Abstract
Introduction
The rate-limiting step in orthodontic treatment is often the rapidity with which teeth move. Using biological agents to modify the rate of tooth movement has been shown to be effective in animals. Relaxin is a hormone present in both males and females. Its main action is to increase the turnover of fibrous connective tissues. Thus, relaxin might increase the amount and rate of tooth movement through its effect on the periodontal ligament (PDL). The purpose of this study was to measure the effect of relaxin on orthodontic tooth movement and PDL structures.
Methods
Bilateral orthodontic appliances designed to tip maxillary molars mesially with a force of 40 cN were placed in 96 rats. At day 0, the animals were randomized to either relaxin or vehicle treatment. Twelve rats in each group were killed at 2, 4, 7, and 9 days after appliance activation. Cephalograms were taken at appliance placement and when the rats were killed. Tooth movement was measured cephalometrically in relation to palatal implants. Fractal analysis and visual analog scale assessments were used to evaluate the effect of relaxin on PDL fiber organization at the tension sites in histologic sections. The in-vitro testing for PDL mechanical strength and tooth mobility was performed by using tissue from an additional 20 rats that had previously received the same relaxin or vehicle treatments for 1 or 3 days (n = 5).
Results
Both groups had statistically significant tooth movement as functions of time. However, relaxin did not stimulate significantly greater or more rapid tooth movement. Fractal and visual analog scale analyses implied that relaxin reduced PDL fiber organization. In-vitro mechanical testing and tooth mobility assessments indicated that the PDL of the mandibular incisors in the relaxin-treated rats had reduced yield load, strain, and stiffness. Moreover, the range of tooth mobility of the maxillary first molars increased to 130% to 170%, over vehicle-treated rats at day 1.
Conclusions
Human relaxin does not accelerate orthodontic tooth movement in rats; it can reduce the level of PDL organization, reduce PDL mechanical strength, and increase tooth mobility at early time points.
The orthodontic literature includes many reports on force systems that achieve the most ideal tooth movement relative to various needs. Also, there is interest in the combination of biologic mediators with traditional force systems to modify the rate and amount of tooth movement or relapse and to enhance the anchorage potential of specific teeth. Most of this research has focused on agents that influence bone metabolism, such as parathyroid hormone, estrogen, and bisphosphonates.1-3
Relaxin is a hormone in the insulin/relaxin family of structurally related hormones. It has been shown to bind to receptors that are part of the leucine rich repeat G-protein receptor family (LGR7 and LGR8).4 Relaxin is produced in many mammals during pregnancy5; it promotes cervical softening and elongation of interpubic ligaments in mice and cattle. Furthermore, relaxin influences many other physiologic processes such as collagen turnover, angiogenesis, and antifibrosis in both males and females. The latter actions suggested that relaxin might influence orthodontic tooth movement through alterations of the periodontal ligament (PDL). Therefore, our purposes in this study were to evaluate whether relaxin affects orthodontic tooth movement, and the organization and the physical properties of the PDL.
MATERIAL AND METHODS
Animals and procedures
One hundred twenty 45-day-old Sprague-Dawley rats were used. Male rats were chosen because they have relaxin receptors and do not have estrus cycles. They were acclimatized for at least 2 days under experimental conditions, including housing in plastic cages, receiving a diet of ground laboratory chow and distilled water ad libitum, and keeping on a standard 12-hour light/dark cycle. All animal manipulations, including killing, were performed at the same time of day to control for circadian rhythm effects on stem cells and bone remodeling.6,7 Weights were recorded, and anesthesia was given with intramuscular injections of ketamine (76 mg per kilogram) and xylazine (4.8 mg per kilogram). Three days before relaxin administration and orthodontic appliance activation, all 4 incisors were pinned by using a modification of the method described by Beertsen and Hoeben8 and Liu et al9 to prevent further eruption and to minimize movement of the anchorage. The mandibular incisors were reduced slightly to prevent appliance damage, and the mandibular first molars were extracted. Two barb broach implants (barbed broaches, #6, VDW Dental, München, Germany) were placed under the mucosa just palatal to the 2 molars. These served as superimpositional landmarks. The animals were then allowed to recover for 3 days, while wound healing and weight gain were monitored.
On day 0, the orthodontic appliances were placed and activated. Also, pumps were implanted. Each animal was positioned in a head restrainer, and orthodontic springs were placed bilaterally. One end of a nickel-titanium closed-coil spring (light, #10-000-06, GAC, Central Islip, NY) was attached to a ligature bonded to the occlusal surface of the molar, and the other end was attached to a 40-g suspended weight. The anterior end of the coil was then bonded with light-curing adhesive to the acid-etched lateral surface of the maxillary incisors followed by removal of the weight. This method ensured a precise initial orthodontic force, designed to tip the maxillary first molars to the mesial side. The direction of force was previously categorized as primarily compression on the mesial side and tension on the distal surfaces of the molar roots.9,10
Relaxin administration
Recombinant human relaxin (H2 gene product) or vehicle (20 mmoL per liter sodium acetate, pH 5.0) (Connectics, Palo Alto, Calif) was administered with Alzet osmotic minipumps (model 2001, Alzet, Cupertino, Calif) as previously described.9,11 The pumps were filled with relaxin or vehicle and implanted subcutaneously on the back slightly posterior to the scapulae. The contents of the pump were delivered into the local subcutaneous space, and absorption of the compound by local capillaries resulted in systemic administration. Immediately after the orthodontic appliance was placed and activated, the animal's back was shaved under aseptic conditions, and the skin over the implantation site was sterilized with 20% betadine (Purdue, Frederick, Conn). A small midscapular incision was made, and a hemostat was placed in the incision to create a pocket for the pump. The pocket was large enough (about 1 cm longer than the pump) to allow some free movement. The filled pump was inserted into the pocket delivery portal first, and the wound was closed with sutures. The pump delivered relaxin at a rate of 5.3 μg per hour per kilogram. This dose was shown to result in a blood concentration of approximately 150 ng per milliliter.12
The rats were randomly assigned to the relaxin and the vehicle groups, and to 1 of 4 kill times after initial orthodontic appliance activation (days 2, 4, 7, and 9). These time intervals were shown to produce significant changes in tooth movement and bone remodeling so that differences between the groups could be detected.10,13,14 Fifteen rats were assigned to each group at each time point, resulting in a total of 120 rats in the study; 24 rats were not included because of anesthetic death, appliance damage, or unmeasurable radiographs. Therefore, measurements from 96 rats were eventually included in the data set.
