Abstract
Background
A new method for detecting circulating Ewing sarcoma cells using flow cytometry is described. This strategy exploits the nearly universal expression of CD99 and the lack of expression of CD45 by Ewing sarcoma cells.
Procedure
Ewing sarcoma cell line A673, peripheral blood mononuclear cells (PBMCs), and bone marrow mononuclear cells (BMMCs) were stained for CD99 and CD45 in order to detect CD99+CD45- cells by flow cytometry. Known quantities of A673 Ewing sarcoma cells were spiked into control PBMCs to test the accuracy of this method. Control PBMCs were evaluated to assess the level of background staining.
Results
Flow cytometry was accurate at frequencies as low as one A673 cell per 500,000 PBMCs. The background rate of CD99+CD45- cell detection was low in PBMCs from 9 healthy volunteers (median 0.0001% of total cells; range 0-0.00046%) and was further reduced by incorporating stains to exclude dead cells, progenitor cells, and monocytes. In one subject with newly diagnosed localized Ewing sarcoma, CD99+CD45- cells were detected in both blood (0.0021%) and bone marrow (0.048%).
Conclusions
Multicolor flow cytometry for CD99+CD45- cells provides a new strategy for detecting circulating Ewing sarcoma cells. Clinical evaluation and validation of this method is ongoing.
Keywords: Ewing sarcoma, flow cytometry, CD99
Introduction
Ewing sarcoma is an aggressive malignant bone tumor seen in children and young adults. Reports from cooperative group studies indicate that approximately 75% of patients present with clinically localized tumors [1]. Patients with clinically localized Ewing sarcoma are presumed to have micrometastatic disease since the vast majority of patients developed distant recurrence with the use of local control measures only [2]. For the 25% of patients with metastatic disease, the pattern of dissemination is hematogenous, with lung, bone, and bone marrow metastasis most common [3]. Only 20-25% of patients with initially metastatic disease survive disease-free 5 years from initial diagnosis [1].
Ewing sarcoma cells are characterized by the presence of a reciprocal translocation, most commonly involving the EWS gene and a member of the ETS gene family [4]. Several groups have attempted to detect circulating Ewing sarcoma EWS fusion transcripts in blood and bone marrow from patients using reverse transcriptase-polymerase chain reaction (RT-PCR) techniques. Two early studies determined that RT-PCR could detect as few as 1 mRNA transcript per million nucleated cells [5,6]. Follow-up work indicated that 25-30% of patients with clinically nonmetastatic tumors have detectable transcript in the peripheral blood and/or bone marrow [5,7,8]. Patients with clinically nonmetastatic disease and detectable transcript in the bone marrow or peripheral blood may have an inferior outcome compared to patients without detectable transcript [9]. Despite limitations in the application of this technique, no alternative methods have been reported for detecting circulating Ewing sarcoma tumor cells.
Ewing sarcoma cells demonstrate nearly universal membranous staining with the cell surface antigen CD99 [10]. In contrast to monocytes, immature lymphocytes, and T-cell lymphoblastic leukemia/lymphoma that also express high levels of surface CD99 [11,12], Ewing sarcoma cells do not express CD45, the leukocyte common antigen. Flow cytometry with CD99 and CD45 has been used to evaluate Ewing sarcoma tumor samples [13,14]. When applied to tumor samples, the CD99+CD45- profile may differentiate Ewing sarcoma from other malignancies. In this report, we describe the use of flow cytometry to detect Ewing sarcoma cells in blood and bone marrow.
Methods
Cell Lines and Antibodies
Ewing sarcoma cell lines RD-ES and A673 were obtained from American Type Culture Collection (ATCC; Manassas, VA) and maintained using the cell culture technique recommended by ATCC. Both RD-ES and A673 have been previously characterized as expressing CD99 and as harboring Ewing sarcoma specific translocations [15-17]. CD99-PE, CD45-FITC, CD14-APC and CD34-PECy5 were obtained from BD Bioscience/Pharmigen (San Jose, California). LIVE/DEAD Fixable Dead Cell Stain Kit, aqua-fluorescent amine reactive dye (AARD), was obtained from Invitrogen (Carlsbad, California). Human Gamma Globulin (HGG) was obtained from BioDesign International (Saco, Maine).
