Abstract
Galanin modulates seizures in the brain through two galanin receptor subtypes, GalR1 and GalR2. To generate systemically-active galanin receptor ligands that discriminate between GalR1 and GalR2, the GalR1-preferring analogue, Gal-B2 (or NAX 5055), was rationally redesigned to yield GalR2-preferring analogues. Systematic truncations of the N-terminal backbone led to [N-Me, des-Sar]Gal-B2, containing N-methyl tryptophan: this analogue exhibited 18-fold preference in binding toward GalR2, maintained agonist activity, and exhibited potent anticonvulsant activity in mice following intraperitoneal administration.
Introduction
The endogenous neuropeptide galanin has been shown to modulate hyperexcitability in the brain through two associated galanin receptor subtypes, GalR1a and GalR2.1-3 Systemically-active agonists of galanin receptors include galmic, galnon and Gal-B2.4,5 Galmic and galnon (entries 8 and 9, Table 1) are the only non-peptidic compounds claimed to be subtype-selective and systemically-active galanin receptor agonists; but, these two compounds display relatively low binding affinity compared to their peptide counterparts and have been shown to bind nonspecifically to several off-target GPCRs.4,6 Gal-B2 is a 17-residue galanin analogue containing a lipoamino acid and several Lys resides at the C-terminus (entry 7, Table 1).5,7 A combination of lipidization and cationization turned out to be the most effective strategy to improve penetration of the galanin analogues across the blood-brain barrier (BBB). Gal-B2 was found to have potent anticonvulsant activity in the 6 Hz corneal stimulation mouse model for epilepsy with an ED50 of 0.8 mg/kg after i.p. administration.5 Gal-B2 was also found to be active in other seizure and epilepsy models.8 The chemical modifications applied to galanin analogues enhanced their in vitro stability in rat serum (serum half-life of Gal-B2 increased from 7 min to 9.4 hrs). Gal-B2 maintained low nanomolar affinity for GalR1 and GalR2 receptors; i.e., 3.5 nM and 51.5 nM, respectively. Thus, Gal-B2 displayed a ∼15-fold preference for GalR1 over GalR2.
Table 1.
Summary of galanin receptor agonists, their structures, and respective binding affinities. All analogues are C-terminal amides.
| KI in nM | ||||||
|---|---|---|---|---|---|---|
| Entry | Ligand | Strucure | hGalR1 | hGalR2 | Ref. | |
| hGalR1 | 1 | Galanin | GWTLNSAGYLLGPHAVGNHRSFSDKNGLTS-CONH2 | 0.03 | 0.9 | 14, 15 |
| 2 | C7 | GWTLNSAGYLLGPrPKPQQwFwLL-CONH2a | 0.08 | 0.6 | 14, 16 | |
| 3 | M15 | GWTLNSAGYLLGPQQFFGLM-CONH2 | 0.03 | 1.1 | 14, 16 | |
| 4 | M32 | GWTLNSAGYLLGPRHYINLITRQRY-CONH2 | 0.30 | 1.4 | 14, 16 | |
| 5 | M35 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | 0.01 | 1.8 | 14, 15 | |
| 6 | M40 | GWTLNSAGYLLGPPPALALA-CONH2 | 0.10 | 1.5 | 14, 15 | |
| 7 | GalB2 | (Sar)WTLNSAGYLLGPKK(KP)K-CONH2b | 3.50 | 52 | 5 | |
| 8 | Galmic |  |
34,200 | >100,000c | 4 | |
| 9 | Galnon | 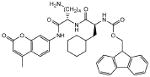 |
11,700 | 34,100c | 4 | |
| hGalR2 | 10 | Gal(2-30) | WTLNSAGYLLGPHAVGNHRSFSDKNGLTS-CONH2 | 51d | 9.8d | 10 |
| 11 | Gal(2-11) | WTLNSAGYLL-CONH2 | 870d | 1.74d | 9 | |
| 12 | [D-Trp2]Gal | GwTLNSAGYLLGPHAVGNHRSFSDKNGLTS-CONH2a | 545d | 218d | 10 | |
Lower case letters denote D-amino acids;
