Abstract
Infrequently omitting a sound from a repetitive sequence elicits the mismatch negativity (MMN) ERP response when the stimulus onset asynchrony (SOA) is less than 200 ms. We contrasted two alternative explanations of omission MMN. (1) Each sound starts a separate temporal integration process. Omissions violate the constancy of the temporal structure within the integration window. (2) Sounds preceding an omission are perceived to be louder than those followed by a sound within the integration period, because omissions allow the full stimulus aftereffect to be included in perceived loudness. We varied the SOA between 117 and 217 ms. For this case, the temporal structure explanation predicts that no MMN will be elicited, whereas the loudness summation explanation predicts that MMN will be elicited. MMN was elicited by tone omissions with random SOA, suggesting that loudness summation plays an important role in the elicitation of omission MMN.
Keywords: Temporal integration, Temporal window of integration, Loudness summation, Mismatch negativity (MMN), Auditory event-related potentials (ERP), Sensory memory
Results of various experiments indicate that sound energy is integrated in the human brain within an approximately 200-ms-long time window starting at the onset of abruptly commencing sounds. Cowan (1984) as well as Massaro (1975) consider temporal integration as the first (short or preperceptual) phase of sensory memory, during which sound information goes through a series of rapid transformations. These transformations establish the main sound features and prepare the way for a more stable integrated sound representation, one that is accessible to a wide range of processes, including those that underlie conscious perception.
The most well-known perceptual phenomena associated with temporal integration are the persistence of auditory sensation, loudness summation, and masking (Cowan, 1984). The persistence of auditory sensation is a stimulus aftereffect brought about by the inertial properties of auditory system. There are two lines of research investigating auditory persistence. In one type of experiment, subjects were required to judge the perceived offset of a sound (Efron, 1970a, 1970b, 1970c). Results showed that, for very short (<130 ms) sounds, subjects overestimated the sound duration. The moment of sound offset was perceived at 130–170 ms from stimulus onset, depending on the procedure used in the test. Auditory persistence has also been studied with gap detection procedures and by perceptual judgments about the sound offset ramp (Békésy, 1933; Miller, 1948; Plomp, 1964). For example, in Békésy's experiment, the steepness of the offset ramp of a continuous 800-Hz tone was increased until participants could detect no further change in the stimulus. Regardless of tone intensity, no perceptual changes were found between offset ramps shorter than 140 ms.
Loudness perception is also based on temporal integration. Several studies showed that for short sounds (<200 ms duration), perceived loudness correlated with overall sound energy, the power of the stimulus integrated in time (Scharf, 1978). Increasing the duration of a sound beyond 200 ms does not affect its loudness (for a review, see Cowan, 1984). Compatible results were obtained in detection masking experiments. Between 20 and 200 ms, sound duration interacts with sound intensity (Green, Birdsall, & Tanner, 1957). That is, detection of short sounds presented concurrently with noise depends on stimulus energy. Thus it appears that the human auditory system integrates the sensory effect of short sounds within an approximately 200-ms long period from sound onset (Zwislocki, 1969). The integration period has been termed the temporal window of integration (Näätänen, 1990). The integrated effect of a sound is encoded into the auditory sensory memory representation of the sound (Cowan, 1984; Massaro, 1975).
Auditory event–related brain potentials (ERP) also reflect effects of temporal integration. For example, the amplitude of the anterior subcomponent of the magnetic N1 response (the N100 mA; Sams, Hari, Rif, & Knuutila, 1993) elicited by the second sound of a sound pair shows a nonmonotonous (reversed U-shaped) function of the time between the onsets of the two tones in the 0–250-ms range (Loveless, Levänen, Jousmäki, Sams, & Hari, 1996). Loveless et al. interpreted the rise of N100 mA amplitude, which peaks at 150 ms stimulus onset asynchrony (SOA; onset-to-onset interval) as reflecting the integration of two consecutive sounds arriving within a single temporal window of integration. Another ERP component showing temporal window of integration specific response patterns is the mismatch negativity response (MMN; for a recent review, see Picton, Alain, Otten, Ritter, & Achin, 2000). MMN is an ERP component elicited by sounds violating some acoustic regularity (Näätänen & Winkler, 1999), such as an infrequent deviant sound appearing among the regular (standard) sounds in the auditory oddball paradigm. MMN is elicited whether or not subjects attend the sounds (Näätänen, 1990; Sussman, Winkler, & Wang, 2003). MMN has been shown to reflect mismatch between the deviant sound and the memory representation of the acoustic regularity (Näätänen, 1990; Näätänen & Winkler, 1999). Because temporal integration is assumed to precede the creation of the sound representations underlying MMN, one can expect to find temporal window of integration related effects on the MMN response.
Indeed, results of recognition masking studies using the MMN measure (Winkler, Reinikainen, & Näätänen, 1993; Winkler & Näätänen, 1994) as well as the pattern of MMN responses when two deviations occur within a single temporal window of integration (Czigler & Winkler, 1996: Sussman, Winkler, Ritter, Alho, & Näätänen, 1999) provided evidence suggesting that temporal integration can affect MMN. The most striking MMN phenomenon that is related to the temporal window of integration was obtained when sounds were occasionally omitted from an isochronous sequence of a repeating sound. Sound omissions only elicited MMN when the SOA was shorter than ca. 200 ms (Yabe, Tervaniemi, Reinikainen, & Näätänen, 1997; Yabe et al., 1998, 1999; for a similar result based on tone pairs presented with a constant within-pair interval, see Tervaniemi, Saarinen, Paavilainen, Danilova, & Näätänen, 1994).