After these procedures, powdered and moist food was provided. The animals were killed under anesthesia by cardiac perfusion at each specified time interval. The Institutional Animal Care and Use Committee of the University of Washington approved all animal manipulation procedures.
Tooth movement assessment
Tooth movement was assessed cephalometrically by determining the change in molar location along the molar-incisor vector by using the submucosal palatal implants as superimpositional landmarks. Each rat was positioned with the mandible down in the cephalometric head-holding device with an intraoral film packet placed under the mandible. Radiographic exposures were taken vertically from a distance of 127 cm. Two radiographs were taken at appliance placement and at the end of each time point. Thus, bilateral maxillary first molar movements in each rat were measured twice: at activation and at kill.
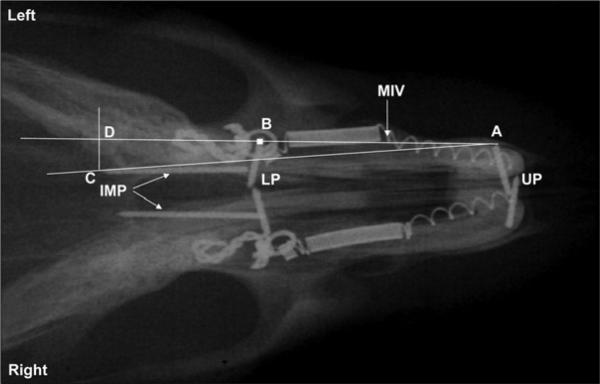
The radiographs were first scanned onto a computer (Apple PowerBook G4 laptop, Cupertino, Calif) at 600 dpi by using VueScan software (Hamrick Software, Phoenix, Ariz). One investigator (M.S.M.), who was blinded to treatment groups, performed all scanning and measuring of radiographs. Scanned images were adjusted in Adobe Photoshop (Adobe Systems, San Jose, Calif) by changing the color to grayscale and saving as TIFF files. This allowed the radiographic files to be opened by the Scion Image software (Scion, Frederick, MD), which was used to measure and compute tooth movement. The following landmarks were used (Fig 1): the tip of the maxillary incisor pin (A), the center of the first molar crown (B), and the end point of the palatal implant (C). A piece of wire was attached to each film packet before exposure as a calibration device (known width, 0.44 mm). The calibration scale was reset for each radiograph measured.
Fig 1.
Cepahalometric analysis for measurement of tooth movement. UP and LP, horizontal pins in roots of upper and lower incisors; IMP, sagittal palatal implants; A, most lateral tip of left UP; B, center of left bonded ligature; C, most posterior tip of the left IMP; D, intersection on line AB created by line drawn from point C perpendicular to line AC. Length B-D represents sagittal distance changes caused by forward tooth movement of molar. Line AB indicates molar-incisor vector (MIV).
The center of each first molar crown was first located on the radiographs. To consistently choose the same place as the center, the preradiographs and postradiographs were scanned together in the same file. The first center was marked, and this image was copied and pasted onto the subsequent radiographs of the same rat.
On each radiograph (Fig 1), a line was drawn from the most lateral point of the incisor pin to the center of the molar (molar-incisor vector, MIV). Another line was drawn from the lateral point of the pin to the most posterior point on the palatal implant. A third line was drawn perpendicular to the first line from the end of the palatal implant, intersecting the MIV. Thus, the distance along the MIV, from the center of the tooth (B)to the intersecting point (D) increased with mesial movement of the molar during the various time intervals.
Histologic evaluations
The maxillary molar segments from both sides were harvested. All segments from the left side were processed for general histology, including trimming all exposed crowns and decalcifying specimens by immersion in Immunocal and Decal Arrest (Decal Chemical, Tallman, NY) for 3 days. The specimens were embedded in paraffin and sectioned cross sectionally at a thickness of 8 μm. These sections were stained with hematoxylin and eosin.
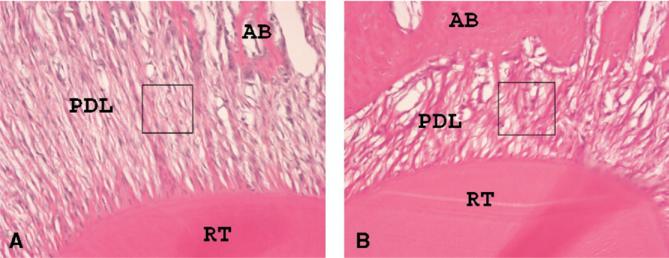
Histological sections at the root trunk of the maxillary first molar were examined by using the fractal analysis software developed by Chen et al.15 The tension side of the root trunk (distal) was chosen as the region of interest (ROI) for this analysis because it shows the most alignment of the principal PDL fibers during tipping. Histological sections from the root trunk were available from 34 specimens (Fig 2). A 5 × 5-mm ROI was cropped from the PDL images of these 34 molars (17 from each group). These images were converted to grayscale by using Adobe Photoshop 7.0 and saved as a bitmap file. All ROIs were selected from approximately the same location with the same dimensions (360 × 360 pixels). The fractal analysis was used to assess the level of complexity of the PDL structure at these ROIs.16
Fig 2.