Sar = sarcosine (N-methyl glycine), Kp = lysine-palmitoyl;
Data taken from rat GalR2;
Derived from IC50 values using the Cheng-Prusoff equation.17
To engineer GalR2 preferring analogues, N-terminal modifications of galanin have been reported in the literature.9,10 First, the des-Gly analogues (table 1, entries 10 and 11) were found to possess decreased affinity for GalR1 while maintaining a GalR2 binding preference, providing a significant clue for generating GalR2 selective agonists. Supporting the des-Gly hypothesis, the Gal(2-11) fragment was found to be a potent GalR2 selective agonist.9 Further characterization using alanine displacement studies on the Gal(1-13) fragment showed that Gly1, Trp2, Tyr9, and Gly12 are important for high affinity binding to galanin receptors;11 however, an Ala-walk on Gal(2-11) fragments showed that residues Trp2, Asn5, Gly8, and Tyr9 are necessary for high affinity binding towards GalR2.12 A second strategy for improving GalR2 over GalR1 affinity was to use D-tryptophan at position 2 of human galanin (table 1, entry 12).10 This inversion of stereochemistry led to a significant loss of binding to both receptors, though the analogue did have a slight preference towards GalR2.
Site directed mutagenesis and molecular modeling of human GalR1 was performed to identify the key residues needed for high affinity binding towards galanin.10,13 The major interactions were found to be His264 and His267 towards Trp2, Phe292 towards Try9, and Phe115 towards Gly1. Currently, GalR2 has not been characterized in this way and thus the research community is still uncertain as to what structural features define receptor subtype selectivity. Since modeling studies are not predictive, more SAR studies are needed to better understand how galanin discriminates between receptor subtypes. Here, we report the results of a SAR study with the N-terminal part of the Gal-B2 analogue that suggest a strategy for developing GalR2 preferring systemically-active galanin analogues.
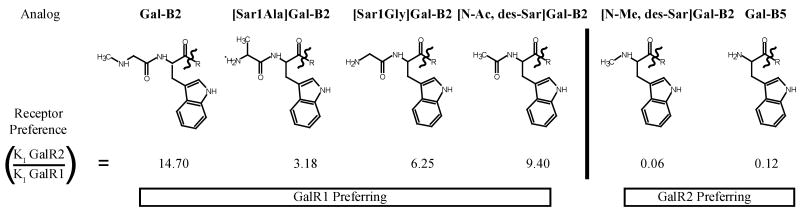
The rational design of GalR2 preferring galanin analogues was achieved using a new approach, a backbone atom shaving technique, as illustrated in Figure 1. It is known in the literature that removal of the N-terminal Gly residue results in GalR2-preferring agonist activity, yet very little explanation is given as to why this pharmacophore has preference for GalR2 over GalR1.9 The backbone atom shaving allowed for identification of key groups at the N-terminus of Gal-B2 that were necessary for discriminating affinities toward GalR1 or/and GalR2 receptors. Starting with the full length Gal-B2 analogue that contains a N-terminal sarcosine, we shaved atoms by successively altering the N-terminus with derivatives introduced during solid phase peptide synthesis. All of our analogues maintain the galanin(2-13) core with the oligo-lysine C-terminus, where the penultimate lysine is conjugated with palmitic acid (KK[Lys-palmitoyl]K) and the amidated C-terminus. The C-terminal derivatives included sarcosine (Gal-B2), alanine ([Sar1Ala]Gal-B2), glycine ([Sar1Gly]Gal-B2), N-acetyl tryptophan ([N-Ac, des-Sar]Gal-B2), N-methyl tryptophan ([N-Me, des-Sar]Gal-B2), and tryptophan (Gal-B5).5
Figure 1.