Two explanations have been offered for the omission–MMN phenomenon. The first one suggests that omissions violate a regularity, which is based on the temporal structure of sounds within the temporal window of integration (Yabe et al., 1997). Yabe and his colleagues assume that each sound in the sequence starts a new temporal window of integration, which lasts for about 200 ms (this notion includes the possibility of overlapping temporal windows of integration). Because the sequence is presented with a uniform SOA, the temporal structure of the stimulation falling into a single temporal window of integration is constant throughout the regular part of the sound sequence. Therefore, when the SOA is shorter than the duration of the temporal window of integration, the memory trace, which is created from the temporal window of integration, contains the succession of two tones. When a sound is omitted, the temporal window of integration initiated by the sound preceding the omission contains only one sound. Therefore, the (temporal) structure of the resulting auditory memory trace differs from that of the standards, thus violating the repetition of the standard event and triggering an MMN. When the SOA is longer than 200 ms, the temporal window of integration contains only one sound for regular stimuli and, therefore, omissions do not result in temporal window of integration segments, whose content would differ from that of the standards. Thus no MMN is elicited with >200-ms SOAs. Note that an explanation based on the idea that omissions violate the regularity of the temporal schedule of stimulus delivery would not explain the lack of omission MMN at >200-ms SOAs, because other temporal violations, such as occasionally shortening the SOA in an isochronously presented sequence elicits MMNs even at SOAs exceeding the duration of the temporal window of integration (Nordby, Roth, & Pfefferbaum, 1988).
An alternative explanation of the omission–MMN phenomenon is based on the loudness–summation process. As shown by auditory persistence phenomena, the effect of a sound in the human auditory system lasts longer than the sound itself. Previous results demonstrated that the perceived loudness of short (<200 ms) tones depends on the length of the following silent period (see Cowan, 1987). The pattern of results was partly explained by the growth of sensation in the poststimulus period. That is, when other factors are kept constant, a tone is perceived as being louder when it is followed by a longer silent interval and this effect is limited to the temporal window of integration period. Thus it is likely that loudness summation includes the full effect of the stimulus, including the persisting part of the stimulus aftereffect (Cowan, 1995). Because MMN is based on perceived stimulus features rather than on acoustic stimulus parameters (e.g., Winkler, Tervaniemi, & Näätänen, 1997), infrequent changes in the perceived loudness of a repeating sound should elicit MMN. When sounds are regularly presented with an SOA that is shorter than the temporal window of integration, each sound terminates the summation of loudness for the preceding sound. Thus, sound omissions may cause deviation from the standard by allowing the full aftereffect of the sound preceding the omission to be integrated into the perceived loudness of this sound. In other words, those sounds that precede an omission may be perceived as being louder than the other sounds of the sequence and, therefore, would elicit MMN. This does not occur when the SOA is longer than 200 ms, because the loudness summation process stops at 200 ms.
Randomizing the SOA within the range of the temporal window of integration leads to different predictions from the above two explanations. In this case, the loudness-summation explanation predicts that MMN will be elicited by stimulus omissions, because the sound preceding the omission should be louder than any of the sounds that are followed by another sound within the temporal window of integration period. This prediction is supported by results showing that when the sounds in a stimulus sequence vary in some feature, those sounds that differ most from the center of the variation elicit the MMN (Huotilainen et al., 1993; Winkler et al., 1990). In contrast, according to the temporal-structure-violation explanation, no standard will emerge, because the temporal structure of the acoustic stimulation varies within the temporal window of integration. Therefore, no regularity will be detected, and no MMN should be elicited by sound omissions. Thus, by comparing the response to stimulus omissions when sounds are presented with a constant SOA versus when the SOA is randomized, we can dissociate the above two explanations.
One can, however, extend the temporal-structure-violation explanation of omission MMN to account for MMN elicitation by omission with randomized SOA. If a temporal window of integration segment is started by each tone, the ones triggered by tones preceding <200-ms SOAs contain two discrete tones. In contrast, the temporal window of integration segments triggered by tones preceding >200-ms SOAs (the tones preceding an omission) contain only one tone. Assuming that the auditory system can extract abstract regularities from the temporal window of integration segments, omissions break the abstract regularity of having two tones within one temporal window of integration. This would lead to MMN elicitation. Alternatively, SOAs that are shorter than the length of the temporal window of integration may not be represented with fine resolution. This is supported by results showing that the duration of short tones is uniformly perceived to be 130/170 ms (Efron, 1970a, 1970b, 1970c), although we do not know whether the loss of resolution occurs early or late in the path leading to perception. If the exact length of the SOA is lost before the regularities used in the MMN-generating process are extracted, then the temporal window of integration segments triggered by most standard tones in the random-SOA condition are registered as repetitions of the same stimulus event, thus forming a repetitive regularity. These extended versions of the temporal-structure-violation explanation can be contrasted with the loudness-summation violation explanation by checking in the random-SOA condition, whether standards followed by the shortest SOAs elicit MMN. If the temporal window of integration segments containing two tones form an abstract standard or if short SOAs are not distinguished from each other, then tones followed by the shortest SOA should not elicit MMN, because these tones do not violate the emerging regularity (i.e., they are part of the standard). In contrast, the loudness-summation explanation predicts that MMN will be elicited by tones followed by the shortest SOA, because these tones are perceived as the softest within the sequence and, as was mentioned above, when sounds vary in some auditory feature, extreme cases (on both sides of the center) elicit MMN (Huotilainen et al., 1993; Winkler et al., 1990). Thus the loudness-summation explanation can be separated also from these extended versions of the temporal-structure-violation explanation of omission MMN.