Samples of hematoxylin and eosin-stained histologic sections. Cervical region and tension side were chosen for analysis; 5 × 5 mm ROI was cropped from this area. A, Sample from vehicle-treated rat; B, sample from relaxin-treated rat. RT, tooth root; AB, alveolar bone; PDL, periodontal ligament. Small boxes indicate ROI for fractal analysis.
A visual analog scale (VAS) also was devised to subjectively rate the level of organization of the PDL. Fourteen observers were asked to score the selected PDL images. Images of the rats treated with both the relaxin and the vehicle at days 2 and 7 were presented in random order. Each observer was asked to place a mark, indicating perception of the level of organization, on an 85-mm line anchored by drawings of complete fiber organization (0) and complete fiber disorganization (85). The mean score per picture was determined. The scores for each group at days 2 and 7 were determined.
Mechanical testing and tooth mobility assessment
An additional 20 rats were used to examine PDL physical properties and tooth mobility. Relaxin and vehicle were delivered as described above, and the rats were killed at 1 day and 3 days after the minipump implantation. Thus, there were 5 rats in each treatment/day group.
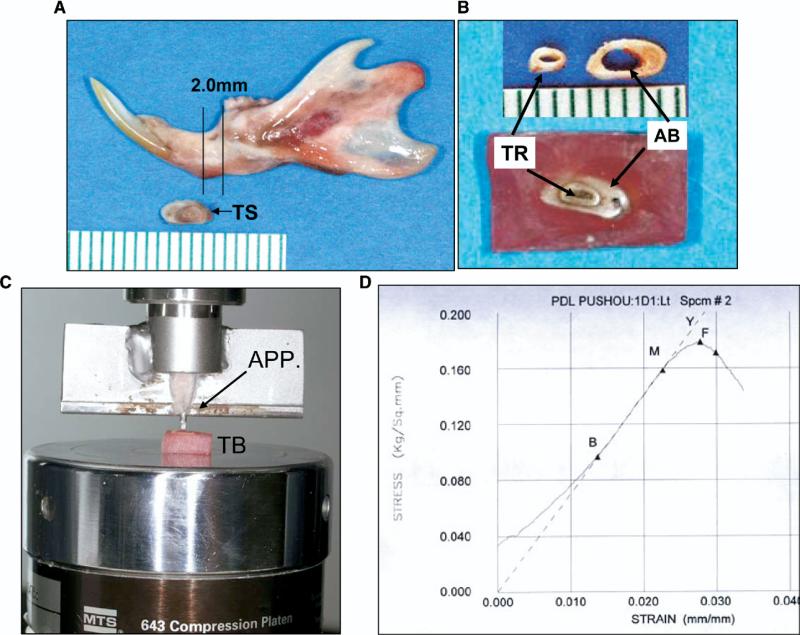
To test the PDL's mechanical strength, a 2-mm-thick transverse section containing the alveolar bone and the root of a mandibular incisor was cut (Fig 3, A) and embedded in orthodontic self-curing resin, with the root intentionally exposed without resin covering (Fig 3, B). A custom-made loading apparatus was connected to a 5-pound loading cell of an MTS machine (Material Testing Systems, Cary, NC) (Fig 3, C). The attached loading tip was a stainless steel rod, 0.8 mm in diameter (slightly smaller than the exposed root section). A continuous compressive load pushed the exposed root section down until failure. Failure was defined as >20% drop from maximal loading stress. Visual inspection after testing showed that failure took place at the PDL without obvious distortion of the tooth root. This procedure produced a stress-strain curve (Fig 3, D) to calculate peak, yield, and failure loads (kg), strains (%) at peak and failure loads, and Young's modulus (stiffness, kg per square millimeter). All samples were fresh and kept moist with 0.9% saline-solution drip during testing.
Fig 3.
PDL mechanical strength testing apparatus. A, Location of test section; B, resin-embedded testing block; C, loading on testing machine; D, strain-stress curve created from testing. TS, testing section; TR, root; AB, alveolar bone; TB, testing block; APP, custom-made loading apparatus. Definitions in stress-strain curve: B-M, steepest slope, indicating Young's modulus (stiffness); Y, yield point: zero slope, indicating transition from elastic to plastic deformation under compressive loading; F, failure point: <20% drop from maximal stress.
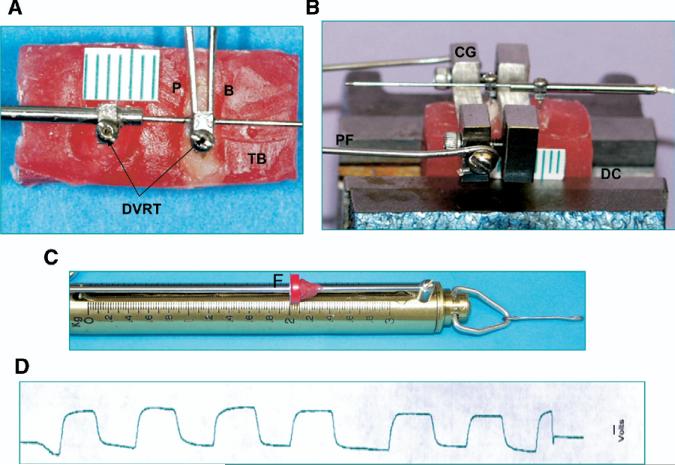
To test tooth mobility, the harvested maxillary molar segments were embedded in an orthodontic self-curing resin block, and the crowns of the second and third molars were trimmed. Two small holes (0.5 mm in diameter) were made in the molar crown and the resin block with a carbide bur. Two barbs of a differential variance reluctant transducer (DVRT) (MicroStrain, Williston, Vt) were inserted into these holes17,18 (Fig 4, A). The block was stabilized with a desk clamp, and the exposed crown of the maxillary first molar was clamped by using a custom-made grip. A repeated 2.0-kg horizontally oriented load was exerted on the crown through a force gauge connected to the grip (Fig 4, B and C). This load wiggled the molar in the buccolingual direction. The signals during molar wiggle were recorded by a computer running AcqKnowledge (Biopac Systems, Goleta, Calif) through a DVRT demodulator, and the range of molar mobility (in millimeters) was measured with a calibration equation (Fig 4, D).
