Abstract
The behavioral effects of nicotine withdrawal are lower in adolescent versus adult rats. However, the neurochemical mechanisms that mediate these developmental differences are unknown. Previous studies have shown that extracellular levels of dopamine in the nucleus accumbens (NAcc) are reduced in adult rats experiencing withdrawal. This study compared dopamine levels in the NAcc of male adolescent and adult rats experiencing nicotine withdrawal. Animals were prepared with subcutaneous pumps that delivered an equivalent nicotine dose in these age groups. Following 13 days of nicotine exposure, rats were implanted unilaterally with microdialysis probes into the NAcc and ipsilateral ventral tegmental area (VTA). The next day, dialysate levels were collected following systemic administration of the nicotinic-receptor antagonist mecamylamine to precipitate withdrawal. Mecamylamine produced an average % decrease in NAcc dopamine that was lower in adolescents (20%) versus adults (44%). Similar developmental differences were observed with the dopaminergic (DOPAC and HVA) but not serotonergic (5-HIAA) metabolites. A follow up study compared NAcc dopamine in adolescent and adult rats receiving intra-VTA administration of bicuculline, which reduces gamma-aminobutyric acid (GABA) inhibition of dopamine transmission. The results revealed that blockade of GABAA receptors in the VTA produced a 2-fold increase in NAcc dopamine of adults but not adolescents. These results provide a potential mechanism involving dopamine that mediates developmental differences in nicotine withdrawal. Specifically, they suggest that GABA systems are underdeveloped during adolescence and this reduced inhibition of dopamine neurons in the VTA may lead to reduced decreases in NAcc dopamine of young animals experiencing withdrawal.
Keywords: development, adolescence, nicotinic, in-vivo microdialysis, HPLC-EC, extracellular, DOPAC, HVA, 5-HIAA, rat, abstinence, GABA, bicuculline, ventral tegmental area
INTRODUCTION
Much research suggests that tobacco dependence in adults is mediated, in large part, by avoiding the negative consequences of nicotine withdrawal (Benowitz 2008; Buchhalter et al., 2008; Carmody et al., 2007). However, the contribution of withdrawal to tobacco use during adolescence has not been well established. Pre-clinical animal studies have suggested that both the physical and negative affective properties of nicotine withdrawal are lower in adolescent relative to adult rats. For example, work in our laboratory has demonstrated that adolescent rats display fewer physical signs of nicotine withdrawal and reduced place aversion to environmental cues previously associated with nicotine withdrawal relative adult rats (O’Dell et al., 2004, 2006, 2007). Work in other laboratories has further established that the behavioral effects of nicotine withdrawal are lower in adolescent rats (Shram et al., 2008) and mice (Kota et al., 2007, 2008) relative to their adult counterparts. Collectively, these studies suggest that adolescence is a period of development characterized by reduced sensitivity to nicotine withdrawal. Also, these findings suggest that the underlying neural mechanisms that mediate withdrawal are different across various stages of development. However, the neural mechanisms that mediate developmental differences in nicotine withdrawal are presently unclear.
The reinforcing effects of nicotine are mediated in large part by enhanced dopamine neurotransmission in the mesolimbic pathway (Corrigall, 1991; Mansvelder and McGehee, 2002; Watkins et al., 2000). This pathway originates in the ventral tegmental area (VTA) and projects to various forebrain structures, including the nucleus accumbens (NAcc), which plays a critical role in mediating the rewarding effects of drugs of abuse.
Recent studies have demonstrated that the neurochemical effects of nicotine withdrawal are opposite to the increases in extracellular levels of NAcc dopamine following nicotine administration. Studies investigating the neurochemical effects of nicotine withdrawal have used mecamylamine, a pharmacological agent that blocks nicotinic acetylcholine receptors and precipitates withdrawal in animals previously exposed to nicotine for at least 5-7 days. For example, studies examining the neurochemical effects of nicotine withdrawal have demonstrated that adult rats showing physical signs of nicotine withdrawal display a 20-35% decrease in extracellular levels of dopamine in the NAcc relative to baseline levels (Carboni et al., 2000; Gaddnas et al., 2002; Hildebrand et al., 1998, 1999; Rada et al., 2001). The latter studies also found that nicotine withdrawal produces a decrease in NAcc levels of the dopamine metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA).
The hypothesis that the neural mechanisms of withdrawal involve decreased NAcc dopamine levels is consistent with studies showing reduced levels of NAcc dopamine during withdrawal from alcohol, opiates, and cocaine (Pothos et al., 1991; Rada et al., 2004; Weiss et al., 1992). However, to our knowledge, changes in NAcc dopamine levels have not been compared in adolescent and adult rats experiencing nicotine withdrawal. Thus, the goal of this study was to compare developmental differences to changes in extracellular levels of dopamine and the dopamine metabolites, DOPAC and HVA, in the NAcc of adolescent and adult rats experiencing nicotine withdrawal. In order to assess the role of serotonergic systems in mediating developmental differences in nicotine withdrawal, this study also included a comparison of changes in one of the major serotonin metabolites, 5-hydroxyindoleacetic acid (5-HIAA).
Much work has shown that dopamine release in the NAcc is inhibited by gamma-aminobutyric acid (GABA) neurotransmission in the dopamine cell body region of the VTA. The inhibition of dopamine in the VTA occurs via a population of GABA interneurons that form synapses onto VTA dopamine neurons that project to the NAcc (Johnson and North, 1992; Kalivas, 1993; Mansvelder and McGehee, 2002; Mansvelder et al., 2002). Microdialysis studies demonstrate that intra-VTA infusions of a GABAA receptor antagonist increase NAcc dopamine levels (Ikemoto et al., 1997; Westerink et al., 1996), whereas intra-VTA infusions of a GABAA agonist decrease NAcc dopamine levels (Westerink et al., 1996). Although the inhibition of dopamine release via GABA systems has been well established, to our knowledge no one has compared the ability of GABA systems to inhibit dopamine release in the NAcc of adolescent and adult rats. Thus, this study also examined developmental differences to neurochemical changes in NAcc dopamine produced by intra-VTA administration of a GABAA antagonist.