In addition, we tested in yet another way whether or not temporal expectation plays a role in the elicitation of omission MMN. For this, we added a condition in which a visual stimulus appeared synchronously with each sound as well as in the exact moment when the omitted sound should have appeared. If temporal expectation of the sounds helps the processes leading to MMN elicitation by sound omissions, then the effects of randomizing the SOA can be avoided or attenuated in the audiovisual compared with the auditory-only condition.
Method
Participants and Procedure
Thirteen healthy adult volunteers (5 men, mean age 21.5 years) were studied in the sound-attenuated and electrically shielded chamber of the Institute for Psychology, Budapest. Participants gave written informed consent after the procedures of the experiment were explained to them. Participants were instructed to keep their eyes on the cross on the screen and to ignore the sounds.
Stimuli
Complex tones were summed from three sine-wave tones (frequencies 700, 840, and 1610 Hz, relative intensities 0 dB: – 10 dB: – 20 dB, respectively). Tone duration was 50 ms, including 7.5 ms rise and 7.5 ms fall time, and the intensity was 50 dB (SPL). In the constant-SOA conditions, the stimulus onset asynchrony was 150 ms.
In the random-SOA conditions, SOAs of 117, 133, 150, 167, 183, 200, and 217 ms (values adjusted to the screen refresh periods because of the audiovisual conditions) were used with equal probability in randomized order. In all conditions, tone omissions occurred pseudorandomly within the sequence with 0.1 probability, excluding the possibility of two omissions occurring in a row.
In the audiovisual conditions, a white cross (20 mm height and 20 mm width) was presented together with each tone. The cross appeared over a black background at the center of a computer screen placed 0.65 m in front of the participant. During the interstimulus interval, the cross turned gray at 50% of the full intensity. The cross was white also for omitted tones, thus marking the time when the tone should have appeared. In the auditory-only conditions the cross remained white throughout the stimulus blocks.
During the experiment, participants were presented with four blocks of 2000 stimuli, each corresponding to one of the four conditions. The order of four blocks was randomized across participants.
Data Acquisition and Analysis
Electroencephalogram (EEG) was recorded from scalp locations at Fz, Cz, Pz, F3, F4, C3, C4, P3, P4 (10–20 system) and from the left and right mastoids (Lm and Rm, respectively) with Ag-AgCl electrodes, referred to the common reference electrode placed on the tip of the nose. Horizontal and vertical electrooculogram (HEOG and VEOG, respectively) were measured with two pairs of bipolarly connected electrodes. HEOG was recorded between electrodes placed lateral to the outer canthi of the two eyes, VEOG between electrodes below and above right eye. After amplification (band pass 0.1–100 Hz, – 3 dB) the signals were digitized with a sampling frequency of 250 Hz.
EEG signals were off-line low-pass filtered below 20 Hz and epochs of 600 ms duration (including 100 ms prestimulus interval) were averaged separately for each condition and stimulus class. Omission-related responses were evaluated from epochs triggered by the preceding standard stimulus. This allowed us to correctly align the omission-related responses elicited in the random-SOA condition and to compare them with the responses elicited in the constant-SOA condition. Epochs with an amplitude change exceeding 100 μVat any EEG or EOG channel were omitted from processing. The MMN response was estimated by subtracting the response elicited by the standard stimulus events from the corresponding omission response. To estimate the amplitude of the full MMN response, including its polarity-reversed part appearing at the mastoids, ERPs were rereferenced to the average of the signals measured at the two mastoids.
Amplitude measurements were referred to the average voltage in the100- to 0-ms interval relative to the onset of the tone preceding the omission (baseline). MMN and P3a amplitudes were measured from windows of 20 ms duration centered on the negative and positive (MMN and P3a, respectively) difference peaks found in the grand-average waveforms. Elicitation of these components was tested with Student's t test. The effects of the experimental conditions on the MMN and P3a amplitude and latency were tested with two-way dependent repeated measures ANOVAs (Timing [constant-SOA vs. random-SOA] × Stimulation [auditory-only vs. audiovisual]). When testing the effects of the poststimulus SOA on the standard-stimulus responses (only for the random-SOA auditory-only condition), some waveforms showed no MMN- or P3a-like deflections. Therefore, MMN and P3a were assessed from local minima in the 200–300 ms and local maxima in the 280–400 ms latency ranges, respectively. Effects of the prestimulus SOA were statistically tested with one-way ANOVAs and planned comparisons. Where applicable, sphericity violations were corrected using the Greenhouse–Geisser method (ε values and the uncorrected degrees of freedom are given together with the test results).
