Abstract
The presence of circulating tumor cells (CTC) accompanies tumor invasion into the bloodstream. Detection, monitoring and molecular analysis of these rare cancer cells shed into blood will provide a powerful and noninvasive approach for detection of early disease, assessing prognosis and therapeutic response in established cancers, and targeting metastatic precursor cells. We review current and emerging technologies for CTC isolation, with a focus on capture efficiency, purity and viability of CTCs, and their potential clinical applications.
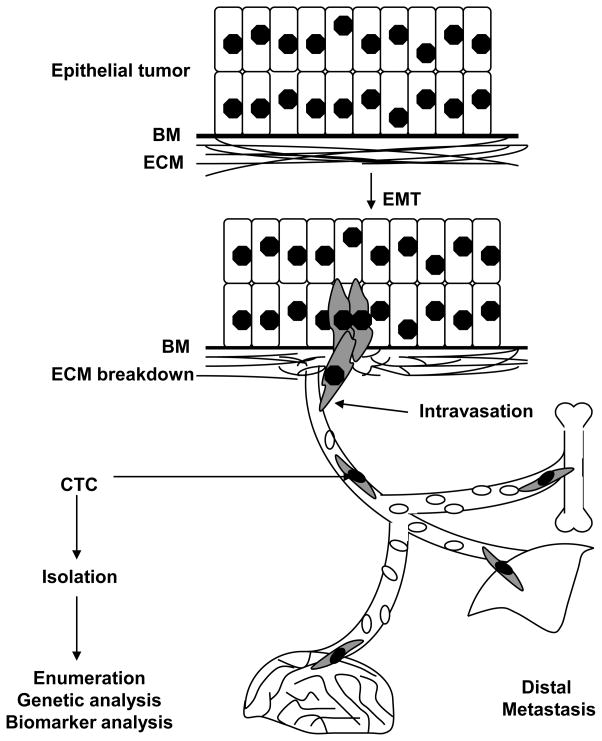
Epithelial cancers initially arise as an organ-confined lesion, but eventually spread to distant sites (eg. lung, liver, bone and/or brain) through the bloodstream, generating metastases that are mainly responsible for their lethality. The process by which human tumor cells exit from their primary site, intravasate into the vasculature and then extravasate into distal organs is not well understood. Mouse models of tumor spread have implicated the process of epithelial to mesenchymal transition (EMT), by which adherent epithelial cells acquire migratory cell fates. Activation of proteases which compromise the integrity of the basement membrane and extracellular matrix is also thought to contribute to cancer metastasis (Fig 1; Kalluri and Weinberg, 2009). However, mouse models of tumor invasion differ significantly from human cancer metastasis, and approaches to studying the spread of human tumors have been very limited. It is in this context that the isolation and characterization of individual tumor cells from the blood of patients known to have metastatic cancer, so-called circulating tumor cells (CTCs), hold tremendous potential for new biological insight with very real clinical applications.
Figure 1.
Schematic representation of epithelial to mesenchymal transition (EMT), by which adherent epithelial cells are thought to acquire migratory cell fates, combined with the activation of proteases which compromise the integrity of the basement membrane (BM) and the extracellular matix (ECM), leading to intravasation of tumor cells into the bloodstream. The rare tumor cells that are present in the bloodstream, admixed with billions of normal blood cells, are defined as circulating tumor cells (CTC). A subset of CTCs are thought to extravasate at distal sites such as the lung, liver, bone and brain to establish metastatic lesions. Isolation, enumeration, and genetic and biomarker analysis of CTCs will provide insight into the biology of these putative metastatic precursors.