Structures of the N-terminus and GalR2/GalR1 receptor preference for galanin analogues. Analogues generated using the backbone “atom shaving” technique. All Analogues maintain the Gal(2-13) pharmacophore and have the C-terminal KK(Lys-palmitoyl)K motif [R = TLNSAGYLLGPKK(Lys-palmitoyl)K-conh2].
Results and Discussion
The galanin analogues were synthesized using Fmoc-based solid phase peptide synthesis and purified by preparative RP-HPLC, as described previously.5,7 Peptide masses were confirmed by MALDI-TOF MS and the peptides were initially characterized by their HPLC retention times (Supplementary Table S2), which were then used to calculate logD values, as described elsewhere.5 All analogues studied here, Table 2, exhibited similar logD values ranging from 1.22 to 1.30, suggesting that small structural changes at the N-terminus did not significantly alter their physicochemical profile. Since previously characterized analogue Gal-B2 had logD = 1.24, all new analogues were expected to have comparable CNS bioavilability to that of this GalR1-preferring analogue.
Table 2.
Peptide sequences, calculated log D, and their stability in rat serum described in this work. KP is palmitoylation of Nζ of lysine. Calculated log D was determined from HPLC retention times. Serum stability was determined by incubation of peptide in 25% rat serum at 37° C.
| Analogue | Structure | Calc. log D | Rat Serum Stability, t½ (hour) |
|---|---|---|---|
| Gal-B2 | (Sar)WTLNSAGYLLGPKK(Kp)K | 1.24 ± 0.02 | 9.3 |
| [Sar1Ala]Gal-B2 | AWTLNSAGYLLGPKK(Kp)K | 1.28 ± 0.01 | n.d. |
| [Sar1Gly]Gal-B2 | GWTLNSAGYLLGPKK(Kp)K | 1.30 ± 0.04 | > 10 |
| [N-Ac, des-Sar]Gal-B2 | (N-Ac-Trp)TLNSAGYLLGPKK(Kp)K | 1.28 ± 0.02 | n.d. |
| [N-Me, des-Sar]Gal-B2 | (N-Me-Trp)TLNSAGYLLGPKK(Kp)K | 1.29 ± 0.01 | 9.0 |
| Gal-B5 | WTLNSAGYLLGPKK(Kp)K | 1.22 ± 0.02 | 10.0 |
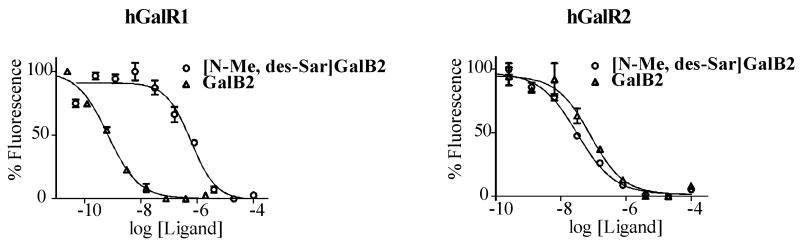
To test the hypothesis that atom shaving in Gal-B2 analogue will result in GalR2-preferring analogues, we determined affinities of the analogues toward human GalR1 and GalR2. Galanin receptor membrane preparations were purchased from Millipore® or Perkin-Elmer©. The receptor binding studies were carried out using time-resolved fluorescent competitive binding assay with Eu3+ labeled galanin as the competitor (see Supporting Information).5 The representative binding curves are shown in Figure 2 and the calculated KI values are summarized in Table 3. All the analogues maintained relatively high affinities toward hGalR2. However, as the N-terminus was truncated from the (N-acetyl)Trp analogue to the (N-methyl)Trp, a significant loss in hGalR1 binding affinity was observed. Analogue [N-Me, des-Sar]Gal-B2 had a KI value of 364 nM for hGalR1 and 20 nM for hGalR2, which was an 18-fold preference for hGalR2. Further removal of methyl group from the N-terminus (Gal-B5) reduced the binding toward GalR2 by 2-fold. When comparing the binding affinities of [N-Me, des-Sar]Gal-B2 and Gal-B5 to Gal-B2 (Figure 2 and Table 3), it is apparent that the first two analogues are Gal-R2-preferring, with [N-Me, des-Sar]Gal-B2 having a greater preference toward this subtype.
