University of Cincinnati. Surg Neurol. 2002;58:131–8. doi: 10.1016/s0090-3019(02)00789-9. [DOI] [PubMed] [Google Scholar]
- Coussios CC, Holland CK, Jakubowska L, Huang SL, MacDonald RC, Nagaraj A, McPherson DD. In vitro characterization of liposomes and Optison by acoustic scattering at 3.5 MHz. Ultrasound Med Biol. 2004;30:181–90. doi: 10.1016/j.ultrasmedbio.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandino J, Kaku Y, Schuknecht B, Valavanis A, Yonekowa Y. Improvement of cerebral oxygenation patterns and metabolic validation of superselective intraarterial infusion of papaverine for the treatment of cerebral vasospasm. Journal of Neurosurgery. 1998;89:93–100. doi: 10.3171/jns.1998.89.1.0093. [DOI] [PubMed] [Google Scholar]
- Firlik K, Kaufmann A, Firlik A, Jungreis C, Yonas H. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surgical Neurology. 1999;51:66–74. doi: 10.1016/s0090-3019(97)00370-4. [DOI] [PubMed] [Google Scholar]
- Fry DW, White JC, Goldman ID. Rapid separation of low molecular weight solutes from liposomes without dilution. Anal Biochem. 1978;90:809–15. doi: 10.1016/0003-2697(78)90172-0. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Huang SL, Wamick D, Stein A, Rabbat M, Madhav T, Kane B, Nagaraj A, Klegerman M, McDonald R, McPherson D. Left ventricular thrombus enhancement after intravenous injection of echogenic immunoliposomes: Studies in a new experimental model. Circulation. 2002;105:2772–8. doi: 10.1161/01.cir.0000017500.61563.80. [DOI] [PubMed] [Google Scholar]
- Hamilton AJ, Huang SL, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43:453–60. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- Huang S, McPherson DD, MacDonald RC. Multi-functional echogenic liposomes for image-guided and ultrasound-controlled PPAR agonist delivery. J Am Coll Cardiol. 2007;49:365A. [Google Scholar]
- Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman ME, McPherson DD, MacDonald RC. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci. 2001;90:1917–26. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]
- Huang SL, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochim Biophys Acta. 2004;1665:134–41. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Juliano R, Layton D. Drug delivery systems: Characteristics and biomedical applications. London: Oxford University Press; 1980. pp. 189–236. [Google Scholar]
- Kassell NF, Helm G, Simmons N, Phillips CD, Cail WS. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg. 1992;77:848–52. doi: 10.3171/jns.1992.77.6.0848. [DOI] [PubMed] [Google Scholar]
- Kopechek JA, Abruzzo TM, Wang B, Chrzanowski SM, Smith DA, Kee P, Huang S, Collier J, McPherson DD, Holland CK. Ultrasound-mediated release of hydrophilic and lipophilic agents from echogenic liposomes. J Ultrasound Med. 2008 doi: 10.7863/jum.2008.27.11.1597. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HM, Tu YK. The efficacy of papaverine administration by different routes for the treatment of experimental acute cerebral vasospasm. J Clin Neurosci. 2002;9:561–5. doi: 10.1054/jocn.2001.1036. [DOI] [PubMed] [Google Scholar]
- Liu JK, Tenner MS, Gottfried ON, Stevens EA, Rosenow JM, Madan N, MacDonald JD, Kestle JR, Couldwell WT. Efficacy of multiple intraarterial papaverine infusions for improvement in cerebral circulation time in patients with recurrent cerebral vasospasm. J Neurosurg. 2004;100:414–21. doi: 10.3171/jns.2004.100.3.0414. [DOI] [PubMed] [Google Scholar]
- Meltzer RS, Tickner EG, Popp RL. Why do the lungs clear ultrasonic contrast? Ultrasound Med Biol. 1980;6:263–9. doi: 10.1016/0301-5629(80)90022-8. [DOI] [PubMed] [Google Scholar]
- Milburn J, Moran C, Cross D, Diringer M, Philgrim T, Dacey R. Increase in diameters of vasospastic intracranial arteries by intraarterial papaverine administration. J Neurosurg. 1998;88:38–42. doi: 10.3171/jns.1998.88.1.0038. [DOI] [PubMed] [Google Scholar]