Recent technological advances in the detection and analysis of CTCs have generated considerable attention and renewed efforts to understand the biology and significance of these cells. Among the leading applications for CTC analyses are “real time” genetic analysis of tumors, before, during, and after treatment, a subject that has become critical in the new era of genetically targeted cancer therapies. Such targeted therapies aim to match the right drug to the right patient through stages of disease progression, from drug sensitivity to the acquisition of drug resistance. Longitudinal monitoring of CTC-derived genotypes may thus provide a noninvasive approach to identify drug-sensitivity and resistance associated markers, guiding therapeutic decisions. In addition to their potential use in directing the treatment of patients with advanced epithelial cancers, CTCs may also hold the key to monitoring for early dissemination of cancer. The possibility that invasive but localized tumors may shed CTCs into the bloodstream before any bona fide metastases are established suggests that a highly sensitive CTC detection approach may provide important information in individuals at risk due to environmental exposures or genetic predisposition. Finally, the proposed existence of rare cancer stem cells within a more abundant population of tumor cells with only limited proliferative potential predicts that clonal metastatic lesions are initiated by such rare immortal progenitors (Reya et al., 2001). The population of CTCs harvested from a peripheral blood specimen may therefore be enriched for such cancer stem cells or metastatic precursors. Detailed molecular and functional analysis of such rare cells may provide insight into the biology of cancer metastasis and identify novel therapeutic targets for the treatment and prevention of blood borne cancer dissemination.
Given their rarity in the circulation (estimated as one tumor cell per billion normal blood cells in patients with known metastatic cancer), our understanding of CTCs is in fact very much dependent upon the technological approaches used for their detection and isolation. A number of strategies have been employed and many more are at various stages of development. To date, the most successful approaches have made use of the fact that epithelial cells commonly express the cell adhesion protein EpCAM, which is absent in normal blood cells. Immunomagnetic bead-based capture involves treating blood specimens with antibody to EpCAM that has been conjugated with magnetic particles, followed by separation of tagged cells in a magnetic field. Isolated cells are then stained with antibody to another epithelial marker, cytokeratin, as well as a common leukocyte marker CD45, so as to distinguish rare CTCs from contaminating white blood cells. This approach, while robust and semi-automated, typically identifies CTCs in only half of patients known to have metastatic disease, with an average yield of approximately 1 CTC/mL and a purity of 0.1% (Allard et al., 2004). More recent technological developments have applied microfluidic rare cell detection strategies, with a significant increase in both yield and purity. The microfluidic-based CTC capture device we have called the CTC-Chip involves flowing whole blood through a chamber embedded with 80,000 microposts that have been rendered functional by coating with antibody to EpCAM. Flow kinetics are optimized to minimize shear stress, while enhancing the probability of collisions between CTCs and antibody-coated microposts. CTCs are then stained with secondary antibodies against either cytokeratin or tissue specific markers, such as PSA in prostate cancer or HER2 in breast cancer and are visualized by automated scanning of microposts in multiple planes along three dimensional coordinates. In initial experiments, the CTC-chip identified cytokerating-positive circulating tumor cells in virtually all patients with a variety of metastastic cancers, with a median yield of 50 cells/ml and purity ranging from 1–80% (Nagrath et al., 2007). EpCAM-directed antibody-mediated capture of CTCs is effective, but it selects for cells expressing this epithelial marker, which is downregulated during the process of EMT. As such, the CTC-Chip provides a versatile platform to test additional capturing antibodies directed against tumor-specific cell surface antigens.
Antibody based “negative selection”, ie. removing leukocytes from a peripheral blood sample to leave behind uncaptured CTCs, has been tested as a selection strategy that is not biased by the expression of known antigens on the tumor cells themselves (Yang et al., 2009). However, given the extremely rare prevalence of CTCs in blood specimens and the incomplete removal of white blood cells even with the most rigorous techniques, negative selection approaches suffer from relatively low purity of CTC-enriched populations. Non-antibody based capture methods have also been explored, including size based purification using filters of variable pore size, based on the premise that CTCs are often larger than normal leukocytes (Zheng et al., 2007). However, the size range of different tumor cells is highly variable and does overlap with that of normal blood cells. Additional promising techniques involve high speed microscopic scanning of all nucleated blood cells (Hsieh et al., 2006; Krivacic et al., 2004), as well as in vivo imaging of tagged circulating cells (He et al., 2007). While technical challenges remain considerable, the recent application of diverse bioengineering tools to the problem rare cancer cell detection in the blood is likely to radically alter this field, bringing powerful new enabling platforms for clinical and molecular studies. To the extent that such techniques, like microfluidic CTC capture, allow the isolation of viable CTCs, these new bioengineering platforms may enable an array of molecular studies that capitalize on new insights about tumor cell signaling and drug susceptibility.