Figure 2.
Representative binding curves for Analogues Gal-B2 (triangles) and [N-Me, des-Sar]Gal-B2 (circles) against hGalR1 (left) and hGalR2 (right). Each binding assay was performed in triplicate to generate one 10-point binding curve. Binding affinities are reported as the average of three independent binding curves. Binding affinities (KI) for Gal-B2 are 3.5 nM hGalR1 and 52 nM hGalR2 and for [N-Me, des-Sar]Gal-B2 are 364 nM hGalR1 and 20 nM hGalR2.
Table 3.
In vivo pharmacology and in vitro receptor binding results. Antiepileptic activity was assessed after CF-1 mice were treated i.p. with analogues and challenged with corneal stimulation at 15, 30, 60, 120, and 240 min. The number of mice protected out of the sample group is presented; integrated area under the curve (AUC) is shown for each analogue. Competitive receptor binding was performed on receptor membrane preps (Millipore or Perkin Elmer) against Eu-labeled galanin in a time resolved fluorescence based assay.
| Analogue | Primary Screening at 4 mg/kg (6 Hz, 32 mA) | Primary Screening (AUC) |
GalR1 KI (nM) |
GalR2 KI (nM) |
|---|---|---|---|---|
| Gal-B2 | 3/4 4/4 4/4 4/4 0/4 | 16313 | 3.5 ± 1.0 | 51.5 ± 34.4 |
| [Sar1Ala]Gal-B2 | 4/4 4/4 4/4 1/4 0/4 | 9750 | 11.0 ± 6.0 | 35.0 ± 8.0 |
| [Sar1Gly]Gal-B2 | 1/4 3/4 3/4 0/4 0/3 | 5250 | 2.8 ± 0.9 | 17.5 ± 4.0 |
| [N-Ac, des-Sar]Gal-B2 | n.d. | n.d. | 1.7 ± 0.8 | 15.9 ± 3.3 |
| [N-Me, des-Sar]Gal-B2 | 0/4 3/4 3/4 1/4 0/3 | 7313 | 364.5 ± 58.4 | 20.2 ± 2.1 |
| Gal-B5 | 2/4 1/4 2/4 2/4 0/3 | 9375 | 387.0 ± 123.0 | 48.0 ± 11.3 |
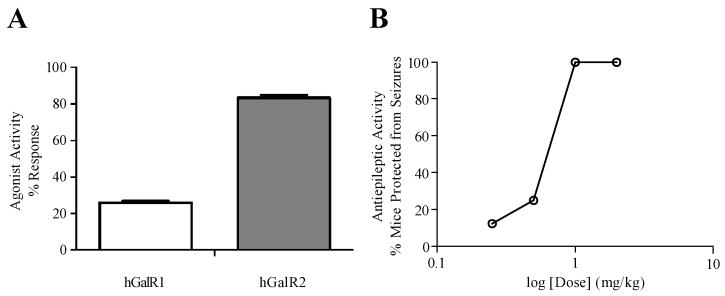
Analogue [N-Me, des-Sar]Gal-B2 was selected to further characterize its properties in greater detail. To demonstrate that analogue [N-Me, des-Sar]Gal-B2 is a galanin receptor agonist, a calcium mobilization assay (GPCRProfiler® Screening, Millipore, St. Charles, MO) was carried out using CHO-K1 cells over-expressing galanin receptors. The receptor activation was normalized to known galanin receptor agonists; Gal(1-30) for GalR1 and Gal(2-29) for GalR2. As shown in Figure 3A, [N-Me, des-Sar]Gal-B2 activated GalR2 receptors with an apparent preference toward GalR2 at the concentration tested (1.25 μM). This finding supports the binding data that showed a preference of [N-Me, des-Sar]Gal-B2 for hGalR2 over hGalR1.