MATERIALS AND METHODS
Animals
Male Wistar adolescent and adult rats (n=7-8 per group) were used. Adolescents were between post-natal day (PND) 28-30 and adults were between PND 60-75 at the time of the pump implantation surgery. All rats were handled for 5 days prior to the start of experimentation and were given free access to food and water throughout the study. Rats were housed in groups of 2-3 per cage in a humidity- and temperature-controlled (20-22°C) vivarium using a 12-/12-hour light/dark cycle with lights on at 8:00 AM. The home cage consisted of a rectangular Plexiglas® hanging cage (41.5 cm long × 17 cm wide × 21 cm high) with pine bedding. The food and water were located above the animals’ living space on a wire platform encased within a filtered top cover. Testing procedures were conducted during the light phase of the rats’ light/dark cycle. The rats were bred in the Psychology Department from a stock of outbred Wistar rats from Harlan, Inc (Indianapolis, IN). Rats were bred onsite to minimize stress in adolescents that might have occurred if they had been shipped and tested close in time. All procedures were approved by the University of Texas at El Paso Animal Care and Use Committee and followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
The drugs used in this experiment were (-) nicotine-hydrogen tartrate and mecamylamine hydrochloride purchased from Sigma Aldrich Inc. (St. Louis, MO), and bicuculline methochloride purchased from Tocris Biosciences Inc (Ellisville, MO). Mecamylamine was dissolved in 0.9% sterile saline and injected via the intraperitoneal (IP) route of administration in a volume of 1 ml/kg. Bicuculline was dissolved in artificial cerebral spinal fluid (ACSF) and administered via reverse dialysis through the microdialysis probe that was surgically implanted into the VTA.
Surgical Preparation of Osmotic Pumps
Rats were anesthetized with an isofluorane/oxygen mixture (1-3% isofluorane) prior to surgical preparation of 14-day Alzet osmotic pumps purchased from Durect Corporation (model 2ML2; 1.0 μl/hour; Cupertino, California) that were implanted subcutaneously on the back of the animal parallel to the spine. Pumps were filled with nicotine (4.7 mg/kg/day for adolescents or 3.2 mg/kg/day for adults; expressed as base). The concentration of nicotine in the pump was adjusted according to the weight of the rat at the time of the surgery. The nicotine concentrations were based on previous studies demonstrating that the infusion rate of nicotine was 1.5 times lower in adolescent versus adult rats after 17 days of exposure to the same nicotine dose as used in the present study (see Trauth et al., 2000). After surgery, the surgical incision was closed with 9-mm stainless steel wound clips and treated with a topical antibiotic ointment.
Stereotaxic Implantation of Microdialysis Probes
Thirteen days after the pump surgery, rats were implanted unilaterally with 2 probes into the NAcc and the ipsilateral VTA. Rats were implanted between PND 40-42 for adolescents and PND 72-87 for adults. The probes were purchased from CMA-Microdialysis (model CMA 11; Solna, Sweden) with an active membrane length of 2 mm in the NAcc and 1 mm in the VTA. The probes were perfused for at least 1 hour prior to implantation at a rate of 0.5 μl/minute with ACSF composed of 145 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 5.4 mM d-glucose, and 0.25 mM ascorbic acid and adjusted to a pH of 7.2-7.4. The probes were stereotaxically implanted into the brain regions using the following coordinates for the NAcc from bregma [adolescent placements- anterior-posterior (AP) = +2.2, medial-lateral (ML) = ±0.8, dorsal-ventral (DV) = −7.1; and adult placements- AP = +1.7, ML = ±1.4, DV = −8.1] and the ipsilateral VTA [adolescent placement- AP = −4.0, ML = ±0.6, DV = −7.4; and adult placements- AP = −4.8, ML = ±0.8, DV = −8.5]. Adolescent placements were derived from Philpot et al. (2001) and Pistis et al. (2004) and adult placements were derived from O’Dell and Parsons (2004). The hemisphere that was implanted with the probe was randomized across treatment groups to control for possible hemispheric differences across age groups.
Following surgery, adolescent and adult animals were transferred to similar-sized test cages that consisted of a square Plexiglas® cage (24 cm long × 24 cm wide × 31 cm high) with pine bedding. Food and water were available throughout dialysis testing. When comparing adolescent and adult rats, some researchers are careful to adjust for the size of the cage since this factor has been shown to influence exploratory behaviors such as sniffing, rearing, and locomotor activity. This may be particularly important for studies assessing developmental differences in affective measures such as anxiety-like behavior that are influenced by exploratory behavior. However, previous work in our laboratory using different sized chambers has consistently revealed that adolescent and adult rats tested in chambers of different sizes and shapes (round versus square) display similar basal and somatic signs of withdrawal (O’Dell et al., 2004 and 2006). Furthermore, our measures of the physical signs of withdrawal do not include exploratory behaviors and thus are not likely influenced by cage size.
Microdialysis Testing
The next day after probe implantation, the perfusate flow rate was increased to 1.0 μl/minute for 1 hour to allow equilibration of the probes. Samples were then collected in 10-minute intervals for 1 hour to establish a baseline period, and then following a systemic injection of saline and 2 doses of mecamylamine in increasing order (1.5 and 3.0 mg/kg, expressed as salt; IP). The doses of mecamylamine were chosen based on previous studies demonstrating that they produce a place aversion to environmental cues previously associated with withdrawal in nicotine-dependent rats (O’Dell et al., 2007). In addition, reports from other laboratories have demonstrated that similar doses of mecamylamine produce decreases in extracellular levels of NAcc dopamine (approximately 20-35% from baseline) in nicotine-dependent adult rats experiencing withdrawal (Carboni et al., 2000; Gaadnas et al., 2002; Rada et al., 2001).
The last series of dialysate samples were collected during a 1-hour perfusion of bicuculline-methochloride into the VTA probe (100 μM). This manipulation was done in order to compare developmental differences in the ability of GABAA receptor blockade to increase NAcc dopamine levels. All dialysate samples collected from the NAcc probe were diluted with 10 μl of a perchloric-acid solution (0.05 N) in order to preserve our samples and prevent degradation of dopamine and the metabolites. After sample collection, the samples were immediately frozen on dry ice and then stored in a −70°C freezer until they were analyzed within the next 1-3 weeks.