Collection of Peripheral Blood and Bone Marrow
Healthy adult controls provided a 5 mL peripheral blood sample collected into acid citrate dextrose (ACD) or ethylenediaminetetraacetic acid (EDTA) tubes. These adult controls provided informed consent using a UCSF Committee on Human Research-approved consent form.
Control aspirated bone marrow material was obtained from UCSF patients with malignancies other than Ewing sarcoma or lymphoblastic lymphoma undergoing scheduled bone marrow aspirates as part of their clinical care. Residual bone marrow aspirate material in EDTA tubes that were slated to be discarded was used as control bone marrow. Use of this discarded material was exempt from UCSF Committee on Human Research review.
A newly diagnosed patient with clinically localized Ewing sarcoma provided 10 mL of peripheral blood and 5 mL of aspirated bone marrow in EDTA tubes prior to the initiation of chemotherapy. Parental consent was obtained using a UCSF Committee on Human Research-approved consent form.
Flow Cytometry Methods
Mononuclear cells were isolated from blood and bone marrow on a ficoll density gradient. Isolated cells were counted and the viability assessed on a Guava PCA using the ViaCount procedure (Guava Technologies; Hayward, CA). For two-color panel studies of control samples, one to five million peripheral blood and bone marrow mononuclear cells were washed in calcium, magnesium free PBS with 1% bovine serum albumin (wash buffer), incubated in 1 mg/mL HGG buffer for 10 minutes at room temperature to block FC receptors, and then stained with commercially available monoclonal antibodies, CD99-PE and CD45-FITC, for 30 minutes at 4° C. Cells were then washed and fixed in 0.5% formaldehyde. One to five million cells were collected on a FACSCalibur (BD Biosciences; San Jose, CA) within 18 hours of fixation. For six-color panel studies, one to eight million cells were washed in wash buffer, labeled for 20 minutes at room temperature with 4 times the recommended volume of AARD, blocked with HGG buffer, stained with CD99-PE, CD45-FITC, CD14-APC, and CD34-PECy5, and then washed and fixed as above. Cells were collected on a customized LSRII Flow Cytometer (BD Biosciences; San Jose, CA) within 18 hours. For both panels, data were compensated and analyzed using FlowJo software (Tree Star; Ashland, OR).
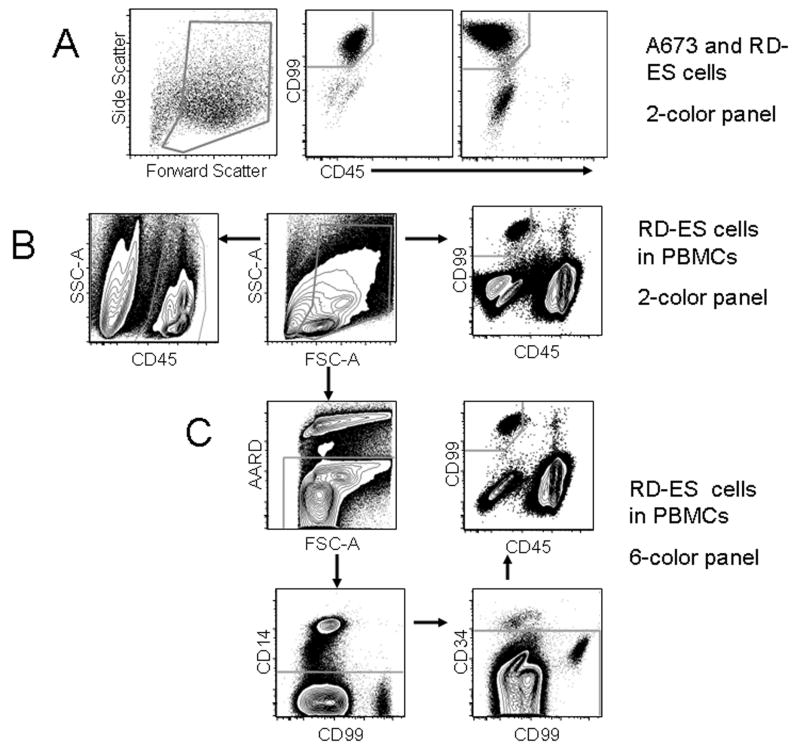
The gating strategy for both panels is illustrated in Figure 1. Two-color panel gating is illustrated in figure 1B. A debris exclusion gate was set on a forward and side scatter plot. A CD45+ gate was then drawn on all CD45+ cells to define total PBMCs on a CD45 vs. side scatter plot. A CD99+CD45- gate was set to include Ewing sarcoma cells but exclude CD45- cells on a CD99 vs. CD45 plot. The proportion of CD99+CD45- cells was expressed as a percent of the total CD45+ PBMCs. The gating strategy for the six-color panel is shown in figure 1C. Following debris exclusion on the forward and side-scatter plot, AARD-positive dead cells, CD14+ monocytes, and CD34+ progenitors were sequentially removed. A CD99+CD45- gate was defined as for the two-color panel and cells were expressed as a percent of total CD45+ PBMC.