Figure 3.
(A) Agonist activity of Analogues determined by calcium mobilization assay (Millipore). The % response is normalized to galanin receptor agonists, Gal(1-30) for hGalR1 and Gal(2-29) for hGalR2. At 1.25 μM ligand concentration, Analogue [N-Me, des-Sar]Gal-B2 preferentially activates hGalR2 over hGalR1, to 83% ± 3.8% and 26% ± 1.9% respectively (n = 4, p < 0.01). (B) The dose response curve for Analogue [N-Me, des-Sar]Gal-B2; CF-1 mice (n = 8) were treated with the Analogue and challenged by with 6 Hz (32 mA) corneal stimulation 1 hour after i.p. administration.% animals protected is (#mice protected/#mice tested)*100.
Although seizures are controlled by GalR1 and GalR2 galanin receptor subtypes, we cannot exclude a possibility that the systemically-active galanin analogues studied here also interact with the third galanin receptor subtype, GalR3. It is known from the literature that truncated galanin analogue, Gal(2-11), maintains GalR2 binding, and has moderate GalR3 receptor binding, with a 12-fold preference for towards GalR2 above GalR3.18 We acknowledge that more experimentation is needed to verify that [N-Me, des-Sar]Gal-B2 will have similar binding profiles towards GalR3 as Gal(2-11).
We have previously shown that the combination of cationization and lipidization improved stability in rat serum of our galanin analogues (including Gal-B2) and thereby contributed to increased systemic bioavailability.7 The analogues described herein were also found to be metabolically stable when incubated in 25% rat serum at 37° C. For [N-Me, des-Sar]Gal-B2, half-life was determined to be >10 hrs, compared to 9.4 hrs for Gal-B2. (see Supplemental Table S2). It is noteworthy that, having an N-terminal modification such as methylation or acetylation did not affect the in vitro serum stability of the galanin analogues.
To evaluate the anticonvulsant activity of the galanin analogues, they were administered intraperitoneally (i.p.) to CF-1 mice at a dose of 4 mg/kg, as described previously.5,19 The mice were then challenged with a 6 Hz corneal stimulation (32 mA for 3 sec delivered via corneal electrodes) 15, 30, 60, 120, and 240 min after i.p. administration. Mice not displaying any characteristic limbic seizure activity (jaw chomping, vibrissae twitching, forelimb clonus, or Straub tail) were considered protected; protection is graded as all or none.19 All studied analogues exhibited anticonvulsant activity, with maximal protection persisting up to 2 hours after administration. Figure 3B shows the dose-dependent response of analogue [N-Me, des-Sar]Gal-B2, where subjects were challenged one hour after dosing. The calculated ED50 of analogue [N-Me, des-Sar]Gal-B2 was 0.77 mg/kg (see Supporting Information). This value is comparable with an ED50 of 0.8 mg/kg for Gal-B2, suggesting that at the doses tested, GalR1- and GalR2-preferring analogues exhibit similar level of anticonvulsant activity. Currently, we are uncertain as to the level of BBB penetration; however, anticonvulsant activity is considered a good surrogate measurement of CNS bioavailability.
Conclusions
Developing a GalR2 subtype-selective receptor agonist will provide an important pharmacological tool for understanding not only the SAR of galanin, but also provide insight into their therapeutic potential as anticonvulsants and analgesics. Generation of Gal(2-11) advanced our knowledge of the galanin signaling; however, this peptide is limited in its use due to its low bioavailability. Here we describe galanin analogues that discriminate between receptor subtypes GalR1 and GalR2 and are systemically-active, which can be used as an even greater pharmacological tool for investigating galanergic signaling.