Assessment of Physical Signs of Nicotine Withdrawal
Rats were monitored for somatic signs of nicotine withdrawal for 3 separate 10-minute observation periods after administration of saline and then again after administration of the 2 doses of mecamylamine during microdialysis testing. The observed signs included blinks, writhes, body shakes, teeth chatters, gasps, and ptosis. These measures of withdrawal have been used as a reliable index of the physical signs of withdrawal in nicotine-dependent adolescent and adult rats receiving a systemic injection of mecamylamine (Malin et al., 1994; O’Dell et al., 2004, 2006; Shram et al., 2008). Animals were continuously observed for 10 minutes, during which time the number of times the animals exhibited any of the above signs were recorded. Multiple successive counts of any sign required a distinct pause between episodes. If present continuously, ptosis was only counted once. The total number of somatic signs was defined as the sum of individual occurrences of the aforementioned withdrawal signs during the entire 10-minute observation period.
Neurochemical Analysis of Dopamine, DOPAC, HVA and 5-HIAA
Dopamine and the metabolites were quantified from a 10-μl sample injected into a HPLC system equipped with an ESA HR-80 80×4.6 mm column (3 μm BetaBasic packing material, C-18 stationary phase, Chelmsford, MA) and eluted using a mobile phase composed of a 75 mM NaH2PO4 (monohydrate, monobasic) buffer (pH 3.75) with 10% acetonitrile, 0.025 mM sodium-EDTA, 0.4% (v/v) triethylamine and 1.7 mM 1-octanesulfonic acid sodium salt delivered at 1 ml/minute by an ESA model 580 syringe pump (Chelmsford, MA). Quantification was achieved via an ESA Coulochem II detector equipped with a coulometric sensor containing dual glassy carbon working electrodes (Chelmsford, MA) set at +350 mV for the metabolites and −150 mV for dopamine. The extracellular levels of dopamine and the metabolites were estimated using external calibration curves with standards containing known concentrations of these neurochemicals.
Histology
At the end of the experiment, all rats were deeply sedated with pentobarbital (100 mg/kg, salt; IP) for systemic perfusion using 0.85% saline and then a 4% paraformaldehyde solution. Following the perfusion, the brains were extracted and stored in formalin solution until they were sectioned. Verification of the probe placements was verified during sectioning using the Paxinos and Watson (1998) atlas. The probe placements were focused in the NAcc core region for both adolescents and adults, as determined during sectioning of the brain tissue. The VTA placements were all confined in this region. As a final elimination criterion, each animals’ baseline values had to fall within a range that was less than 2 standard deviations from the group mean in order to be included in the final analysis. Based on these criteria, n=3 adolescents and n=2 adults were excluded from the study.
Statistical Analyses
Total overt signs of nicotine withdrawal were analyzed using repeated-measures ANOVA with age (adolescent and adult) as a between-subjects factor and drug treatment (saline and mecamylamine) as a within-subject factor. Repeated-measures ANOVA were first conducted to examine whether there were basal differences in dopamine, DOPAC, HVA, and 5-HIAA levels in adolescent and adult rats. The data revealed that there were no differences in basal levels of dopamine or any of the metabolites. Our subsequent analyses were conducted on values that were converted to % change from baseline [i.e., (dialysate value/ average baseline value) × 100%] in order to more clearly illustrate group differences. The data were then analyzed using repeated-measures ANOVA with age (adolescent and adult) as a between-subjects factor and time (10-minute intervals) as a within-subject factor. Changes in neurotransmitter levels during the intra-VTA bicuculline infusion were analyzed separately using repeated-measures ANOVA with age (adolescent and adult) as a between-subjects factor and time (10-minute intervals during the 3 samples prior to and the 6 samples following the intra-VTA bicuculline infusion) as a within-subject factor. Significant interaction effects were further analyzed using Fisher’s least significant difference (LSD) tests and a modified Bonferroni correction factor for alpha inflation.
RESULTS
Physical Signs of Withdrawal
Figure 1 illustrates the physical signs of nicotine withdrawal in adolescent and adult rats. Overall, the results revealed that mecamylamine precipitated physical signs of withdrawal relative to baseline, and this effect was larger in nicotine-dependent adult versus adolescent rats. Baseline withdrawal signs were not different between adolescents (5.8 ± 0.7) and adults (6.4 ± 0.9) [F (1, 12) = 0.4; P = ns]. Our analyses revealed a significant interaction between age and drug treatment [F (2, 22) = 4.3; P < 0.05]. Subsequent post-hoc analyses revealed that both age groups displayed an increase in the physical signs of withdrawal relative to baseline levels following mecamylamine administration, and this effect was larger in adult versus adolescent rats (Ps < 0.05).
Fig. 1.
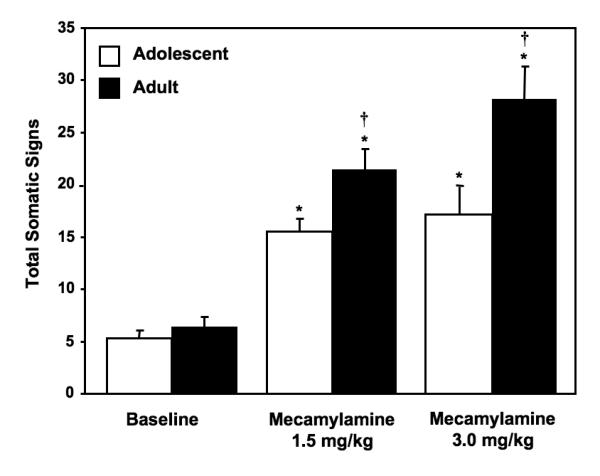
Data reflect total somatic signs of withdrawal (±SEM) exhibited in a 10-minute observation period during baseline and following mecamylamine administration in adolescent and adult rats (n=7-8 per group). Asterisks (*) denote significant differences from baseline values (Ps < 0.05), and daggers (†) denote significant differences between age groups (Ps < 0.05).