Results
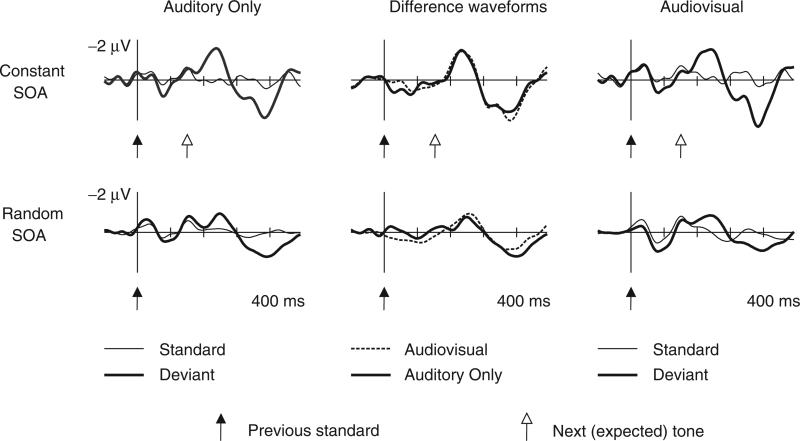
Figure 1 presents the grand-averaged ERP responses for standard and omitted stimuli, together with the respective difference waveforms. Significant MMN and P3a responses were found in all four experimental conditions (Student's t tests were significant at p<.01 for MMN in all four conditions and for P3a in the random-SOA conditions, p<.05 for P3a in the constant-SOA conditions). In individual participants, MMN peaked in the 250–280 ms latency range calculated from the onset of the standard stimulus preceding the omission (100–130 ms from the onset of the omission in the constant-SOA conditions), whereas P3a peaked in the 330–370 ms latency range (180–220 ms from the onset of omission in the constant-SOA conditions; see Table 1 for the grand-averaged MMN and P3a amplitudes and peak latencies measured in all four conditions). MMN amplitudes were significantly higher and latencies significantly shorter in the constant- than the random-SOA conditions, F(1,12)=8.99, p<.02 and F(1,12)=58.90, p<.01 for the MMN amplitude and latency, respectively; no other main effects or interactions reached significance. The latency of the P3a response was significantly shorter in the constant- than in the random-SOA conditions, F(1,12)=12.43, p<.01; no other main effects or interactions reached significance. The P3a amplitude was not significantly affected by the current experimental variables.
Figure 1.
Grand–averaged (N=13) frontal (Fz) ERP responses elicited by the test tones (thin lines) and tone omissions (thick lines) in all four experimental conditions: constant-SOA in the top row, random-SOA in the bottom row, auditory-only in the left column, and audiovisual in the right column. The deviant-minus-standard (omission-minus-tone) difference waveforms are shown in the central column. The difference waveforms are overplotted for the auditory-only (continuous line) and audiovisual conditions (dashed line), separately for the constant-SOA and the random-SOA conditions. The zero time point coincides with the delivery of a tone (standard) or the delivery of the tone preceding the omission (deviant). For the constant-SOA condition, the time of the delivery of the next tone (expected time of delivery of the omitted tone) is marked with empty arrows (the time of delivering the next tone varies in the random-SOA condition).
Table 1.
Grand–Averaged (N = 13) Frontal (Fz) MMN and P3a Amplitude (in Microvolts) and Latency Measurements (in Milliseconds) in the Four Experimental Conditions
| Condition |
||||
|---|---|---|---|---|
| Constant SOA |
Random SOA |
|||
| Auditory–only | Audiovisual | Auditory–only | Audiovisual | |
| MMN | ||||
| Amplitude | –1.6 (0.8)** | –1.4 (0.9)** | –0.7 (0.7)** | –1.1 (0.9)** |
| Latency | 254.8 (25.6) | 244.6 (26.9) | 274.0 (25.9) | 274.8 (24.1) |
| P3a | ||||
| Amplitude | 1.1 (1.8)* | 1.4 (1.6)* | 1.2 (1.0)** | 0.9 (0.7)** |
| Latency | 342.2 (39.1) | 337.2 (43.6) | 363.1 (41.0) | 360.0 (39.9) |
Note: Standard deviations are given in parentheses.
Amplitudes that are significantly different from zero at p<.05 or p<.01, respectively.
Amplitudes that are significantly different from zero at p<.05 or p<.01, respectively.