The backbone atom shaving approach used to determine the individual contributions of individual atoms in the backbone towards the galanin receptors is a unique feature of this study. Focus is normally devoted towards side chain interactions with receptor binding, and is often probed via peptide-Ala walks. Our method showed that with the loss of the carbonyl functional group from analogue [N-Ac, des-Sar]Gal-B2 to [N-Me, des-Sar]Gal-B2, the analogues lost affinity towards the GalR1 receptor, but maintain affinity towards GalR2. With this development we find that independent N-terminal or C-terminal modifications are critical for independent engineering of either subtype preference or bioavailability, respectively.5
During the preparation of this manuscript, Runesson independently published compelling results reinforcing N-terminal modifications of galanin-like peptides, for generating GalR2 agonists.18 What is unique about our results is the functional readout of anticonvulsant activity obtained by administering the modified analogue [N-Me, des-Sar]Gal-B2 in vivo, after i.p. administration to CF-1 mice. When taken together with the in vitro receptor binding data, enhanced rat serum stability, and favorable logD, we illustrate the generation of a systemically active galanin analogues that discriminate between receptor subtypes.
Experimental Section
Peptide Synthesis
All analogues were synthesized using Fmoc-based solid-phase synthesis. The peptide analogues were removed from the resin by a 2.5 h treatment with Reagent K (82.5% TFA, 5% nH2O, 5% ethanedithiol, 2.5% thioanisole v/v, 75 mg/mL phenol). After precipitation with ice cold MTBE, a diphenyl preparative HPLC column was used to purify large quantities of the analogues. The HPLC buffers were Buffer A (1 L H2O + 1 mL TFA) and Buffer B (900 mL acetonitrile + 100 mL H2O + 1 mL TFA). Elution of the analogues from the column was carried out over a linear gradient ranging from 20 to 90% Buffer B over 30 min. Purified analogues were quantified by measuring UV absorbance at 279.8 nm (ε = 7,000 M-1 cm-1). Purity of all analogues was found to be >95% by analytical HPLC running a linear gradient of 20% Buffer B to 100% buffer B in 40 min (Table S2). Calculated masses were confirmed by MALDI mass spectrometry analysis at the University of Utah core facility.
LogD
HPLC capacity factor (k′) method.5 The HPLC buffers were Buffer A (1 L H2O + 1 mL TFA) and Buffer B (900 mL acetonitrile + 100 mL H2O + 1 mL TFA). A 5.0 μg standard (run in triplicate) was injected onto a Vydac diphenyl column using a linear gradient starting at 80/20 Buffer A:Buffer B and ending at 10/90 Buffer A:Buffer B in 15 min before immediate return to initial conditions. The retention times are the average of 3 runs. The capacity factors (k′) of the peptides were calculated using the formula below; to is the solvent front, tr is the retention time of the peptide. The logDs obtained from the shake-flask method were plotted against their peptides respective k′ values giving a linear plot. LogDs for all other peptides were calculated using this standard curve.
Stability in Rat Serum
One ml of 25% rat serum was incubated at 37 °C for 10 minutes, prior to addition of the analogue. Reactions were prepared by adding each analogue, dissolved in nH2O to a solution containing 25% rat serum and 0.1M Tris-HCl, pH 7.5 to a final peptide concentration of 20μM. At appropriate time intervals (ranging up to 8 hours), 200μl aliquots were withdrawn and added to 100μl “quenching solution” (15% trichloroacetic acid in 40% isopropanol). 40% isopropanol was added to quenching mixture as it was noted to improve recovery of the Gal-B2 analogue. Upon precipitation with the quenching mixture, the samples were incubated at -20°C for 15 minutes and centrifuged at 12,000 rpm to separate out serum proteins. The supernatant was analyzed using HPLC separation with an YMC ODS-A™ 5μm 120Å column (AA12S052503WT). In cases where analogue peaks overlapped with peaks observed in the “serum-only” control samples, the gradient was optimized by changing the composition of the mobile phases, column temperature or HPLC column (for example, diphenyl, or C8). Recovery of the analogues was assessed by spiking “serum-only” control samples after the TCA precipitation with known amounts of the analogue. Metabolic stability was assessed by monitoring the disappearance of the analogues over a period of 8 hours. This was accomplished by comparison of the area under the curve for the peak corresponding to the intact peptide at each time point. These values were then used to calculate half-life values for each analogue.