NAcc Dopamine During Nicotine Withdrawal
Figure 2 illustrates % change from baseline levels of NAcc dopamine (± SEM) in adolescent and adult rats experiencing nicotine withdrawal. Overall, the results revealed that mecamylamine produced a decrease in extracellular levels of NAcc dopamine that was larger in nicotine-dependent adult versus adolescent rats. There were no age differences in baseline dopamine levels across adolescent (2.8 ± 0.2 nM) versus adult (2.9 ± 0.1 nM) rats [F (1, 14) = 0.3; P = ns]. Our analyses revealed a significant interaction between age and time [F (23, 322) = 2.0; P < 0.05], with both age groups displaying a decrease in NAcc dopamine following mecamylamine administration that was larger in adult versus adolescent rats. Specifically, adult rats displayed a larger decrease in dopamine (average decrease of 44.1 ± 5.5% from baseline levels) versus adolescent rats (average decrease of 20.1 ± 5.3% from baseline levels). Subsequent post-hoc analyses revealed that the adult rats displayed a significant decrease in NAcc dopamine relative to baseline at all time points following mecamylamine except for the sample that was collected before administration of the highest dose of mecamylamine (Ps < 0.05). In contrast, adolescents only displayed a significant decrease from baseline during the 2nd −5th, 9th and 11th −12th time points after mecamylamine administration (Ps < 0.05). Post-hoc analyses examining age differences revealed that adults displayed larger decreases in NAcc dopamine levels relative to adolescents at the 1st, 2nd, 7th and 12th time points after mecamylamine administration (Ps < 0.05).
Fig. 2.
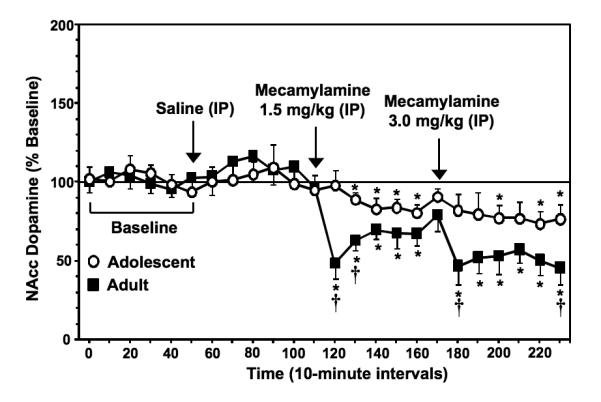
Data reflect % change from baseline levels of NAcc dopamine (± SEM) plotted across 10-minute sample collections during baseline and following administration of saline and then 2 doses of mecamylamine to precipitate withdrawal in adolescent and adult rats (n=7-8 per group). There were no group differences in baseline dopamine levels in adolescent (2.8 ± 0.2 nM) versus adult (2.9 ± 0.1 nM) rats. The arrows indicate the onset of drug administration. Asterisks (*) denote significant differences from baseline levels (Ps < 0.05) and daggers (†) denote significant differences between age groups (Ps < 0.05).
NAcc DOPAC During Nicotine Withdrawal
Figure 3 illustrates % change from baseline levels of NAcc DOPAC (± SEM) in adolescent and adult rats experiencing nicotine withdrawal. Overall, the results revealed that mecamylamine produced a decrease in extracellular levels of NAcc DOPAC that was larger in nicotine-dependent adult versus adolescent rats. There were no age differences in baseline DOPAC levels in adolescent (487.8 ± 52.1 nM) versus adult (517.9 ± 47.9 nM) rats [F (1, 12) = 0.2; P = ns]. Our analyses revealed a significant interaction between age and time [F (23, 276) = 1.6; P < 0.05], with adults displaying a decrease in NAcc DOPAC levels following mecamylamine administration that was larger than the adolescent rats. Specifically, adult rats displayed a decrease in DOPAC levels (average decrease of 20 ± 14.3% from baseline levels) that was not altered in adolescent rats. Post-hoc analyses revealed that adult rats displayed a significant decrease relative to baseline levels during the final time point after mecamylamine administration (P < 0.05). Also, the post-hoc analyses examining age differences revealed that adults displayed larger decreases in NAcc DOPAC levels relative to adolescents during the 1st, 5th, and 12th time points after mecamylamine administration (Ps < 0.05). It should be noted that adults displayed an increase in NAcc DOPAC levels during the 3rd, 5th, and 6th time points after saline administration (Ps < 0.05). However, adolescents did not display this effect following saline administration.
Fig. 3.
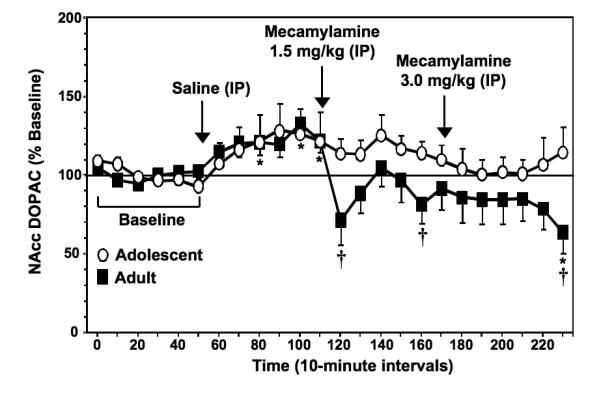
Data reflect % change from baseline levels of NAcc DOPAC (± SEM) plotted across 10-minute sample collections during baseline and following administration of saline and then 2 doses of mecamylamine to precipitate withdrawal in adolescent and adult rats (n=7-8 per group). There were no group differences in baseline DOPAC levels in adolescent (487.8 ± 52.1 nM) versus adult (517.9 ± 47.9 nM) rats. The arrows indicate the onset of drug administration. Asterisks (*) denote significant differences from baseline levels (Ps < 0.05) and daggers (†) denote significant differences between age groups (Ps < 0.05).