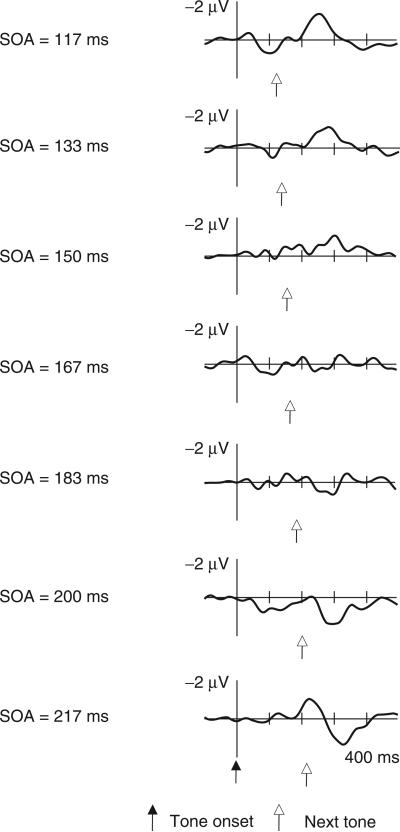
Figure 2 presents the grand-averaged ERP responses elicited by standard tones followed by different SOAs in the random–SOA auditory-only condition. Low-amplitude responses were elicited by standards followed by intermediate SOAs, whereas standards followed by the extreme SOAs (both at the short and long end of the range) elicited a negative deflection followed by a positive one (see Table 2). On the basis of our previous study, which varied the intensity of the standard tones in a passive oddball design (Winkler et al., 1990), we expected the extreme substandards to elicit MMN and, possibly, also P3a. Therefore, we identified the succession of negative and positive waves observed for extreme SOAs as MMN and P3a, respectively, and conducted one-way ANOVA tests, separately for the MMN and P3a peak latencies and amplitudes. The MMN and P3a amplitudes were significantly affected by the poststandard SOA, F(6,72)=7.41, p<.01, ε=.64 and F(6,72)=7.64, p<.01, ε=.53, for the MMN and the P3a peak amplitudes, respectively. Planned comparisons were used to compare the amplitude measurements from the extreme SOAs with those obtained for medium–duration SOAs. The MMN amplitude was significantly higher both at the shortest and at the longest SOA than at intermediate SOAs, at which MMN was not elicited, F(1,12)=10.36, p<.01 and F(1,12)=7.38, p<.02, for the shortest and the longest SOA versus the three medium–duration SOAs, respectively. The P3a peak amplitude was significantly higher at the longest than at intermediate SOAs, F(1,12)=21.75, p<.01, whereas the similar planned comparison between the shortest and the medium duration SOAs failed to reach significance, F(1,12)=0.95, p=.35. Both the MMN and P3a peak latencies were significantly shorter at the long compared with the short SOAs, F(6,72)=72.89, p<.01, ε=.58 and F(6,72)=309.68, p<.01, ε=.74 for the MMN and the P3a peak latencies, respectively.
Figure 2.
Grand-averaged frontal (Fz) ERP responses elicited by tones preceding different SOAs in the random-SOA auditory-only condition. The zero time point coincides with the delivery of the tone. The time of delivery of the next tone is marked with empty arrows.
Table 2.
Grand-Averaged (N = 13) Frontal (Fz) MMN and P3a Amplitude (in Microvolts) and Latency Measurements (in Milliseconds) as a Function of the Poststimulus SOA in the Random-SOA Auditory-Only Condition
| SOA |
|||||||
|---|---|---|---|---|---|---|---|
| 117 ms | 133 ms | 150 ms | 167 ms | 183 ms | 200 ms | 217 ms | |
| MMN | |||||||
| Amplitude | –1.3 (1.2)** | –1.0 (0.6)** | –0.5 (0.8)* | 0.4 (1.0) | 0.4 (1.1) | –0.1 (0.6) | –1.1 (1.2)** |
| Latency | 262.2 (4.5) | 269.2 (7.9) | 250.8 (6.0) | 248.3 (8.1) | 243.7 (6.0) | 229.2 (6.2) | 225.9 (6.5) |
| P3a | |||||||
| Amplitude | 0.5 (0.7)* | 0.1 (1.1) | –0.3 (0.6) | –0.1 (0.8) | –0.3 (0.7) | 1.5 (1.4)** | 1.1 (0.7)** |
| Latency | 386.8 (6.8) | 386.2 (7.2) | 344.3 (7.7) | 348.3 (5.3) | 344.3 (7.7) | 305.5 (7.6) | 308.6 (6.5) |
Note: Standard deviations are given in parentheses.
Amplitudes that are significantly different from zero at p<.05 or p<.01, respectively.
Amplitudes that are significantly different from zero at p<.05 or p<.01, respectively.
Note that the responses elicited by the “standard” tones preceding the longest SOA (217 ms) were similar to those elicited by tones preceding omissions (see Figure 2). This suggests that the 217-ms SOA fell outside the temporal window of integration. As a result, these “standards” may have behaved as omissions, possibly increasing the effective probability of omissions to about 0.23 (compared to 0.1).
Discussion
Significant MMNs were found in all four experimental conditions. The MMN amplitude was significantly lower with the random stimulus onset asynchrony condition than with the isochronous stimulus presentation mode. Tones followed by the shortest and longest SOAs elicited MMN and, possibly, also the P3a response. The timing of stimulus presentation affected the amplitude but not the elicitation of the MMN component. Concurrent presentation of a visual stimulus, which marked the expected timing of the omitted tones, had no significant effect on the ERP responses.
The elicitation of MMN in the random-SOA auditory-only condition suggests that loudness summation plays a role in the elicitation of the mismatch negativity response by stimulus omissions when the SOA is shorter than the duration of the temporal window of integration. The explanation based on the constancy of the temporal structure of the contents of the temporal window of integration (the original version of the temporal-structure-violation explanation) is not supported by the current results, because the temporal structure of the stimulation varied, and, therefore, the contents of the temporal window of integration segments assumed to be triggered by each tone also varied.