Figure 1.
(A) Evaluation of Ewing sarcoma cell lines for the CD99+CD45- immunophenotype. More than 95% of A673 cells (middle panel) and 87% of RD-ES cells (right panel) were CD99+CD45-. (B) Gating strategy used to detect CD99+CD45- cells in PBMCs spiked with 1% RD-ES cells using the two-color panel. The proportion of CD99+CD45- cells to CD45+ cells was calculated by dividing the CD99+CD45- count by the CD45+ PBMC count. (C) Gating strategy used to detect CD99+CD45- cells in PBMCs spiked with 1% RD-ES cells using the six-color panel. Cells positive for AARD, CD14, and CD34 were excluded before gating for CD99+CD45-.
For titration experiments, known quantities of A673 cells were “spiked” into control PBMC and BMMC samples. Samples were stained and flow cytometry on these “spiked” samples was performed and analyzed as above. Increasing amounts of Ewing sarcoma cells were gradually titrated to identify an appropriate gate that included the “spiked” cells and excluded normal blood or bone marrow cells. The proportion of Ewing sarcoma cells detected in each titration experiment was measured by gating on CD99+CD45- cells and reported as the percentage of total CD45+ PBMCs or BMMCs.
Results
Accuracy of Ewing Sarcoma Cell Detection in Peripheral Blood by Flow Cytometry
Greater than 95% of A673 and 87% of RD-ES Ewing sarcoma cells expressed CD99 but did not express CD45 (Figure 1A). These cell lines served as positive controls for remaining experiments.
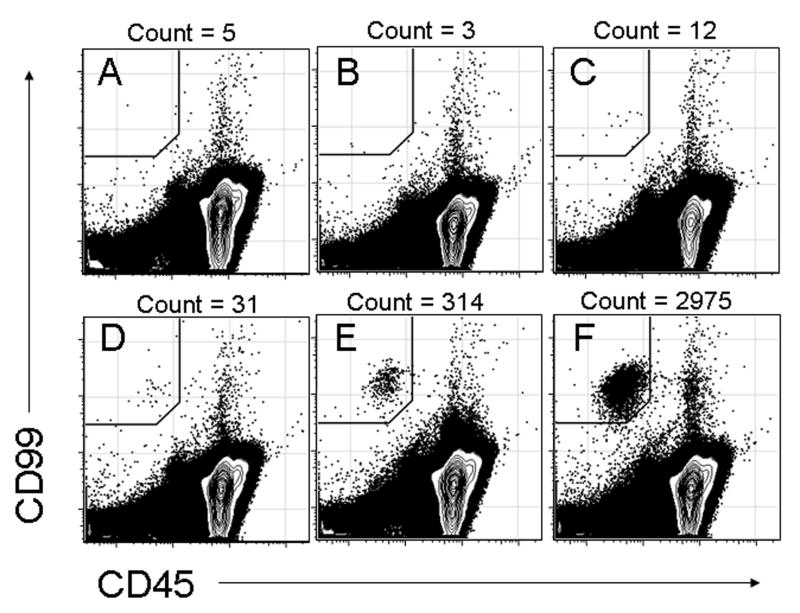
Given the expected low frequency of circulating Ewing sarcoma cells, experiments were performed to determine if two-color flow cytometry could accurately detect low frequencies of Ewing sarcoma cells in a background of normal PBMCs. Increasing numbers of A673 cells were spiked into PBMCs at dilutions ranging from 1 in 1,000 to 1 in 10 million (Figure 2). When no A673 cells were added, a background frequency of 0.00015% CD99+CD45- cells was detected (Figure 2A). At a dilution of 1 A673 cell per 10 million PBMCs, the input frequency (0.00001%) was below the background detection frequency (Figure 2B). At a dilution of 1 A673 cell per 1 million PBMCs, the frequency of CD99+CD45- cells detected (0.00035%) was very close to the sum of the background frequency plus the frequency of added cells (0.00015% + 0.0001%), demonstrating that flow cytometry could accurately detect CD99+CD45- cells added at very low frequencies (Figure 2C). As increasing numbers of A673 cells were added, the number of CD99+CD45- cells detected increased as expected (Figures 2D-2F).
Figure 2.
Detection of increasing proportions of CD99+CD45- A673 cells spiked into a background of normal PBMCs and evaluated with the two-color panel. (A) Control with no A673 Ewing sarcoma cells added. (B) 1 A673 cell per 10 million control PBMCs. (C) 1 A673 cell per 1 million control PBMCs. (D) 1 A673 cell per 100,000 control PBMCs. (E) 1 A673 cell per 10,000 control PBMCs. (F) 1 A673 cell per 1,000 control PBMCs.