NAcc HVA During Nicotine Withdrawal
Figure 4 illustrates % change from baseline levels of NAcc HVA (± SEM) in adolescent and adult rats experiencing nicotine withdrawal. Overall, the results revealed that mecamylamine produced a decrease in extracellular levels of NAcc HVA that was larger in nicotine-dependent adult versus adolescent rats. There were no age differences in baseline HVA levels in adolescent (395.7 ± 41.3 nM) versus adult (328.7 ± 38.6 nM) rats [F (1, 12) = 1.4; P = ns]. Our analyses revealed a significant interaction between age and time [F (23, 276) = 1.9; P < 0.05] with adults, but not adolescents displaying a time-dependent decrease in NAcc HVA levels. Specifically, adult rats displayed a decrease in HVA levels (average decrease of 21 ± 8.4% from baseline levels) that was not observed in adolescents. Post-hoc analyses revealed that adults displayed a significant decrease in NAcc HVA levels relative to baseline during the final 3 time points after administration of the highest mecamylamine dose (Ps < 0.05). Also, post-hoc analyses examining age differences produced by mecamylamine administration revealed that adults displayed a larger decrease in HVA levels relative to adolescents during the last time point after administration of the highest mecamylamine dose (P < 0.05). It should be noted that adults displayed an increase in NAcc HVA levels during the 5th and 6th time points after saline administration (Ps < 0.05). However, adolescent rats did not display this effect following saline administration.
Fig. 4.
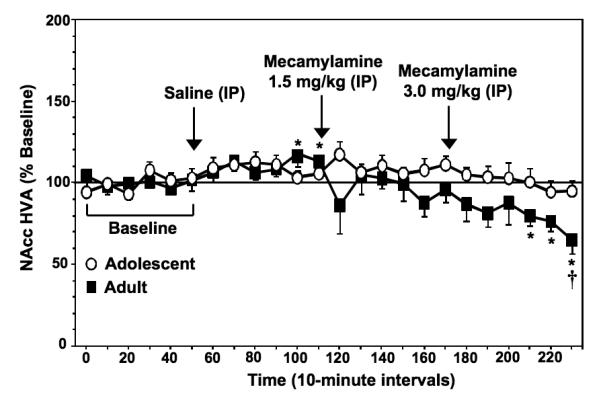
Data reflect % change from baseline levels of NAcc HVA (± SEM) plotted across 10-minute sample collections during baseline and following administration of saline and then 2 doses of mecamylamine to precipitate withdrawal in adolescent and adult rats (n=7-8 per group). There were no group differences in baseline HVA levels in adolescent (395.7 ± 41.3 nM) versus adult (328.7 ± 38.6 nM) rats. The arrows indicate the onset of drug administration. Asterisks (*) denote significant differences from baseline levels (Ps < 0.05) and the dagger (†) denotes a significant difference between age groups (P < 0.05).
NAcc 5-HIAA During Nicotine Withdrawal
Figure 5 illustrates % change from baseline levels of NAcc 5-HIAA (± SEM) in adolescent and adult rats experiencing nicotine withdrawal. The results revealed that mecamylamine did not produce changes in extracellular levels of NAcc 5-HIAA in nicotine-dependent adolescent or adult rats.
Fig. 5.
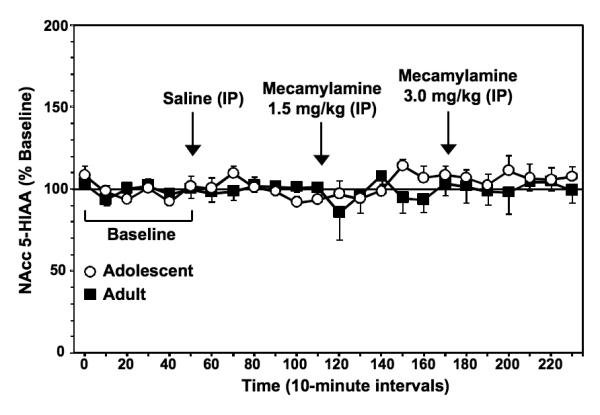
Data reflect % change from baseline levels of NAcc HVA (± SEM) plotted across 10-minute sample collections during baseline and following administration of saline and then 2 doses of mecamylamine to precipitate withdrawal in adolescent and adult rats (n=7-8 per group). There were no group differences in baseline 5-HIAA levels in adolescent (169.2 ± 29.0 nM) versus adult (225.3 ± 49.8 nM) rats. The arrows indicate onset of drug administration.
NAcc Dopamine During Intra-VTA Administration of Bicuculline
Figure 6 illustrates % change from baseline levels of NAcc dopamine during the 3 samples prior to and the 6 samples following intra-VTA administration of bicuculline in adolescent and adult rats. Overall, the results revealed that blockade of GABAA receptors in the VTA produced an increase in extracellular levels of NAcc dopamine of adult but not adolescent rats. There were no age differences in dopamine levels during the 3 samples prior to bicuculline administration across adolescent (2.2 ± 0.2 nM) versus adult (1.6 ± 0.2 nM) rats [F (1, 14) = 3.8; P = ns]. Our analyses revealed a significant interaction between age and time [F (8, 88) = 17.9; P < 0.05], with adult rats displaying increases in NAcc dopamine that were higher versus adolescents. Specifically, adults displayed a 2-fold increase in dopamine following bicuculline infusion (i.e., from 56.0 ± 7.5% to 125.9 ± 10.7), whereas adolescents only showed a slight increase (i.e., from 77.4 ± 5.5% to 87.9 ± 7.3%) in these measures. Post-hoc analyses revealed that during the final 3 time points, adults displayed significant increases in NAcc dopamine relative to the 3 samples prior to bicuculline (Ps < 0.05). In contrast, adolescents only displayed a significant increase in NAcc dopamine during the final time point relative to the 3 samples prior to bicuculline (P < 0.05). Also, post-hoc analyses examining age differences revealed that adults displayed significantly higher NAcc dopamine relative to adolescents during the final 3 time points after bicuculline (Ps < 0.05).
Fig. 6.