Fig 4.
Tooth mobility testing apparatus: A, embedded molars and device; B, setup; C, force gauge; D, raw recording indicating range of molar palate-buccal mobility produced by 2 kg horizontal load. DVRT, differential variance reluctance transducer; CG, custom-made grip; DC, desk clamp; TB, testing block; P, palatal side; B, buccal side; PF, pulling force; F, force stop.
Error of the method and statistical analysis
The reproducibility of the measurements from tooth movement was assessed in 20 randomly chosen rats by analyzing the difference between duplicate measurements taken at least 14 days apart by 1 investigator (M.S.M.). Measurement error was calculated with Dahlberg's equation Sx = √Σ(D2/2N), where D is the difference between 2 measurements, and N is the sample size. The measurement error was 0.09 mm, or about 1.8%, for tooth movement; this was considered acceptable.
The Kruskal-Wallis test was used to test the effects of relaxin on tooth movement at 4 time points. The Mann-Whitney test with the Bonferroni adjustment was then used as a post-hoc test. The Mann-Whitney test was also used to detect differences between relaxin and vehicle at each time point and to test biomechanical differences. The Spearman correlation coeffient was used to identify associations between Young's modulus and tooth mobility.
RESULTS
Twenty-four rats were excluded from the study because of loss of initial radiographs or anesthetic death (5 in the relaxin and 9 in the vehicle groups). The 14 anesthetic deaths were distributed evenly throughout each time point, averaging approximately 2 per time point per group. Thus, there was no systematic effect on the final data set. Data from 6 rats were also excluded from the final data set of tooth movement because the values were physiologically impossible (outliers). Four were in the vehicle group (1 at day 4, 1 at day 7, and 2 at day 9), and 2 were in the relaxin group (1 each at days 2 and 4). These were minimal and evenly distributed across groups and times. Moreover, their exclusion did not impact the conclusions drawn from the data.
Both groups experienced temporary weight losses (10%-12%) after appliance activation; the weight loss was not statistically significant. The rats in both groups healed uneventfully after surgery, and the pumps and appliances were well tolerated throughout the experiment. The transitory loss in body weight suggests that procedures associated with appliance placement influenced the rats systemically, but this was not specific to group.
Tooth movement
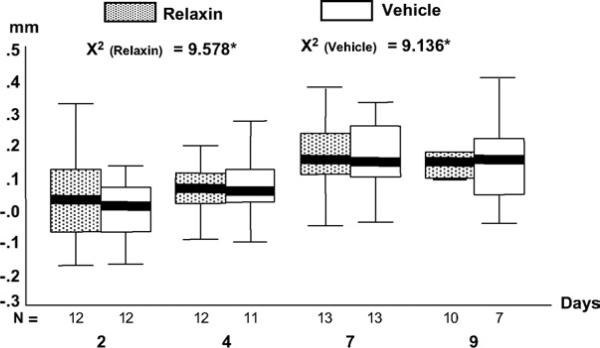
Measurements of both the maxillary right and left first molars were first compared by using the Mann-Whitney test. Because the difference between sides was insignificant, we averaged them to determine mean tooth movement per rat. After this, mean tooth movement per group was calculated. There were no differences between the 2 groups at any time point. However, significant differences over time were found in both treatment groups (Fig 5, P <.05). The differences in the relaxin group were between days 4 and 7 (P = .011) and between days 4 and 9 (P = .009). In the vehicle group, the differences were between days 2 and 7 (P = .014) and between days 2 and 9 (P = .013).
Fig 5.
Boxplots of measured molar movement at 4 time points. N, sample size of each group at each time point. Upper and lower limits of box represent 75th and 25th percentiles, respectively. Horizontal line in each box represent median. Outliers and extreme values were excluded (see text).
PDL structure
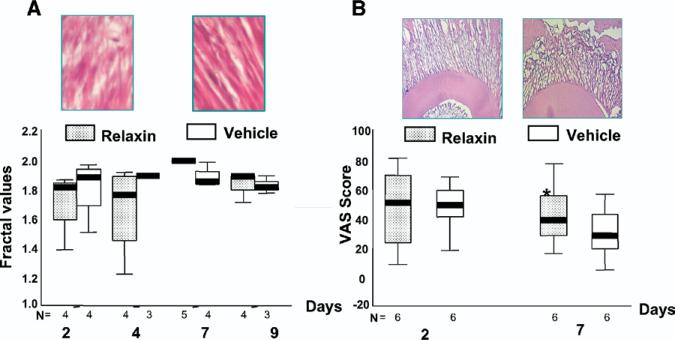
Fractal analysis (Fig 6, A) of the PDL images showed that the relaxin group had greater variability in scores at days 2 and 4 with a tendency toward lower fractal scores, suggesting less organization of the PDL fibers. These trends were not apparent in the vehicle group or at the later time points in either group. No significant difference was identified between the 2 groups at either time point.
Fig 6.
A, Boxplots of fractal values at 4 time points. Top 2 pictures represent typical ROI selected for fractal analysis from relaxin-treated (left) and vehicle-treated (right) samples. B, Boxplots of VAS values at 2 time points. Top 2 pictures represent typical hematoxylin and eosin-stained pictures for visual test from relaxin-treated (left) and vehicle-treated (right) samples.