The results obtained for standard stimuli in the random-SOA condition rule out both of the extended versions of the temporal-structure-violation explanation of omission MMN: the abstract regularity of the temporal structure of the temporal window of integration segments and the decreased temporal sensitivity within the temporal window of integration explanations (see the introduction). If the regularity extracted from the temporal window of integration segment stated only that two tones should fall within a temporal window of integration period or that SOAs lower than the length of temporal window of integration cannot be distinguished, then no MMN should have been elicited with the shortest SOA, because the temporal window of integration triggered by the preceding tone covered two tones and/or the SOA should have been treated the same as the majority of the SOAs (which have been shorter than the temporal window of integration duration). Therefore, the shortest SOA should have been processed as a standard event eliciting no MMN. However, MMN was elicited with the shortest SOA in the random-SOA condition. The argument against the abstract temporal-structure explanation of omission MMN is supported by the results of Paavilainen, Simola, Jaramillo, Näätänen, and Winkler (2001), who found no MMN elicited by extreme members of an abstract category, which were presented in majority within the sound sequence. In contrast, rare stimuli that did not fit the abstract category elicited MMN, even though acoustically they differed less from the center of the abstract category than the extreme standards did. That is, if an abstract structural regularity emerged in the random-SOA condition, no MMN should have been elicited by tones followed by the shortest SOA.
The loudness-summation explanation of omission MMN can account for all of the current MMN results. According to this explanation, the tones delivered in the random–SOA conditions varied in their perceived intensity. Thus the situation was very similar to the one set up by Winkler et al. (1990), who varied the intensity of short tones, which were presented with an SOA that was longer than the duration of the temporal window of integration. In all respects, the current results are consistent with those of Winkler et al. In both experiments, MMN was elicited by tones whose loudness substantially differed from the range within which most tones varied as well as by the extreme exemplars of the varying standard tones. Therefore we suggest that omission MMN is at least partly based on violating the regularity of perceived loudness.
The standard-stimulus responses yielded another interesting result. Whereas the MMN elicited with the shortest SOA was probably time locked to the tone following the SOA (262 ms from the onset of the preceding tone, 145 ms from the onset of the tone following the 117-ms SOA), this was not the case for the longest SOA (226 ms from the onset of the preceding tone, whereas the SOA was altogether 217 ms). Because the longest SOA fell outside the temporal window of integration, it behaved similarly to the omissions, eliciting an MMN by violating the loudness regularity. Because this MMN is elicited by the tone that preceded this SOA (which is perceived as louder than most other tones), the MMN response is time locked to the tone preceding the SOA, not to the tone following it. In contrast, with the shortest SOA, loudness summation for the tone preceding the SOA is terminated by the tone following it. Thus, in this case, the moment at which the loudness of the preceding tone can be evaluated (and from which deviation can be detected) is at the onset of the tone following the SOA. Therefore, MMN is time locked to the onset of the tone following the shortest SOA. The difference between the two cases is that with the long SOAs, evidence about deviance is gradually accumulated during the temporal integration process until it exceeds some difference threshold, whereas with the short SOAs, there is a definite point in time (some constant time after the onset of the next abruptly commencing sound, when the integration process is terminated) at which features analysis is complete and deviance is evaluated. The MMN elicited with the shortest SOA may also be explained by violating a temporal regularity. It has been previously found for SOAs longer than the temporal window of integration that SOA decrements elicit MMN, whereas SOA increments do not (Ford & Hillyard, 1981; Yabe et al., 1999). If MMN at the shortest SOA was elicited due to the shortening of the SOA, then again it should be time locked to the post-SOA tone, and its latency cannot be compared with the MMN latency found with the longest SOA, because the two MMNs are elicited by different processes. Please note, however, that although regarding only the results obtained for standards in the random–SOA condition, these can also be explained by temporal expectation (i.e., by assuming that some intermediate SOA value acted as the prototype), the temporal expectation hypothesis cannot explain why no omission MMN is elicited when the standard SOA exceeds the duration of the temporal window of integration. Furthermore, strengthening temporal expectation by concurrent visual stimuli had no effect on the current results.
Finally, one may consider whether the negative differences elicited by the extreme substandards reflected the variation of the N1 amplitude observed by Loveless and his colleagues (1996). However, the amplitude of that N1 m subcomponent showed a reversed U-shaped curve as the function of the SOA, whereas the current negative difference showed the opposite U–shaped function. Further, we found a shortening of the peak latency of the negative difference from the shortest to the longest SOA, which also argues against the N1 explanation of this response, whereas similar MMN latency differences were found for shorter versus longer duration deviants (Winkler et al., 1996). In addition, the amount of deviation is probably larger for the shortest than for the longest SOA substandard due to the nonlinear intensity contour of the persisting stimulus aftereffect (Plomp, 1964), and the MMN latency has been found to decrease with increasing amounts of deviations (Näätänen, 1990).
In summary, the results obtained for standards in the random-SOA condition ruled out the extended versions of the temporal-structure-violation explanation (not all temporal window of integration segments with two tones were treated as standards and the shortest SOA was distinguished from the medium SOAs), whereas they are fully compatible with the loudness-summation explanation of the omission MMN. Although these results bring up the possibility that some temporal regularities were also detected (the violation of which could lead to MMN), the omission MMN could not be explained by these regularities alone.