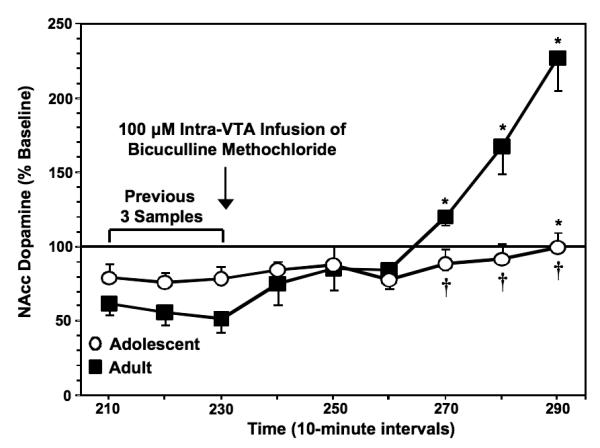
Data reflect % change from baseline levels of NAcc dopamine plotted across 10-minute sample collections during the 3 samples prior to and the 6 samples following intra-VTA bicuculline administration in adolescent and adult rats (n=7-8 per group). Asterisks (*) denote significant differences relative to the 3 samples collected prior to bicuculline administration (Ps < 0.05), and daggers (†) denote significant differences between age groups (Ps < 0.05).
DISCUSSION
The major finding of this report is that the physical signs of nicotine withdrawal and decreases in extracellular levels of dopamine in the NAcc were lower in adolescent versus adult rats. The pattern of developmental differences in dopamine was also consistent with the metabolites of this neurotransmitter, as decreases in extracellular levels of DOPAC and HVA were also lower in the NAcc of adolescent versus adult rats. The present report also demonstrated that intra-VTA administration of a GABAA antagonist produced an increase in NAcc dopamine that was lower in adolescent versus adult rats. Thus, one possible mechanism to explain reduced changes in NAcc dopamine during withdrawal in adolescent rats is that inhibition of dopamine in the VTA is underdeveloped such that adolescents display less of a decrease in NAcc dopamine during withdrawal.
The present finding that the physical signs of nicotine withdrawal are lower in adolescent versus adult rats is consistent with previous behavioral studies. For example, adolescent rats display fewer physical signs of withdrawal relative to adults across a range of nicotine doses to produce dependence, and across a range of mecamylamine doses to precipitate withdrawal (O’Dell et al., 2006). Furthermore, work in our laboratory and others have demonstrated that the negative affective properties of nicotine withdrawal are also lower in adolescent versus adult rats (O’Dell et al., 2007) and mice (Kota et al., 2007, 2008). Based on these previous studies and the present findings, we suggest that adolescence is a period of development characterized by reduced sensitivity to the behavioral and neurochemical effects of nicotine withdrawal.
Our neurochemical results extend previous behavioral studies by providing a potential mechanism for reduced sensitivity to nicotine withdrawal during adolescence. This mechanism involves reduced dopamine transmission in the NAcc, an effect that has been well established in adult rats experiencing withdrawal from nicotine (Carboni et al., 2000; Gaddnas et al., 2002; Hildebrand et al., 1998, 1999; Rada et al., 2001) as well as other drugs of abuse (Pothos et al., 1991; Rada et al., 2004; Weiss et al., 1992). The latter studies regarding nicotine withdrawal have reported a 20-35% decrease in extracellular levels of NAcc dopamine during nicotine withdrawal, and the magnitude of this effect is consistent with the 44% decrease in NAcc dopamine observed in the present study. The major contribution of this report to the literature; however, is that adolescent rats only displayed a 20% decrease in extracellular levels of NAcc dopamine during nicotine withdrawal.
Consistent with the developmental differences in the dopamine data, adolescent rats also displayed decreases in the dopaminergic metabolites DOPAC and HVA that were lower relative to adults. The metabolite data are useful for several reasons. First, they provide a supplementary (albeit, indirect) verification of the changes we observed with dopamine neurotransmission since the metabolites generally produced similar patterns of developmental differences during withdrawal. Thus, our metabolite data provided a verification of the developmental differences observed with dopamine using a measure that produced a large neurochemical signal. Second, the metabolite data allowed us to detect differences that were not observed with dopamine, such as the increases in DOPAC and HVA levels observed in adult rats receiving saline. The saline-induced increases in the metabolites might reflect acute stress in the adult animal produced by a systemic injection. This effect has also been observed in nicotine-dependent adult animals following intra-VTA administration of saline (Hildebrand et al., 1999). Third, the metabolite data suggest that our developmental differences are specific to dopaminergic systems, since neither age group displayed changes in 5-HIAA levels during withdrawal. This is consistent with previous studies showing that 5-HIAA levels are not altered in adult rats experiencing nicotine withdrawal (Gaddnas et al., 2002; Hildebrand et al., 1998).
In this study, mecamylamine was used as a pharmacological tool to compare developmental differences in the neurochemical effects of withdrawal. Thus, our comparisons focused on adolescent and adult rats that were exposed to nicotine and then given mecamylamine to precipitate withdrawal. It may be argued that our observed changes in dopamine and its metabolites reflect age-dependent differences in response to mecamylamine given in combination with chronic nicotine treatment versus mecamylamine given alone. However, our previous place conditioning studies revealed that adolescents chronically exposed to nicotine still demonstrate less sensitivity to mecamylamine-precipitated withdrawal versus adults, even in separate groups of adolescents that received a 2-fold higher dose of mecamylamine or 7 additional days of nicotine exposure (O’Dell et al., 2007). Furthermore, in the absence of mecamylamine, the removal of a nicotine pump still produces less spontaneous signs of withdrawal in adolescent versus adult rats (Shram et al., 2008) and mice (Kota et al., 2007).
It is also unlikely that developmental differences observed in this study can be attributed to the effects of mecamylamine alone, since several reports have shown that this drug has little behavioral or neurochemical effects in the absence of nicotine. For example, administration of the mecamylamine doses used in the present study do not alter the physical signs of withdrawal or produce place aversion in naïve adolescent and adult rats (O’Dell et al., 2007; Shram et al., 2008) or mice (Kota et al., 2007). Also, separate laboratories have shown that mecamylamine alone does not alter extracellular levels of dopamine in the NAcc of naïve adult rats (Carboni et al., 2000; Gaddnas et al., 2002; Hildebrand and Svensson, 2000; Rada et al., 2001). Taken together, these studies suggest that our results are not influenced by developmental differences in response to mecamylamine alone. However, this potential limitation in the interpretation of the present findings might be addressed in future empirical studies comparing developmental differences in nicotinic receptor function.