The VAS scores (Fig 6, B) indicated greater perceived disorganization at day 7 in the vehicle group (P = .01). At day 2, the scores were comparable.
PDL mechanical strength
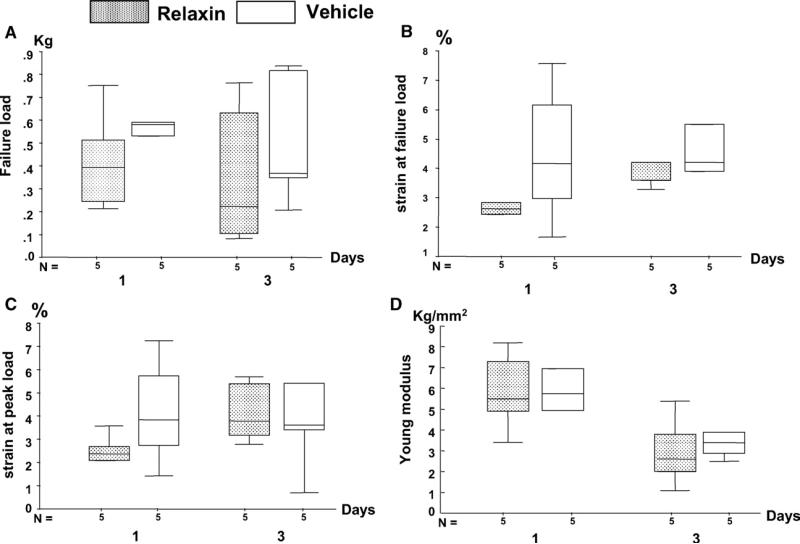
Failure load, percentage of strain at failure, peak load, and Young's modulus were all lower in the relaxin-treated animals, although no statistical significance was found because of limited sample size and large variations (Fig 7). These differences were more obvious in the 1- vs 3-day treatment groups.
Fig 7.
Boxplots of values of PDL mechanical strength at 2 time points: A, failure load; B, percentage of strain at failure load; C, percentage of strain at peak load; D, Young's modulus (stiffness). All values were lower in relaxin-treated than in vehicle-treated groups.
Tooth mobility
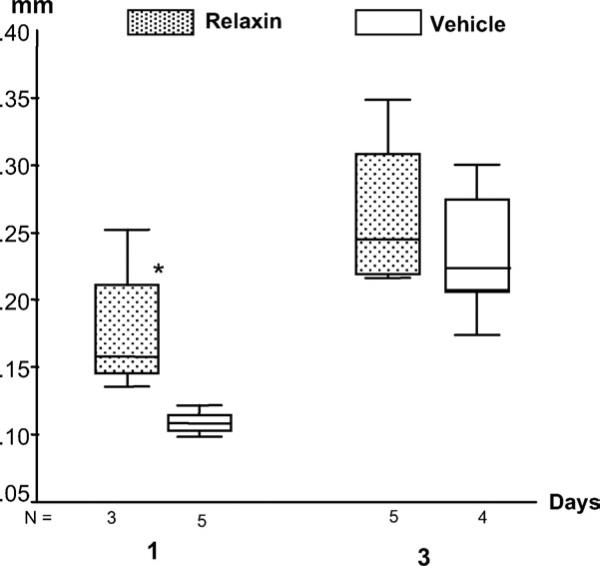
Compared with the vehicle-treated controls, the mobility ranges of the maxillary first molars increased up to 170% and 130% for 1-day and 3-day relaxin-treated rats, respectively, with a significant increase at day1 (P = .036) (Fig 8). Furthermore, a significantly negative association was found between the mobility range of the maxillary first molars and the PDL stiffness (Young's modulus) of the mandibular incisors (r2 = – 0.54, P = .025).
Fig 8.
Boxplots of values of tooth mobility at 2 time points. Significantly larger mobility in relaxin-treated molars was found at day 1.
DISCUSSION
The results of this study clearly show that relaxin does not affect either the rate or the amount of tooth movement in this animal model system. Both groups generated 3-part tooth movement kinetics consisting of initial deflection (day 2), lag period (day 4), and continued postlag movement (day 9) in the direction of force, as shown in other tooth-movement studies.10,19 This finding provides confidence that tooth movement occurred in a predictable fashion with both relaxin and vehicle.
Despite its failure to demonstrate enhancement of orthodontic tooth movement, we offer 5 related lines of evidence from this study suggesting that relaxin has an effect on the PDL. We focused on the sites of greatest tension in the PDL (ie, disto-occlusal aspects of the maxillary first molars). The rationale for this choice was based on the expectation that a relaxin effect on fiber morphology would be more readily apparent at tensile sites due to fiber stretching and orientation of the principal fibers in the direction of force.
First, there were significant increases in tooth movement between days 4, 7, and 9 in the relaxin-treated group, whereas significant increases occurred between days 2, 7, and 9 in the vehicle-treated group. This finding suggests that the increase in tooth movement in the relaxin-treated animals began later than in the controls. Assuming that the orthodontic strains were being transmitted to the alveolar bone through the principal PDL fibers, it is reasonable to postulate that alterations in these fibers in the relaxin group might interfere with the transduction of tensile signals.20
Second, fractal analysis showed trends toward lower fractal dimension and greater variability at the earlier times in the relaxin-treated group. This suggests a more amorphous structure at day 2 in both groups and a delay in recovery of fiber organization in the relaxin group that persists at day 4 but is no longer apparent by day 7. This implies that orthodontic tooth movement can affect fiber organization at tensile sites, but relaxin might interfere with short-term recovery.