To date, biological and clinical insights into CTCs have been dependent upon the parameters of the isolation technologies used. Given the relatively low yield and purity of immunomagnetic bead-based CTC capture, initial insights have focused on general prognostic implications of CTC counts above a level determined in patient survival studies (typically 5 CTCs/7.5mL of blood). In studies of breast, colon and prostate cancer, patients with metastatic disease who were “CTC positive” had a worse prognosis than those without detectable CTCs (Cristofanilli et al., 2004; Cristofanilli et al., 2005; Danila et al., 2007; Scher et al., 2009; Shaffer et al., 2007; Vidaurreta et al., 2007). While these studies have reinforced the clinical significance of CTC load, they have not provided sufficiently robust information in individual patients to guide therapeutic decisions. In this context, initial studies of microfluidic-based CTC detection appear to be particularly promising, in that the higher number of isolated CTCs provides a dynamic range that can be monitored during therapy. Thus in an initial pilot study, chemotherapy-induced responses in patients with a variety of epithelial cancers was associated with a profound decline in CTCs, coincident and even preceding measured radiographic evidence of tumor response (Maheswaran et al., 2008; Nagrath et al., 2007). Should these studies be extended in larger patient studies, it is conceivable that rapid measurements of CTC numbers following therapy (and eventually more sophisticated measures of drug-induced alterations in CTCs) may allow more effective selection of successful therapies.
The most promising near term application of CTC monitoring relates to the advent of targeted cancer therapies, and the need to tailor such treatments to individual tumor characteristics. The identification of tumor genotypes that inform selection of targeted therapies is usually performed on the initial diagnostic specimen. However, these may not be readily available or sufficient for molecular analyses (eg. fine needle aspirates are increasingly used to diagnose cancers, providing minimal cytological specimens). Further, the initial primary tumor specimen may not always be representative of the metastatic deposits, which may arise many years after resection of the initial cancer. A primary example is prostate cancer, which often presents with multifocal localized disease, and may recur many years later with bony lesions that are not readily biopsied. As such, molecular analysis of CTCs may provide a “real time” noninvasive approach for tumor cell genotyping (often described as a “liquid biopsy”), which can be repeated during the course of therapy to monitor the acquisition of novel genetic abnormalities in response to drug exposure.
A powerful example of the application of CTC genotyping to targeted cancer therapy is provided by the subset of non-small cell lung cancer (NSCLC) that is responsive to selective kinase inhibitors of the Epidermal Growth Factor Receptor (EGFR). Only ~10% of NSCLC have somatic activating mutations in EGFR, but these mutations identify patients whose tumor is highly likely to exhibit a dramatic response to selective EGFR kinase inhibitors (Lynch et al., 2004). Similar paradigms are emerging with other experimental kinase inhibitors that target the EML4-ALK translocation in another subset of lung cancer, and B-RAF mutations in melanoma. While less dramatic in terms of tumor lysis reflecting so-called “oncogene addiction”, the effectiveness of antibody directed against HER2 amplified breast cancer is well established. In lung cancer, we initially established the accuracy of CTC-based genotyping in a study of patients with EGFR-mutant NSCLC, where the driving EGFR mutation was correctly identified in 12/13 cases. Most significantly, acquisition of the recurrent T790M-EGFR drug resistance mutation was evident in CTCs during the course of therapy, coincident with the development of clinically refractory disease (Maheswaran et al., 2008). As second line targeted therapies emerge in clinical trials, matching these drugs to different mechanisms of acquired resistance (including the T790M-EGFR mutation, as well as amplification of other growth factor receptors, such as c-MET) is likely to become a critical application of CTC-based molecular analyses.