Anticonvulsant Activity
Each galanin analogue was administered intraperitoneally to 5 groups of CF-1 mice (n = 4 mice) at a dose of 4 mg/kg. At various times (i.e., 15, 30, 60, 120, and 240 min) after ip admistration, mice were challenged with a 6 Hz corneal stimulation (32 mA for 3 sec delivered via corneal electrodes). Mice not displaying a characteristic limbic seizure (e.g., jaw chomping, vibrissae twitching, forelimb clonus, Straub tail), graded as all or none, were considered protected. The percent of animals protected at each time-point was plotted against time and the area under the curve (AUC) for the time-response study was calculated for each analogue.
Receptor binding
Competitive binding assays were performed in 96-well format on AcroWell filter plates (Perkin-Elmer®) using receptor membrane preparations, purchased from Millipore® or Perkin-Elmer (hGalR1 and hGalR2) and Eu-galanin (Perkin-Elmer), using the DELFIA® Assay Buffers (Perkin-Elmer). The samples were performed in quadruplicate. Binding assay was carried out with 6 micrograms of receptor membrane preparations (1.4 pmol/mg protein) and 2 nM of Eu-galanin, in a volume of 100 μL of the DELFIA L*R Binding Buffer (50 mM Tris-HCl pH 7.5, 5 mM MgCl2, 25 μM EDTA, and 0.2 % BSA). Samples were incubated at room temperature for 90 min, followed by washing (4×) with 200 μL DELFIA Wash Buffer (50 mM Tris-HCl pH 7.5 and 5 mM MgCl2). DELFIA Enhancement Solution (200 μL) was added, and the plates were incubated at room temperature for 30 min. The plates were read on a VICTOR3 spectrofluorometer using a standard Eu-TRF measurement (excitation at 340 nm, delay for 400 μs, and emission at 615 nm for 400 μs). Competition curves were analyzed with GraphPad Prism software using the sigmoidal dose-response (variable slope) equation for nonlinear regression analysis.
Supplementary Material
Acknowledgments
The authors thank Karen White and Daniel R. McDougle for their technical assistance. We also like to thank Professor Bob Shackmann and Scott Endicott from the DNA/Peptide Synthesis Core Facility at the University of Utah. This work was funded by the University of Utah startup funds and the Epilepsy Research Foundation, and NIH grant R21 NS059669. Conflict of interest disclosure, G. B. and H. S. W. are scientific cofounders of NeuroAdjuvants, Inc.
Footnotes
Abbreviations: BBB, blood-brain barrier; Kp, lysine-palmitoyl; Sar, sarcosine; GalR1, galanin receptor subtype 1; GalR2, galanin receptor subtype 2, GalR3, galanin receptor subtype 3; GPCR, G-protein coupled receptor; i.p., intraperitoneal
Supporting Information Available: Physicochemical characterization, MALDI, peptide purity, and HPLC retention times found in supplementary data. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Mazarati A, Lundstrom L, Sollenberg U, Shin D, Langel Ü, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- 2.Mitsukawa K, Lu X, Bartfai T. Galanin, galanin receptors and drug targets. Cell Mol Life Sci. 2008;65:1796–1805. doi: 10.1007/s00018-008-8153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu XJ, Hokfelt T, Wiesenfeld-Hallin Z. Galanin and spinal pain mechanisms: where do we stand in 2008? Cell Mol Life Sci. 2008;65:1813–1819. doi: 10.1007/s00018-008-8155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartfai T, Xiaoying L, Badie-Mahdavi H, Barr AM, Mazarati A, Hua XY, Yaksh T, Haberhauer G, Ceide SC, Trembleau L, Somogyi L, Kröck L, Rebek J. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc Natl Acad Sci, USA. 2004;101:10470–10475. doi: 10.1073/pnas.0403802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulaj G, Green BR, Lee HK, Robertson CR, White K, Zhang L, Sochanski M, Flynn S, Scholl EA, Pruess T, Smith MD, White HS. Design, synthesis and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activity. J Med Chem. 2008;51:8038–8047. doi: 10.1021/jm801088x. [DOI] [PubMed] [Google Scholar]