As an additional test of the validity of these results, cells from cell line RD-ES were also evaluated by two-color flow cytometry when added at a dilution of 1%. The distribution of CD99+CD45- cells seen with RD-ES cells was nearly identical to the distribution of CD99+CD45- cells seen with A673 cells (Figure 1B).
In order to test the reproducibility of these findings and to determine if detection could be enhanced by reducing the background detection rate, A673 cells were spiked into control PBMCs in triplicate and stained with both the two-color panel and a six-color panel designed to remove non-specific staining. A minimum of 3 million cells was collected for each of these experiments. The results are shown in the Table. Using the two-color panel, the background frequency of CD99+CD45- cells in this experiment ranged from 0.0002 to 0.00036% (mean 0.00027%). At a dilution of 1 A673 cell per million PBMCs, the mean frequency of CD99+CD45- cells detected was approximately 1.8 times the mean background rate. At a dilution of 1 A673 cell per 100,000 PBMCs, the actual frequency detected (range 0.0011-0.0014%) was close to the sum of the input frequency of 0.001% plus background, indicating that most of the added A673 cells were captured in the CD99+CD45- gate.
Table.
Results of titration experiment with increasing numbers of A673 Ewing sarcoma cells spiked in volunteer peripheral blood mononuclear cells. Experiments were performed in triplicate. Two-color flow cytometry indicates evaluation only for CD45 and CD99 while six-color flow cytometry indicates evaluation for CD45, CD99, CD14, CD34, and AARD as described in the Methods.
| A673 Dilution | Frequency of CD99+CD45-Cells with Two-color Flow Cytometry | Frequency of CD99+CD45-Cells with Six-color Flow Cytometry |
|---|---|---|
|
Control (No A673 cells) |
0.00020% | 0.00014% |
| 0.00036% | 0.00018% | |
| 0.00027% | 0.00016% | |
| Mean Detection Rate | 0.00027% | 0.00011% |
|
0.0001% A673 cells (1 in million cells) |
0.00054% | 0.00036% |
| 0.00037% | 0.00021% | |
| 0.00056% | 0.00035% | |
| Mean Detection Rate | 0.00049% | 0.00031% |
|
0.001% A673 cells (1 in 100,000 cells) |
0.00105% | 0.00083% |
| 0.00123% | 0.00103% | |
| 0.00140% | 0.00118% | |
| Mean Detection Rate | 0.00123% | 0.00102% |
Using the six-color flow cytometry panel, the background frequency of CD99+CD45-CD34-CD14- cells was reduced (mean 0.00011%) compared with the two-color panel. At a dilution of 1 A673 cell per million PBMCs, the mean frequency of cells detected with the multicolor panel (0.00031%) was approximately 2.8 times the mean background rate. At a dilution of 1 A673 cell per 100,000 PBMCs, the actual frequency detected (range 0.00083-0.00118%) was again very close to the sum of the input frequency of 0.001% plus background rate.