The amplitude of the MMN elicited by tone omissions decreased and the peak latency increased in the random-SOA compared with the constant-SOA conditions. One possible explanation of this effect is that a part of the omission-elicited MMN response in the constant-SOA conditions was caused by violating a temporal regularity. However, the same decrease of the MMN amplitude was also observed by Winkler et al. (1990), when comparing between the constant- and varying-standard conditions. In line with the explanation offered by Winkler et al. it is possible that the decrease of the MMN amplitude when the standard stimulus varies, compared with a constant standard stimulus, reflects a decrease of the predictive power of the regularity representation (the standard), because variance among the standard stimuli reduces the sharpness of the predictions that can be drawn from such regularities. The MMN amplitude difference may also have resulted from the MMN response elicited by some of the standards in the random-SOA condition (i.e., those that followed the short and long SOAs), because the MMN amplitude has been estimated from the deviant-minus-standard difference waveforms. This latter explanation receives further support from the observation that standards followed by the longest SOA probably behaved similarly to omissions. Thus the probability of omissions was increased to about 0.23 in the random-SOA condition, compared to 0.1 in the constant-SOA condition. Increasing the deviant-even probability reduces the MMN amplitude (Näätänen, Simpson, & Loveless, 1982).
The fact that synchronous presentation of visual information did not affect the MMN amplitude probably reflects that temporal expectation does not play a significant role in the elicitation of the omission MMN. However, it is also possible that visual information has no predictive value for the MMN-generating processes, as was also found by Ritter, Sussman, Deacon, Cowan, and Vaughan (1999) and Sussman, Winkler, and Schröger (2003).
In summary, the current results strongly suggest that omission MMN at least partly reflects loudness summation within the temporal integration period. Subsequent research may test whether omission MMN can be fully explained by violation of a loudness regularity or other regularities are also violated along with it.
Acknowledgments
This research was supported by the Hungarian National Research Fund (OTKA T048383) and the National Institutes of Health (DC04263).
References
- Békeśy G. von. Über die Hörsamkeit der Ein– und Ausschwingvorgänge mit Berücksichtigung der Raumakustik. Annalen der Physik. 1933;16:844–860. [Google Scholar]
- Cowan N. On short and long auditory stores. Psychological Bulletin. 1984;96:341–370. [PubMed] [Google Scholar]
- Cowan N. Auditory sensory storage in relation to the growth of sensation and acoustic information extraction. Journal of Experimental Psychology: Human Perception and Performance. 1987;13:204–215. doi: 10.1037//0096-1523.13.2.204. [DOI] [PubMed] [Google Scholar]
- Cowan N. Attention and memory. An integrated framework. Oxford University Press; Oxford: 1995. [Google Scholar]
- Czigler I, Winkler I. Preattentive auditory change detection relies on unitary sensory memory representations. NeuroReport. 1996;7:2413–2417. doi: 10.1097/00001756-199611040-00002. [DOI] [PubMed] [Google Scholar]
- Efron R. Effects of stimulus duration on perceptual onset and offset latencies. Perception and Psychophysics. 1970a;8:231–234. [Google Scholar]
- Efron R. The relationship between the duration of a stimulus and the duration of a perception. Neuropsychologia. 1970b;8:37–55. doi: 10.1016/0028-3932(70)90024-2. [DOI] [PubMed] [Google Scholar]
- Efron R. The minimum duration of a perception. Neuropsychologia. 1970c;8:57–63. doi: 10.1016/0028-3932(70)90025-4. [DOI] [PubMed] [Google Scholar]
- Ford JM, Hillyard SA. Event related potentials, ERPs, to interruptions of steady rhythm. Psychophysiology. 1981;18:322–330. doi: 10.1111/j.1469-8986.1981.tb03043.x. [DOI] [PubMed] [Google Scholar]
- Green DM, Birdsall TG, Tanner WP. Signal detection as a function of signal intensity and duration. Journal of the Acoustical Society of America. 1957;29:523–531. [Google Scholar]
- Huotilainen M, Ilmoniemi RJ, Lavikainen J, Tiitinen H, Alho K, Sinkkonen J, Knuutila J, et al. Interaction between representations of different features of auditory sensory memory. Neuro-Report. 1993;4:1279–1281. doi: 10.1097/00001756-199309000-00018. [DOI] [PubMed] [Google Scholar]
- Loveless N, Levänen S, Jousmäki V, Sams M, Hari R. Temporal integration in auditory sensory memory: Neuromagnetic evidence. Electroencephalography and Clinical Neurophysiology. 1996;100:220–228. doi: 10.1016/0168-5597(95)00271-5. [DOI] [PubMed] [Google Scholar]
- Massaro DW. Experimental psychology and information processing. Rand McNally; Chicago: 1975. [Google Scholar]
- Miller GA. The perception of short bursts of noise. Journal of the Acoustical Society of America. 1948;20:160–170. [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral and Brain Sciences. 1990;13:201–288. [Google Scholar]