Fractal analysis has been used extensively to assess bone trabeculation or dental microwear.16,21,22 A few other studies have applied fractal analysis to evaluate soft tissues, including quantification of skin pigmented lesion boundaries and the complexity of the epithelial connective tissue interface of pseudoepitheliomatous hyperplasia associated with granular cell tumors.23,24 Fractal analysis was also used to quantify the complexity of the PDL-bone interface in orthodontic tooth movement, suggesting that PDL remodeling in response to force might manifest as increased complexity at this interface.16
Third, viewer perceptions or the level of organization, quantified by a VAS, also showed greater variability in the relaxin group on day 2. In addition, this approach detected greater disorganization in the relaxin group at day 7. This difference was not detectible in the fractal data at day 7, suggesting that fractal analysis might be less sensitive to these changes than the visual approach.
Fourth, PDL mechanical testing quantified lower failure loads, strains at peak and failure, and stiffness in the relaxin-treated group at both early time points, particularly at day 1. Relaxin was shown to have a role in connective-tissue regulation by enhancing collagen turnover.25 In-vitro data show that relaxin disorganizes and loosens the arrangement of the PDL from the tooth to the bone surface, and dissolves Sharpey's fiber insertions of the PDL.26 These relaxin effects might weaken the mechanical strength of the PDL, resulting in a more fragile and looser PDL over the short term.
Fifth, increased molar mobility was identified in the relaxin group. This difference was more obvious at day 1 than at day 3. Because the ranges of tooth mobility reflect the rigidity of the PDL, this finding is considered the consequence of a disorganized PDL. The finding of a significant negative association between PDL stiffness and tooth mobility further supports this conclusion.
The fibers of the PDL begin to remodel as soon as tooth movement starts. There is extensive breakdown of PDL fibers in the areas of both pressure and tension. Also, intense vascular activity in the PDL and the alveolar bone occurs in areas of tension.27 The expression of type I collagen increases at 72 hours after the initiation of tooth movement and is sustained at a high level at 1 week, whereas an increase in the expression of type XII collagen is first noted by 1 week. This temporal sequence of types I and XII collagen expression occurs in a pattern similar to that found during the development of the PDL, suggesting that type XII collagen expression might be closely associated with the functional regeneration of the PDL.28 The findings from this study support the idea that relaxin has an effect on PDL remodeling within a few days.
During pregnancy in rats, relaxin is detectable by day 10 of a 23-day gestation period, with levels rising rapidly to about 100 ng per milliliter by day 20. A few days before birth, luteal cells degranulate, and serum relaxin levels surge to maximal levels that frequently exceed 150 ng per milliliter.5 We administered a dosage of 5.3 μg per kilogram per hour. Even at these target levels, we cannot be certain that the human relaxin administered would bind to receptors in the rat model. Since there is great diversity among species with respect to structure, tissue source, and physiologic effects of relaxin, the rats in this study might not have responded to the human relaxin 2 as they would have to endogenous relaxin. Furthermore, we assumed that the periodontal structures have receptors that would bind the human relaxin 2. Unfortunately, due to limited funds, serum levels of relaxin and PDL relaxin receptors could not be examined in this study.
The relaxin receptors belong to a family of LGRs designated LGR7 and LGR8, which are the receptors for relaxin as well as INSL3/RLF.29 Both relaxin and INSL3/RLF elicit bioactivity through stimulation of the Gs-cAMP-protein kinase A (PKA)-dependent signaling pathway. Human relaxin 2 also induces cAMP production in cells that contain only LGR8 receptors.29 Relaxin is well known to have diverse effects in male and female rats—ie, kidneys, heart, lungs, liver, skin, and brain.5 Some evidence suggests that relaxin also binds to cranial sutures.26 Relaxin has been immunolocalized on PDL fibers and cranial sutures in both male and female mice.26 Moreover, there is a link between relaxin, matrix metalloproteinases, and matrix degradation; relaxin was found to induce degradative mediators in the nonreproductive, fibrocartilaginous synovial discs of temporomandibular joints.30 In an effort to discern the effect of relaxin on bone metabolism, we found relaxin to increase tumor necrosis factor- and interleukin-1β secretion in supernatants from peripheral blood mononuclear cells.31 Based on this, relaxin might have effects on osteoclastic behavior resulting in increased bone resorption, but there is no direct evidence for this effect.
Most studies on relaxin focus on its effect on fibrous tissues. Relaxin was shown to reduce the overexpression of collagen in ventricular fibroblasts that were stimulated by profibrotic mediators, transforming growth factor-beta (TGF-beta) and angiotensin II, as well as in excessive collagen-producing human scleroderma fibroblast lines.32,33 Prolonged administration of relaxin results in decreased deposition of collagen in rodent models of fibrosis.32 Pigs treated with human relaxin 2 experienced a decrease in fibrosis, due to decreased collagen production, suggesting that it has an effect on collagen remodeling.34 The proposed mechanism includes both down-regulation of fibroblast activity resulting in decreased collagen synthesis and up-regulation of matrix metalloproteinase 2 expression resulting in collagen degradation.33
A plausible hypothesis is that relaxin reduces the synthesis and increases the rate of degradation of extracellular fibrous connective tissues, resulting in more rapid turnover. Increased collagen deposition in response to tensional forces and decreases in type I collagen and fibronectin protein in response to compressional forces were reported in tooth movement.35 Interestingly, an increase in collagen synthesis during tooth movement on the compression side also has been documented.36 At first, the latter might seem contradictory, but it is possible that total collagen turnover is increased at compression sites with a net loss due to an imbalance between synthesis and degradation. It was also suggested that 4% stretching of PDL fibroblasts results in a dramatic increase of type I collagen gene expression.37 Since that study was done in vitro, it is unclear whether this amount of elongation occurs in vivo and precisely where. It seems intuitive that stretching would occur on the tension side of the tooth, but recent finite element modeling studies38 found that, when the PDL was modeled as a nonlinear material, taking into account the influence of geometry/morphology, material properties, and boundary conditions, strains could not be explained just based on stretching or compressing PDL fibers. The calculations showed that tension in the alveolar bone itself was far more common than compression, suggesting that bending of alveolar bone might be a more important predictor of bone remodeling in tooth movement than strains in the PDL fibers.