Frequency of CD99+CD45- Cells Detected in PBMCs from Healthy Controls
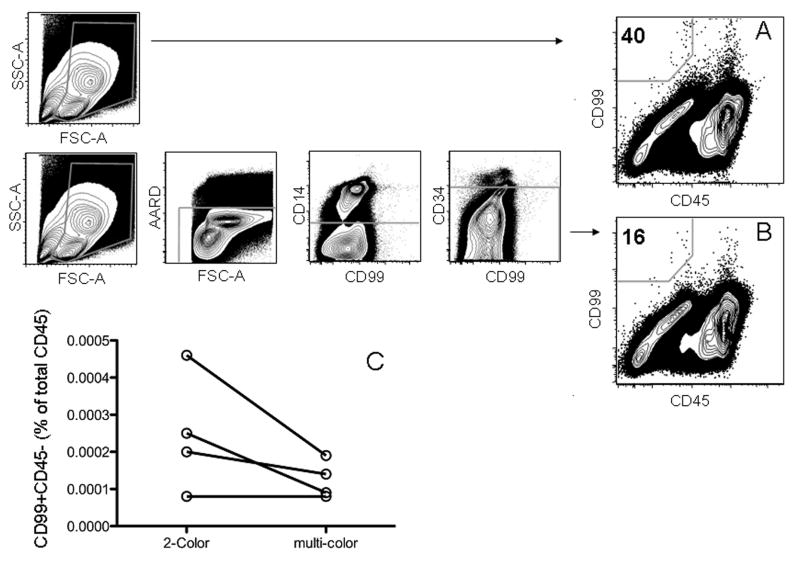
PBMCs from 9 healthy adult volunteers were tested to determine the background frequency of CD99+CD45- cells in people without Ewing sarcoma. A minimum of one million cells was collected for these experiments. The frequency of CD99+CD45- cells detected by two-color flow cytometry ranged from 0 to 0.00046% (median 0.0001%). Three of the samples had no detectable CD99+CD45- cells. These results together with the previous control experiments from the titration studies indicated that no healthy control had a CD99+CD45- event rate greater than 0.00046% by 2-color flow cytometry. In order to evaluate the ability of the six-color panel to reduce the background frequency of CD99+CD45- cells from healthy controls, PBMCs from 4 of the volunteers with background rates of 0.00008%, 0.0002%, 0.00025% and 0.00046% by two-color flow cytometry were also stained with the six-color panel to exclude non-specific staining (3A and 3B). These exclusions resulted in corresponding background rates of 0.00008%, 0.00014%, 0.00009%, and 0.00019%, respectively. The frequency of CD99+CD45- cells declined the most in the subject with the highest background in the two-color panel (Figure 3C).
Figure 3.
Detection of CD99+CD45- cells in control PBMCs from a representative healthy control using either a two-color (A) or six-color panel (B), demonstrating a reduction in the background frequency of CD99+CD45- cells using the six-color panel. (C) Plot of CD99+CD45- cell detection in four healthy controls evaluated with both the two-color and six-color panels. Normal controls with the highest background frequency of CD99+CD45- with the two-color panel show the most reduction in background when stained with the six-color panel.