- Näätänen R, Simpson M, Loveless NE. Stimulus deviance and evoked potentials. Biological Psychology. 1982;14:53–98. doi: 10.1016/0301-0511(82)90017-5. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Winkler I. The concept of auditory representation in cognitive neuroscience. Psychological Bulletin. 1999;125:826–859. doi: 10.1037/0033-2909.125.6.826. [DOI] [PubMed] [Google Scholar]
- Nordby H, Roth WT, Pfefferbaum A. Event-related potentials to time-deviant and pitch-deviant tones. Psychophysiology. 1988;25:249–261. doi: 10.1111/j.1469-8986.1988.tb01238.x. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Simola J, Jaramillo M, Näätänen R, Winkler I. Preattentive extraction of abstract feature conjunctions from auditory stimulation as reflected by the mismatch negativity (MMN). Psychophysiology. 2001;38:359–365. [PubMed] [Google Scholar]
- Picton TW, Alain C, Otten L, Ritter W, Achin A. Mismatch negativity: Different water in the same river. Audiology and Neuro-Otology. 2000;5:111–139. doi: 10.1159/000013875. [DOI] [PubMed] [Google Scholar]
- Plomp R. Rate of decay of auditory sensation. Journal of the Acoustical Society of America. 1964;36:277–282. [Google Scholar]
- Ritter W, Sussman E, Deacon D, Cowan N, Vaughan HG., Jr. Two cognitive systems simultaneously prepared for opposite events. Psychophysiology. 1999;36:835–838. [PubMed] [Google Scholar]
- Sams M, Hari R, Rif J, Knuutila J. The human auditory sensory memory trace persists about 10 s: Neuromagnetic evidence. Journal of Cognitive Neuroscience. 1993;5:363–370. doi: 10.1162/jocn.1993.5.3.363. [DOI] [PubMed] [Google Scholar]
- Scharf B. Loudness. In: Carterette EC, Friedman MD, editors. Handbook of perception. Vol. 4. Academic Press; New York: 1978. pp. 187–242. [Google Scholar]
- Sussman E, Winkler I, Ritter W, Alho K, Näätänen R. Temporal integration of auditory stimulus deviance as reflected by the mismatch negativity. Neuroscience Letters. 1999;264:161–164. doi: 10.1016/s0304-3940(99)00214-1. [DOI] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Schröger E. Top-down control over involuntary attention-switching in the auditory modality. Psychonomic Bulletin & Review. 2003;10:630–637. doi: 10.3758/bf03196525. [DOI] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Wang WJ. MMN and attention: Competition for deviance detection. Psychophysiology. 2003;40:430–435. doi: 10.1111/1469-8986.00045. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Saarinen J, Paavilainen P, Danilova N, Näätänen R. Temporal integration of auditory information in sensory memory as reflected by the mismatch negativity. Biological Psychology. 1994;38:157–167. doi: 10.1016/0301-0511(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Winkler I, Karmos G, Näätänen R. Adaptive modeling of the unattended acoustic environment reflected in the mismatch negativity event–related potential. Brain Research. 1996;742:239–252. doi: 10.1016/s0006-8993(96)01008-6. [DOI] [PubMed] [Google Scholar]
- Winkler I, Näätänen R. The effects of auditory backward masking on event-related brain potentials. In: Karmos G, Csépe V, Czigler I, Molnár M, Desmedt J, editors. Perspectives of ERP research. EEG Supplement. Vol. 44. Elsevier Science B.V.; Amsterdam: 1994. pp. 185–189. [PubMed] [Google Scholar]
- Winkler I, Reinikainen K, Näätänen R. Event related brain potentials reflect traces of the echoic memory in humans. Perception & Psychophysics. 1993;53:443–449. doi: 10.3758/bf03206788. [DOI] [PubMed] [Google Scholar]
- Winkler I, Paavilainen P, Alho K, Reinikainen K, Sams M, Näätänen R. The effect of small variation of the frequent auditory stimulus on the event-related brain potential to the infrequent stimulus. Psychophysiology. 1990;27:228–235. doi: 10.1111/j.1469-8986.1990.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Winkler I, Tervaniemi M, Näätänen R. Two separate codes for missing fundamental pitch in the auditory cortex. Journal of the Acoustical Society of America. 1997;102:1072–1082. doi: 10.1121/1.419860. [DOI] [PubMed] [Google Scholar]
- Yabe H, Tervaniemi M, Reinikainen K, Näätänen R. Temporal window of integration revealed by MMN to sound omission. NeuroReport. 1997;8:1971–1974. doi: 10.1097/00001756-199705260-00035. [DOI] [PubMed] [Google Scholar]
- Yabe H, Tervaniemi M, Sinkkonen J, Huotilainen M, Ilmoniemi RJ, Näätänen R. The temporal window of integration of auditory information in the human brain. Psychophysiology. 1998;35:615–619. doi: 10.1017/s0048577298000183. [DOI] [PubMed] [Google Scholar]
- Yabe H, Sato Y, Sutoh T, Hiruma T, Shinozaki N, Nashida T, et al. The duration of the integrating windows in auditory sensory memory. Electroencephalography and Clinical Neurophysiology Evoked Potentials and Magnetic Fields, Supplement. 1999;49:166–169. [PubMed] [Google Scholar]
- Zwislocki JJ. Temporal summation of loudness: An analysis. Journal of the Acoustical Society of America. 1969;46:431–440. doi: 10.1121/1.1911708. [DOI] [PubMed] [Google Scholar]