Extrapolating the failure to demonstrate relaxin-enhanced orthodontic tooth movement in this model system to the usefulness of relaxin in human orthodontic treatment should be approached with caution. There might be an additive effect of continuous relaxin administration and repeated appliance reactivations with modification of tooth movement during actual orthodontic treatment in humans. In this model, the appliance was activated only once, at the beginning of treatment. Future studies should examine the effect of relaxin combined with repeated appliance reactivations. Also, based on its effects on PDL fibers, relaxin might find its most significant clinical application in reducing the tendency for postorthodontic tooth relapse, not enhanced tooth movement. The etiology of orthodontic relapse is not well understood but is likely to be multifactorial. One factor is the memory of the PDL fibers. These fibers were shown to play a role in orthodontic relapse. To this end, gingival fiberotomies have been shown to reduce relapse.39 Enhanced PDL fiber remodeling with relaxin treatment might reduce the rate or the amount of relapse associated with orthodontic treatment by chemically mimicking the effect of a gingival fiberotomy.
Acknowledgments
We thank Sue Herring for her helpful comments; Jayoung Shin, a visiting scholar from Korea, and Xia-Qin Bai for their help with the experiments; and Noralyn Altases for her histological expertise.
Supported by BAS Medical, San Mateo, Calif.
REFERENCES
- 1.Yamashiro T, Takano-Yamamoto T. Influences of ovariectomy on experimental tooth movement in the rat. J Dent Res. 2001;80:1858–61. doi: 10.1177/00220345010800091701. [DOI] [PubMed] [Google Scholar]
- 2.Soma S, Iwamoto M, Higuchi Y, Kurisu K. Effects of continuous infusion of PTH on experimental tooth movement in rats. J Bone Miner Res. 1999;14:546–54. doi: 10.1359/jbmr.1999.14.4.546. [DOI] [PubMed] [Google Scholar]
- 3.Adachi H, Igarashi K, Mitani H, Shinoda H. Effects of topical administration of a bisphosphonate (risedronate) on orthodontic tooth movements in rats. J Dent Res. 1994;73:1478–86. doi: 10.1177/00220345940730081301. [DOI] [PubMed] [Google Scholar]
- 4.Yan Y, Cai J, Fu P, Layfield S, Ferraro T, Kumagai J, et al. Studies on soluble ectodomain proteins of relaxin (LGR7) and insulin 3 (LGR8) receptors. Ann N Y Acad Sci. 2005;1041:35–9. doi: 10.1196/annals.1282.007. [DOI] [PubMed] [Google Scholar]
- 5.Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev. 2004;25:205–34. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 6.Sherwood OD, Crnekovic VE, Gordon WL, Rutherford JE. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980;107:691–8. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- 7.Hellsing E, Hammarstrom L. The effects of pregnancy and fluoride on orthodontic tooth movements in rats. Eur J Orthod. 1991;13:223–30. doi: 10.1093/ejo/13.3.223. [DOI] [PubMed] [Google Scholar]
- 8.Beertsen W, Hoeben KA. Movement of fibroblasts in the periodontal ligament of the mouse incisor is related to eruption. J Dent Res. 1987;66:1006–10. doi: 10.1177/00220345870660050201. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZJ, King GJ, Gu GM, Shin JY, Stewart DR. Does human relaxin accelerate orthodontic tooth movement in rats? Ann N Y Acad Sci. 2005;1041:388–94. doi: 10.1196/annals.1282.059. [DOI] [PubMed] [Google Scholar]
- 10.King GJ, Keeling SD, McCoy EA, Ward TH. Measuring dental drift and orthodontic tooth movement in response to various initial forces in adult rats. Am J Orthod Dentofacial Orthop. 1991;99:456–65. doi: 10.1016/S0889-5406(05)81579-3. [DOI] [PubMed] [Google Scholar]
- 11.Liu ZJ, King GJ, Stewart DR. Effect of relaxin on the physical properties of periodontal ligament in the rat. J Dent Res. 2004;83:2594. [abstract] [Google Scholar]
- 12.Garber SL, Mirochnik Y, Brecklin CS, Unemori EN, Singh AK, Slobodskoy L, et al. Relaxin decreases renal interstitial fibrosis and slows progression of renal disease. Kidney Int. 2001;59:876–82. doi: 10.1046/j.1523-1755.2001.059003876.x. [DOI] [PubMed] [Google Scholar]
- 13.Rody WJ, Jr, King GJ, Gu G. Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2001;120:477–89. doi: 10.1067/mod.2001.118623. [DOI] [PubMed] [Google Scholar]
- 14.Noxon SJ, King GJ, Gu G, Huang G. Osteoclast clearance from periodontal tissues during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2001;120:466–76. doi: 10.1067/mod.2001.117912. [DOI] [PubMed] [Google Scholar]
- 15.Chen SK, Oviir T, Lin CH, Leu LJ, Cho BH, Hollender L. Digital imaging analysis with mathematical morphology and fractal dimension for evaluation of periapical lesions following endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:467–72. doi: 10.1016/j.tripleo.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 16.Wagle N, Do NN, Yu J, Borke JL. Fractal analysis of the PDL-bone interface and implications for orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2005;127:655–61. doi: 10.1016/j.ajodo.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Liu ZJ, Herring SW. Masticatory strains on osseous and ligamentous components of the temporomandibular joint in miniature pigs. J Orofac Pain. 2000;14:265–78. [PubMed] [Google Scholar]