Detection of CD99+CD45- Cells in BMMCs
BMMCs from 3 patients with malignancies other than Ewing sarcoma or lymphoblastic lymphoma were evaluated by two-color flow cytometry with CD45 and CD99. A low frequency of CD99+CD45- cells was detected in all 3 cases. BMMCs had a higher background rate of CD99+CD45- cells (range 0.0009-0.0014%) compared to PBMCs. RD-ES cells were added to these control BMMCs and a similar distribution of CD99+CD45- cells was noted as was seen in experiments performed using PBMCs.
CD99+CD45- Cells Detected in Peripheral Blood and Bone Marrow from a Patient with Ewing Sarcoma
PBMCs and BMMCs from a patient with newly diagnosed localized Ewing sarcoma were evaluated by two-color flow cytometry for CD99 and CD45. The frequency of CD99+CD45- cells was 0.0021% in PBMCs, or more than 4 times the highest background rate detected in healthy controls using two-color flow cytometry (Figure 4A). The frequency of CD99+CD45- cells was 0.048% in BMMCs, or more than 30 times the highest background rate detected in control bone marrow (Figure 4B).
Figure 4.
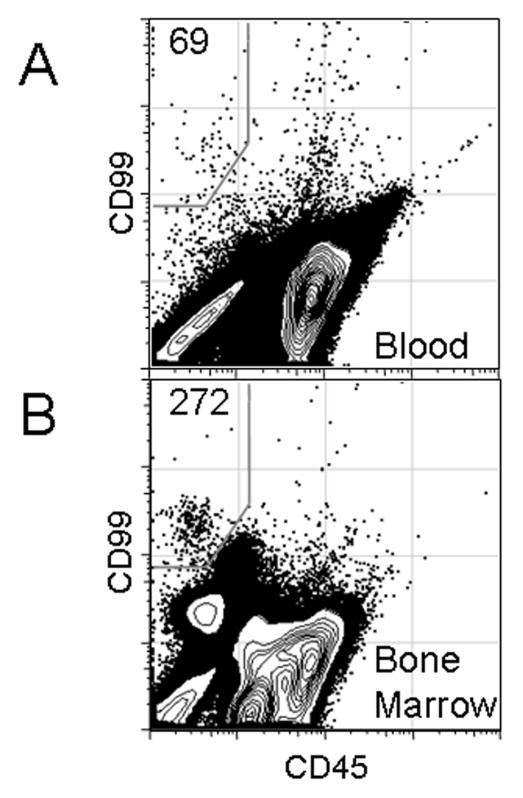
Detection of CD99+CD45- cells in PBMCs (A) and bone marrow mononuclear cells (B) of a patient with newly diagnosed localized Ewing sarcoma using the two-color panel.
Discussion
These results demonstrate that CD99+CD45- cells can be detected amidst a background of peripheral blood or bone marrow mononuclear cells using flow cytometry. Although previously used to evaluate Ewing sarcoma tumor samples [13,14], this report is the first description of flow cytometric detection of circulating Ewing sarcoma cells. Two Ewing sarcoma cell lines were evaluated for expression of CD45 and CD99 and the results suggest that this technique may be applicable to any CD99 positive Ewing sarcoma. As proof of this concept, a patient with newly diagnosed localized Ewing sarcoma was evaluated to determine if CD99+CD45- cells could be detected in a patient sample. The frequency of CD99+CD45- cells was more than 4 and 30 times higher than the background frequency in PBMCs and bone marrow, respectively. These results suggest that detection of CD99+CD45- cells by flow cytometry is specific for the presence of Ewing sarcoma cells.