It should be noted that adolescents display faster weight gain and metabolic breakdown of nicotine than adults. Thus, it may also be suggested that the lack of withdrawal in adolescents is due to lower levels of nicotine on the day of microdialysis testing relative to adults. However, this potential confound was likely avoided because we implanted the adolescent rats with a pump containing a 1.5 fold higher dose of nicotine as compared to adults. This adjustment factor was based on a study showing that after 17 days of nicotine pump exposure, the infusion rates of nicotine were 1.5 times lower in adolescent (3-4 mg/kg/day) versus adult rats (5 mg/kg/day; Trauth et al., 2000). Thus, one might expect that adolescents receiving 1.5 times more nicotine than adults would display equivalent nicotine levels as adults on the test day following 14 days of nicotine exposure. Moreover, we have demonstrated that place aversion produced by nicotine withdrawal is still lower in a group of adolescents that were tested after 21 days of nicotine exposure during which time they received a new pump containing an adjusted nicotine dose 14 days after the initial pump implantation (O’Dell et al., 2007). Also, Kota et al., (2007) demonstrated that adolescent mice given repeated systemic injections of nicotine that were adjusted for weight still display less physical and affective signs of withdrawal as compared to adults. Taken together, these studies suggest that the developmental differences observed in the present study are not likely due to developmental differences in nicotine dosing or tolerance; however, future studies might directly assess this possibility at the time point that was used in the present study.
The present study also explored the hypothesis that developmental differences in NAcc dopamine are mediated via GABAergic inhibition of dopamine cell bodies in the VTA. Our rationale for examining GABAergic systems was based on the finding that blockade of GABAA receptors in the VTA produces a 40-80% increase in NAcc dopamine levels, whereas stimulation of these receptors induces a 60% decrease in this measure (see Ikemoto et al., 1997; Westerink et al., 1996). Indeed, the present findings are consistent with previous reports, as intra-VTA infusions of bicuculline produced a 2-fold increase in dopamine levels in the NAcc of adult rats. However, our results also demonstrated that blockade of GABAA receptors in the VTA did not alter dopamine levels in the NAcc of adolescent rats. Based on this finding, we suggest that the inability of bicuculline to increase NAcc dopamine levels in adolescent rats is due to underdeveloped GABAergic inhibition of VTA dopamine neurons that release dopamine in the NAcc. Our hypothesis is based on previous studies showing that during adolescence, inhibitory postsynaptic potentials in GABA neurons are slower, less frequent, and weaker in response to GABA agonists relative to neurons from mature animals (see Cohen et al., 2000). Also, GABA-mediated inhibition of postsynaptic GABAB receptors is not functional early in life and GABA currents in neonatal rat neurons are insensitive to benzodiazepine activation of GABAA receptors (Cherubini et al., 1991). Thus, it is possible that adolescent rats display reduced decreases in dopamine activity during nicotine withdrawal because of a lack of inhibition in the VTA cell body region. We recognize that our interpretation of these data may be limited on the basis of pharmacological studies, and future studies will need to more directly assess our hypothesis by comparing extracellular levels of GABA in the VTA of adolescent and adult rats experiencing nicotine withdrawal.
To our knowledge, this is the first study to demonstrate that the neurochemical effects of nicotine withdrawal are lower in adolescent versus adult rats. Recent studies have shown that the neurochemical effects of nicotine administration are enhanced in adolescent versus adult rats. For example, a recent study reported that systemic administration of nicotine produces a larger increase in extracellular levels of dopamine and DOPAC in the NAcc of adolescent versus adult rats (Shearman et al., 2008). In addition, Azam et al. (2007) showed that nicotine-stimulated release of 3[H]dopamine is elevated in synaptosomes collected from the NAcc of adolescent versus adult rats. These reports showing that the neurochemical effects of nicotine are enhanced during adolescence are also consistent with several behavioral studies showing that the rewarding effects of nicotine are enhanced during adolescence (Chen et al., 2007; Kota et al., 2007, 2008; Levin et al., 2003, 2007; Shram et al., 2006; Torres et al., 2008; Vastola et al., 2002). Taken together, these studies suggest that the positive rewarding effects of nicotine are enhanced, whereas the negative effects of nicotine withdrawal are reduced during adolescence. These opposite behavioral phenomena appear to be related to changes in dopamine systems, as the neurochemical effects of nicotine are enhanced whereas decreases in dopamine during withdrawal are reduced during adolescence. Thus, adolescent vulnerability to nicotine addiction may be associated with increased sensitivity to the rewarding effects of nicotine and reduced sensitivity to the negative aversive effects of nicotine withdrawal.
In conclusion, the present findings may have clinical relevance for treating adolescent tobacco abuse. Specifically, the finding that adolescents display reduced withdrawal suggests that treatments focusing on alleviating nicotine withdrawal may be less effective in treating adolescent tobacco users. As an example, treatments that enhance dopamine neurotransmission (i.e., bupropion) may be less effective in adolescent smokers that experience less of a decrease in dopamine levels during withdrawal. There is clinical evidence to support our suggestion that treatments focusing on alleviating withdrawal are less effective in adolescent tobacco abusers. For example, it has been demonstrated that long-term abstinence rates do not appear to be closely associated with nicotine replacement therapies in adolescent smokers (Hanson et al., 2003: Hurt et al., 2000; Moolchan et al., 2005). Furthermore, there is evidence to suggest that nicotine replacement does not prevent the expression of nicotine withdrawal symptoms in adolescent smokers (Killen et al., 2001). Moreover, a recent study directly comparing adolescent smokers to non-smokers found that young smokers only exhibit mild symptoms during withdrawal (anger and craving) that do not appear to be associated with self-reports of dependence or biological markers of cigarette use (Smith et al., 2008a). Another report from this laboratory found that withdrawal symptoms on the quit day were not related to relapse behavior in adolescent smokers (Smith et al., 2008b). These studies suggest that abstinence from chronic tobacco abuse only produces mild withdrawal symptoms that are not related to continued use or relapse behavior during adolescence. Thus, treatments focusing on alleviating withdrawal may be less effective in young tobacco abusers. Future studies are needed to examine whether treatments that target nicotine withdrawal via enhanced dopamine neurotransmission are equally effective in adolescent and adult smokers.