- 18.Herring SW, Sun Z, Egbert MA, Rafferty KL, Liu ZJ. Fixation and stability of the pig mandible. Harvard Society for the Advancement of Orthodontics; Boston: 2004. Is distraction the only motion permitted at the osteotomy site? [Google Scholar]
- 19.Gibson JM, King GJ, Keeling SD. Long-term orthodontic tooth movement response to short-term force in the rat. Angle Orthod. 1992;62:211–5. doi: 10.1043/0003-3219(1992)062<0211:LOTMRT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Melsen B. Tissue reaction to orthodontic tooth movement—a new paradigm. Eur J Orthod. 2001;23:671–81. doi: 10.1093/ejo/23.6.671. [DOI] [PubMed] [Google Scholar]
- 21.Yasar F, Akgunlu F. Fractal dimension and lacunarity analysis of dental radiographs. Dentomaxillofac Radiol. 2005;34:261–7. doi: 10.1259/dmfr/85149245. [DOI] [PubMed] [Google Scholar]
- 22.Scott RS, Ungar PS, Bergstrom TS, Brown CA, Grine FE, Teaford MF, et al. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature. 2005;436:693–5. doi: 10.1038/nature03822. [DOI] [PubMed] [Google Scholar]
- 23.Piantanelli A, Maponi P, Scalise L, Serresi S, Cialabrini A, Basso A. Fractal characterization of boundary irregularity in skin pigmented lesions. Med Biol Eng Comput. 2005;43:436–42. doi: 10.1007/BF02344723. [DOI] [PubMed] [Google Scholar]
- 24.Abu Eid R, Landini G. Morphometry of pseudoepitheliomatous hyperplasia: objective comparison to normal and dysplastic oral mucosae. Anal Quant Cytol Histol. 2005;27:232–40. [PubMed] [Google Scholar]
- 25.Ivell R, Einspanier A. Relaxin peptides are new global players. Trends Endocrinol Metab. 2002;13:343–8. doi: 10.1016/s1043-2760(02)00664-1. [DOI] [PubMed] [Google Scholar]
- 26.Nicozisis JL, Nah-Cederquist HD, Tuncay OC. Relaxin affects the dentofacial sutural tissues. Clin Orthod Res. 2000;3:192–201. doi: 10.1034/j.1600-0544.2000.030405.x. [DOI] [PubMed] [Google Scholar]
- 27.Rygh P, Bowling K, Hovlandsdal L, Williams S. Activation of the vascular system: a main mediator of periodontal fiber remodeling in orthodontic tooth movement. Am J Orthod. 1986;89:453–68. doi: 10.1016/0002-9416(86)90001-1. [DOI] [PubMed] [Google Scholar]
- 28.Karimbux NY, Nishimura I. Temporal and spatial expressions of type XII collagen in the remodeling periodontal ligament during experimental tooth movement. J Dent Res. 1995;74:313–8. doi: 10.1177/00220345950740010501. [DOI] [PubMed] [Google Scholar]
- 29.Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–4. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 30.Naqvi T, Duong TT, Hashem G, Shiga M, Zhang Q, Kapila S. Relaxin's induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants. Arthritis Res Ther. 2005;7:R1–11. doi: 10.1186/ar1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kristiansson P, Holding C, Hughes S, Haynes D. Does human relaxin-2 affect peripheral blood mononuclear cells to increase inflammatory mediators in pathologic bone loss? Ann N Y Acad Sci. 2005;1041:317–9. doi: 10.1196/annals.1282.050. [DOI] [PubMed] [Google Scholar]
- 32.Unemori EN, Beck LS, Lee WP, Xu Y, Siegel M, Keller G, et al. Human relaxin decreases collagen accumulation in vivo in two rodent models of fibrosis. J Invest Dermatol. 1993;101:280–5. doi: 10.1111/1523-1747.ep12365206. [DOI] [PubMed] [Google Scholar]
- 33.Mookerjee I, Unemori EN, Du XJ, Tregear GW, Samuel CS. Relaxin modulates fibroblast function, collagen production, and matrix metalloproteinase-2 expression by cardiac fibroblasts. Ann N Y Acad Sci. 2005;1041:190–3. doi: 10.1196/annals.1282.028. [DOI] [PubMed] [Google Scholar]
- 34.Kibblewhite D, Larrabee WF, Jr, Sutton D. The effect of relaxin on tissue expansion. Arch Otolaryngol Head Neck Surg. 1992;118:153–6. doi: 10.1001/archotol.1992.01880020047014. [DOI] [PubMed] [Google Scholar]
- 35.He Y, Macarak EJ, Korostoff JM, Howard PS. Compression and tension: differential effects on matrix accumulation by periodontal ligament fibroblasts in vitro. Connect Tissue Res. 2004;45:28–39. doi: 10.1080/03008200490278124. [DOI] [PubMed] [Google Scholar]
- 36.Bumann A, Carvalho RS, Schwarzer CL, Yen EH. Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur J Orthod. 1997;19:29–37. doi: 10.1093/ejo/19.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Saffar BG, Fotadar U, Sacks P, Taraccio L. The effects of stretch on the expression of collagen I gene in cultured rat gingival connective tissue fibroblasts. J Dent Res. 2003;82:1850. [abstract] [Google Scholar]
- 38.Cattaneo PM, Dalstra M, Melsen B. The finite element method: a tool to study orthodontic tooth movement. J Dent Res. 2005;84:428–33. doi: 10.1177/154405910508400506. [DOI] [PubMed] [Google Scholar]
- 39.Edwards JG. A long-term prospective evaluation of the circumferential supracrestal fiberotomy in alleviating orthodontic relapse. Am J Orthod Dentofacial Orthop. 1988;93:380–7. doi: 10.1016/0889-5406(88)90096-0. [DOI] [PubMed] [Google Scholar]