The accuracy of the flow assay to detect low numbers of Ewing sarcoma cells was tested by spiking normal PBMCs with low numbers of a Ewing sarcoma cell line. After subtracting the number of events detected in a non-spiked sample, the technique could accurately detect 1 Ewing sarcoma cell per 500,000 CD45+ PBMCs. However, since up to 0.00046% CD99+CD45- cells were detected in normal controls using the two-color panel, the sensitivity of the 2-color assay could not be lower than 1 in 200,000 cells. We therefore attempted to reduce the frequency of CD99+CD45- detection in normal controls by adding stains to exclude monocytes, stem cells, and dead cells. Rare event detection by flow cytometry is subject to a number of artifacts that can increase the frequency of background detection, including non-specific staining, cellular autofluorescence, cellular clumping, and instrument noise [18]. We blocked FC receptor binding to decrease non-specific staining but still encountered a fair amount of autofluorescence. Dead cells can bind non-specifically and these were excluded in the multicolor panel by staining with AARD. Auto-fluorescence is often contributed by monocytes and can be gated out based on their forward and side scatter. However, forward and side scatter of the Ewing sarcoma cells overlapped with the monocyte gate. We therefore tested whether specific removal of monocytes by excluding CD14+ cells would reduce the background. With this six-color panel, the highest background rate observed was 0.00019%, just below 1 in 500,000 cells. Additional studies are ongoing to evaluate the sensitivity and specificity of the six-color panel by staining material from additional healthy controls and patients with Ewing sarcoma.
The six-color panel also excludes CD34+ cells in an attempt to remove any hematopoietic cells that express CD99 and do not yet express CD45. One group has reported that a subset of Ewing sarcoma cells may express CD34 [19]. In the current study, exclusion of CD34+ cells in our six-color panel did not eliminate the observed CD99+CD45- signal. This observation may reflect absent or low-level expression of CD34 by the chosen Ewing sarcoma cell lines. In future studies using samples from patients with Ewing sarcoma, we will prospectively evaluate the fraction of CD99+CD45- cells removed by excluding CD34+ cells.
Evaluation of BMMCs from patients without Ewing sarcoma demonstrated a higher rate of CD99+CD45- cells compared to PBMCs. These CD99+CD45- cells may represent early monocytic progenitor cells that have not yet acquired strong CD45 expression [11,20]. Additional studies with the six-color panel are in progress to evaluate CD34 expression in these cells to confirm this hypothesis and to establish the threshold number of CD99+CD45- cells that will provide adequate specificity for Ewing sarcoma. The preliminary result from one patient with Ewing sarcoma suggests that flow cytometry may be a useful modality to detect occult bone marrow disease in patients with Ewing sarcoma.
Several groups have recently reported on their work evaluating circulating tumor cells in adult patients with carcinoma [21-25]. The presence of tumor cells in the bone marrow in newly-diagnosed patients with prostate cancer is an independent prognostic factor [23]. The level of circulating tumor cells at diagnosis is prognostic in women with breast carcinoma and serial measures of circulating tumor cells in this population may predict relapse [21,22,25]. In these studies, circulating tumor cells are enumerated based upon cell surface expression of epithelial markers, such as epithelial cell adhesion molecule or cytokeratin. As a sarcoma, Ewing sarcoma does not typically express these markers and therefore the standard approaches for detecting circulating tumor cells in patients with carcinoma cannot be applied to this disease.
RT-PCR is currently the only technique available for the detection of circulating Ewing sarcoma cells. RT-PCR has several potential disadvantages. This approach requires prior knowledge of the type of EWS fusion oncogene present in the tumor. As such, RT-PCR requires the appropriate primers to detect the appropriate fusion transcript. The type of transcript is not known at initial diagnosis and may never be determined in the clinical care of a patient with Ewing sarcoma. Although PCR has high sensitivity, this methodology may also be prone to contamination and false-positive results. In addition, the stability of mRNA in routinely collected blood and bone marrow specimens is limited. Finally, PCR does not typically provide quantitative information about the number of tumor cells present.
Flow cytometry for CD45 and CD99 circumvents the need to know the type of EWS fusion oncogene before testing a particular patient for circulating tumor cells. Since CD99 is nearly universally expressed on Ewing sarcoma cells, this assay may be applied more generally to patients with these tumors. For those patients with minimal or absent CD99 expression, the flow cytometry assay may yield a false-negative result. Nevertheless, this assay may ultimately be more sensitive than RT-PCR, given that patients with tumors harboring less common EWS fusion oncogenes or samples with degraded mRNA will test negative by RT-PCR but may still be positive by flow cytometry. One final consideration is that flow cytometry requires fresh material for analysis while samples for RT-PCR can be frozen and analyzed in batches. For a rare tumor such as Ewing sarcoma, flow cytometry may therefore be more labor-intensive compared to RT-PCR. However, since samples for flow cytometry are analyzed in real time, the results are available more quickly compared to RT-PCR samples analyzed in batches. This difference may be advantageous if this assay will ultimately be used to make clinical decisions, as is the case for flow cytometry-based minimal residual disease detection in childhood acute lymphoblastic leukemia.