- Minami H, Kuwamura K, Tamaki N. Intraarterial infusion of papaverine and change of cerebral hemodynamics in symptomatic cerebral vasospasm. Kobe J Med Sci. 2001;47:169–79. [PubMed] [Google Scholar]
- Mizushige K, Kondo I, Ohmori K, Hirao K, Matsuo H. Enhancement of ultrasound-accelerated thrombolysis by echo contrast agents: dependence on microbubble structure. Ultrasound Med Biol. 1999;25:1431–7. doi: 10.1016/s0301-5629(99)00095-2. [DOI] [PubMed] [Google Scholar]
- Oskouian RJ, Jr, Martin NA, Lee JH, Glenn TC, Guthrie D, Gonzalez NR, Afari A, Vinuela F. Multimodal quantitation of the effects of endovascular therapy for vasospasm on cerebral blood flow, transcranial doppler ultrasonographic velocities, and cerebral artery diameters. Neurosurgery. 2002;51:30–43. doi: 10.1097/00006123-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004;31:196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Porter TM, Smith DA, Holland CK. Acoustic techniques for assessing the Optison destruction threshold. J Ultrasound Med. 2006;25:1519–29. doi: 10.7863/jum.2006.25.12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroop R, Britz GW, West GA. In: Endovascular Therapy for Vasospasm Associated with Subarachnoid Hemorrhage. Management of Cerebral Aneurysms. Winn HR, LeRoux P, editors. Philadelphia: Saunders; 2004. pp. 489–498. [Google Scholar]
- Smith DA, Porter TM, Martinez J, Huang S, MacDonald RC, McPherson DD, Holland CK. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med Biol. 2007;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Smith DAB, Vaidya S, Kopechek JA, Holland CK. Echogenic liposomes loaded with recombinant tissue-type plasminogen activator (rt-PA) for image-guided, ultrasound-triggered drug release. J Acoust Soc Am. 2007;122:3007. [Google Scholar]
- Smith TP, Enterline DS. Endovascular treatment of cerebral vasospasm. J Vasc Interv Radiol. 2000;11:547–59. doi: 10.1016/s1051-0443(07)61605-4. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Eskridge J, Grady MS, Newell DW, Winn HR. Endovascular therapy for vasospasm. Clin Neurosurg. 2002;49:261–73. [PubMed] [Google Scholar]
- Szebeni J, Fontana JL, Wassef NM, Mongan PD, Morse DS, Dobbins DE, Stahl GL, Bunger R, Alving CR. Hemodynamic changes induced by liposomes and liposome-encapsulated hemoglobin in pigs: a model for pseudoallergic cardiopulmonary reactions to lipo-somes. Role of complement and inhibition by soluble CR1 and anti-C5a antibody. Circulation. 1999;99:2302–9. doi: 10.1161/01.cir.99.17.2302. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148–50. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- Tiukinhoy SD, Khan AA, Huang S, Klegerman ME, MacDonald RC, McPherson Novel echogenic drug-immunoliposomes for drug delivery. Invest Radiol. 2004;39:104–10. doi: 10.1097/01.rli.0000111207.92580.44. [DOI] [PubMed] [Google Scholar]
- Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119:777–84. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Acoustically active lipospheres containing paclitaxel: A new therapeutic ultrasound contrast agent. Invest Radiol. 1998;33:886–92. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Horn P, Bauhuf C, Munch E, Hubner U, Ing D, Thome C, Poeckler-Schoeninger C, Roth H, Schmiedek P. Effect of intra-arterial papaverine on regional cerebral blood flow in hemodynamically relevant cerebral vasospasm. Stroke. 2001;32:498–505. doi: 10.1161/01.str.32.2.498. [DOI] [PubMed] [Google Scholar]
- Wasan KM, Lopez-Berestein G. The past, present, and future uses of liposomes in treating infectious diseases. Immunopharmacol Immunotoxicol. 1995;17:1–15. doi: 10.3109/08923979509052716. [DOI] [PubMed] [Google Scholar]
- Zhao YZ, Liang HD, Mei XG, Halliwell M. Preparation, characterization and in vivo observation of phospholipid-based gas-filled microbubbles containing hirudin. Ultrasound Med Biol. 2005;31:1237–43. doi: 10.1016/j.ultrasmedbio.2005.05.007. [DOI] [PubMed] [Google Scholar]