Improvements in CTC capture efficiency, quantitation, imaging and molecular analyses are likely to bring further clinical applications. In initial studies, we have detected CTCs in some patients with localized prostate cancer, raising the possibility that invasive primary tumors may shed CTCs long before the establishment of distant metastases. Presence or absence of CTCs in such cases have not been correlated with standard histopathological markers of tumor grade, suggesting that CTC quantitation in early cancers may in fact be an independent measure of vascular invasiveness. If confirmed, these studies may pave the way for CTC-based applications in localized cancers, including predictive studies in patients with borderline prostate PSA levels, While these clinical applications are highly promising, probably the greatest insight to be derived from the study of CTCs relates to understanding the process of cancer metastasis. Studies of cancer stem cells or progenitors have focused on the characterization of primary tumors. However, the establishment of metastases by definition requires a fully competent tumor cell, capable of indefinite proliferation. As such, we hypothesize that CTCs may be comprised of variable fractions of tumor cells that are shed into the vasculature with limited viability, as well as a small fraction of metastatic precursors. Analyses that allow the identification of such cells hold the key to identifying relevant drug targets that may suppress metastasis, as well as monitoring the effectiveness of therapies aimed at metastatic precursors.
In summary, the science of CTCs is in its infancy. Little is known about the biology of these cells, and only recently have technological advances provided a window into the composition of this rare cell population, allowing molecularly-designed analyses. Detailed characterization of CTCs may provide the opportunity for clinical impact in the treatment of selected cancers where appropriate targeting of tumor-associated genetic lesions is critical. Technological developments that further enhance the sensitivity of CTC detection may provide new opportunities for early diagnosis of invasive localized cancer. Detailed analysis of rare metastatic precursors may provide unprecedented opportunity to develop therapeutic strategies to effectively treat and prevent cancer metastasis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 3.Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 4•.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. Using the Veridex immunomagnetic CTC isolation technology, the authors demonstrate that baseline CTC numbers are predictive of prostate cancer survival. The results were consistent with the observations reported in breast cancer. [DOI] [PubMed] [Google Scholar]
- 5•.He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci U S A. 2007;104:11760–11765. doi: 10.1073/pnas.0703875104. This manuscript describes a sensitive, and noninvasive technology that involves multiphoton fluorescence imaging of superficial blood vessels to quantify flowing CTCs following the injection of a tumor-specific fluorescent ligand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh HB, Marrinucci D, Bethel K, Curry DN, Humphrey M, Krivacic RT, Kroener J, Kroener L, Ladanyi A, Lazarus N, et al. High speed detection of circulating tumor cells. Biosens Bioelectron. 2006;21:1893–1899. doi: 10.1016/j.bios.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krivacic RT, Ladanyi A, Curry DN, Hsieh HB, Kuhn P, Bergsrud DE, Kepros JF, Barbera T, Ho MY, Chen LB, et al. A rare-cell detector for cancer. Proc Natl Acad Sci U S A. 2004;101:10501–10504. doi: 10.1073/pnas.0404036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10•.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. This is the first report describing the molecular characterization of CTCs in lung cancer: EGFR mutations consistent with those identified in the primary tumor can be detected in CTCs. Serial analysis of CTCs demonstrate the molecular evolution of the tumor during the course of treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. The authors describe microfluidics-based capture of CTCs on a silicon-chip lined with microposts. The technology demonstrates efficient capture of CTCs from several types of tumor with high purity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 13.Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, Heller G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, Gu B, Anand A, Smith K, Maslak P, Doyle GV, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 15.Vidaurreta M, Sastre J, Sanz-Casla MT, Maestro ML, Rafael S, Diaz-Rubio E. Detection and quantification of circulating tumor cells in peripheral blood in patients with colon cancer. Med Clin (Barc) 2007;129:333–334. doi: 10.1157/13109544. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Lang JC, Balasubramanian P, Jatana KR, Schuller D, Agrawal A, Zborowski M, Chalmers JJ. Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnol Bioeng. 2009;102:521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Zheng S, Lin H, Liu JQ, Balic M, Datar R, Cote RJ, Tai YC. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162:154–161. doi: 10.1016/j.chroma.2007.05.064. This manuscript describes a technology that exploits the size difference between CTCs and human blood cells to achieve CTC capture on a filter. [DOI] [PubMed] [Google Scholar]