- 6.Saar K, Mazarati A, Mahlapuu R, Hallnemo G, Soomets U, Kilk K, Hellberg S, Pooga M, Tolf BR, Shi TS, Hökfelt T, Wasterlain C, Bartfai T, Langel Ü. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc Natl Acad Sci, USA. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Robertson CR, Green BR, Pruess TH, White HS, Bulaj G. Structural Requirements for a Lipoamino Acid in Modulating the Anticonvulsant Activities of Systemically Active Galanin Analogues. J Med Chem. 2009;52:1310–1316. doi: 10.1021/jm801397w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White HS, Scholl EA, Klein BD, Flynn SP, Pruess TH, Green BR, Zhang L, Bulaj G. Developing novel antiepileptic drugs: characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics. 2009;6:372–380. doi: 10.1016/j.nurt.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu HX, Brumovsky P, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hokfelt T. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci, USA. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Church WB, Jones KA, Kuiper DA, Shine J, Iismaa TP. Molecular modelling and site-directed mutagenesis of human GALR1 galanin receptor defines determinants of receptor subtype specificity. Protein Eng. 2002;15:313–323. doi: 10.1093/protein/15.4.313. [DOI] [PubMed] [Google Scholar]
- 11.Land T, Langel Ü, Löw M, Berthold M, Undén A, Bartfai T. Linear and cyclic N-terminal galanin fragments and analogs as ligands at the hypothalamic galanin receptor. Int J Pept Protein Res. 1991;38:267–272. doi: 10.1111/j.1399-3011.1991.tb01438.x. [DOI] [PubMed] [Google Scholar]
- 12.Lundström L, Lu X, Langel Ü, Bartfai T. Important pharmacophores for binding to galanin receptor 2. Neuropeptides. 2005;39:169–171. doi: 10.1016/j.npep.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Berthold M, Kahl U, Jureus A, Kask K, Nordvall G, Langel Ü, Bartfai T. Mutagenesis and ligand modification studies on galanin binding to its GTP-binding-protein-coupled receptor GalR1. Eur J Biochem. 1997;249:601–606. doi: 10.1111/j.1432-1033.1997.00601.x. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald LW, Patterson JP, Conklin DS, Horlick R, Largent BL. Pharmacological and biochemical characterization of a recombinant human galanin GalR1 receptor: agonist character of chimeric galanin peptides. J Pharm Exp Thera. 1998;287:448–456. [PubMed] [Google Scholar]
- 15.Bloomquist BT, Beauchamp MR, Zhelnin L, Brown SE, Gore-Willse AR, Gregor P, Cornfield LJ. Cloning and expression of the human galanin receptor GalR2. Biochem Biophys Res Commun. 1998;243:474–479. doi: 10.1006/bbrc.1998.8133. [DOI] [PubMed] [Google Scholar]
- 16.Borowsky B, Walker MW, Huang LY, Jones KA, Smith KE, Bard J, Branchek TA, Gerald C. Cloning and characterization of the human galanin GALR2 receptor. Peptides. 1998;19:1771–1781. doi: 10.1016/s0196-9781(98)00133-8. [DOI] [PubMed] [Google Scholar]
- 17.Cer RZ, Mudunuri U, Stephens R, Lebeda FJ. IC50-to-Ki: a web-based tool for converting IC50 to Ki values for inhibitors of enzyme activity and ligand binding. Nucleic Acids Res. 2009;37:W441–445. doi: 10.1093/nar/gkp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runesson J, Saar I, Lundstrom L, Jarv J, Langel Ü. A novel GalR2-specific peptide agonist. Neuropeptides. 2009;43:187–192. doi: 10.1016/j.npep.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.