ACKNOWLEDGMENTS
This research was supported by the National Institute on Drug Abuse (R01-DA021274; LEO) and the UTEP BBRC-RCMI (5G12RR008124; LEO). This work was also supported by the NIH Ruth L. Kirschstein Fellowship program (F31DA021133; LAN) and the American Psychological Association, Diversity Program in Neuroscience (T32-MH018882-20; LAN). The authors would like to thank Drs. Eddie Castañeda, Kristin Gosselink, Donald Moss, and Christina Sobin for their helpful input during the preparation of this manuscript. The authors would also like to thank Arturo Orona and Francisco Roman for their technical assistance.
REFERENCES
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter AR, Fant RV, Henningfield JE. Novel pharmacological approaches for treating tobacco dependence and withdrawal: current status. Drugs. 2008;68:1067–1088. doi: 10.2165/00003495-200868080-00005. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. American Journal of Medicine. 2008;121:3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. Journal of Neuroscience. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Bortone L, Giua C, Di Chiara G. Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug and Alcohol Dependence. 2000;58:93–102. doi: 10.1016/s0376-8716(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Carmody TP, Vieten C, Astin JA. Negative affect, emotional acceptance, and smoking cessation. Journal of Psychoactive Drugs. 2007;39:499–508. doi: 10.1080/02791072.2007.10399889. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends in Neuroscience. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Coulter DA. Protracted postnatal development of inhibitory synaptic transmission in rat hippocampal area CA1 neurons. Journal of Neurophysiology. 2000;84:2465–2476. doi: 10.1152/jn.2000.84.5.2465. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Understanding brain mechanisms in nicotine reinforcement. British Journal of Addiction. 1991;86:507–510. doi: 10.1111/j.1360-0443.1991.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Gaddnas H, Piepponen TP, Ahtee L. Mecamylamine decreases accumbal dopamine output in mice treated chronically with nicotine. Neuroscience Letters. 2002;330:219–222. doi: 10.1016/s0304-3940(02)00734-6. [DOI] [PubMed] [Google Scholar]
- Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine Tobacco Research. 2003;5:515–526. doi: 10.1080/14622200307243. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilstrom B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Research. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Panagis G, Svensson TH, Nomikos GG. Behavioral and biochemical manifestations of mecamylamine-precipitated nicotine withdrawal in the rat: role of nicotinic receptors in the ventral tegmental area. Neuropsychopharmacology. 1999;21:560–574. doi: 10.1016/S0893-133X(99)00055-X. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Svensson TH. Intraaccumbal mecamylamine infusion does not affect dopamine output in the nucleus accumbens of chronically nicotine-treated rats. Journal of Neural Transmission. 2000;107:861–872. doi: 10.1007/s007020070038. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Croghan GA, Beede SD, Wolter TD, Croghan IT, Patten CA. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Archives of Pediatrics and Adolescent Medicine. 2000;154:31–37. [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. GABA-A receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. Journal of Neurochemistry. 1997;69:137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurons in the rat ventral tegmental area and their synaptic inputs. Journal of Physiology (London) 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Research Reviews. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Killen JD, Ammerman S, Rojas N, Varady J, Haydel F, Robinson TN. Do adolescent smokers experience withdrawal effects when deprived of nicotine? Experimental Clinical Psychopharmacology. 2001;9:176–182. doi: 10.1037//1064-1297.9.2.176. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. Journal of Pharmacology and Experimental Therapeutics. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology. 2008;198:201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani A, Montoya R, Rose J, Swartrzwelder H. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicology and Teratology. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, Conrad DL, Wilson OB. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berlin) 1994;115:180–184. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Moolchan ET, Robinson ML, Ernst M, Cadet JL, Pickworth WB, Heishman SJ, Schroeder JR. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics. 2005;115:407–414. doi: 10.1542/peds.2004-1894. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Parsons LH. Serotonin-1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. Journal of Pharmacological and Experimental Therapeutics. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Annals of the New York Academy of Sciences. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicology and Teratology. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Edition Academic Press; New York: 1998. [Google Scholar]
- Philpot RM, McQuown S, Kirstein CL. Stereotaxic localization of the developing nucleus accumbens septi. Developmental Brain Resesarch. 2001;130:149–153. doi: 10.1016/s0165-3806(01)00225-5. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biological Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Research. 1991;566:348–50. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology. 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rada P, Johnson DF, Lewis MJ, Hoebel BG. In alcohol-treated rats, naloxone decreases extracellular dopamine and increases acetylcholine in the nucleus accumbens: evidence of opioid withdrawal. Pharmacology, Biochemistry and Behavior. 2004;79:599–605. doi: 10.1016/j.pbb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. Journal of Neuroscience. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman E, Fallon S, Sershen H, Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Research Bulletin. 2008;76:626–639. doi: 10.1016/j.brainresbull.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Siu EC, Li Z, Tyndale RF, Le AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology. 2008;198:181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Smith AE, Cavallo DA, Dahl T, Wu R, George TP, Krishnan-Sarin S. Effects of acute tobacco abstinence in adolescent smokers as compared with nonsmokers. Journal of Adolescent Health. 2008a;43:46–54. doi: 10.1016/j.jadohealth.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Cavallo DA, McFetridge A, Liss T, Krishnan-Sarin S. Preliminary examination of tobacco withdrawal in adolescent smokers during smoking cessation treatment. Nicotine and Tobacco Research. 2008b;10:1253–1259. doi: 10.1080/14622200802219357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology, Biochemistry, and Behavior. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Research. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology Behavior. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine and Tobacco Research. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Research. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. Journal of Neuroscience. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