In conclusion, flow cytometry using CD99 and CD45 provides a potential alternative strategy for detecting circulating Ewing sarcoma tumor cells. A clinical study to determine the ability of this methodology to detect circulating Ewing sarcoma tumor cells in the blood and bone marrow of additional patients with newly diagnosed or relapsed disease is ongoing. This study will also compare flow cytometry to RT-PCR in these patients. This technique has the potential for improving risk stratification and early response assessment in patients with this disease.
Acknowledgments
We acknowledge support from the Campini Foundation, the Fighting Childhood Cancer Foundation, the Sarcoma Foundation of America, Hope Street Kids, the UCSF Department of Pediatrics, and NIH/NCRR UCSF-CTSI Grant Numbers RR024131 and KL2 RR024130. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or other funding agencies.
Footnotes
Conflict of Interest Statement: The authors have no relevant conflicts of interest to disclose.
References
- 1.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 2.Chan RC, Sutow WW, Lindberg RD, et al. Management and results of localized Ewing's sarcoma. Cancer. 1979;43(3):1001–1006. doi: 10.1002/1097-0142(197903)43:3<1001::aid-cncr2820430332>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Cangir A, Vietti TJ, Gehan EA, et al. Ewing's sarcoma metastatic at diagnosis. Results and comparisons of two intergroup Ewing's sarcoma studies. Cancer. 1990;66(5):887–893. doi: 10.1002/1097-0142(19900901)66:5<887::aid-cncr2820660513>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Arvand A, Denny CT. Biology of EWS/ETS fusions in Ewing's family tumors. Oncogene. 2001;20(40):5747–5754. doi: 10.1038/sj.onc.1204598. [DOI] [PubMed] [Google Scholar]
- 5.Peter M, Magdelenat H, Michon J, et al. Sensitive detection of occult Ewing's cells by the reverse transcriptase-polymerase chain reaction. Br J Cancer. 1995;72(1):96–100. doi: 10.1038/bjc.1995.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfleiderer C, Zoubek A, Gruber B, et al. Detection of tumour cells in peripheral blood and bone marrow from Ewing tumour patients by RT-PCR. Int J Cancer. 1995;64(2):135–139. doi: 10.1002/ijc.2910640211. [DOI] [PubMed] [Google Scholar]
- 7.West DC, Grier HE, Swallow MM, et al. Detection of circulating tumor cells in patients with Ewing's sarcoma and peripheral primitive neuroectodermal tumor. J Clin Oncol. 1997;15(2):583–588. doi: 10.1200/JCO.1997.15.2.583. [DOI] [PubMed] [Google Scholar]
- 8.Zoubek A, Ladenstein R, Windhager R, et al. Predictive potential of testing for bone marrow involvement in Ewing tumor patients by RT-PCR: a preliminary evaluation. Int J Cancer. 1998;79(1):56–60. doi: 10.1002/(sici)1097-0215(19980220)79:1<56::aid-ijc11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Schleiermacher G, Peter M, Oberlin O, et al. Increased risk of systemic relapses associated with bone marrow micrometastasis and circulating tumor cells in localized ewing tumor. J Clin Oncol. 2003;21(1):85–91. doi: 10.1200/JCO.2003.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Ambros IM, Ambros PF, Strehl S, et al. MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67(7):1886–1893. doi: 10.1002/1097-0142(19910401)67:7<1886::aid-cncr2820670712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Dworzak MN, Fritsch G, Buchinger P, et al. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood. 1994;83(2):415–425. [PubMed] [Google Scholar]
- 12.Dworzak MN, Froschl G, Printz D, et al. CD99 expression in T-lineage ALL: implications for flow cytometric detection of minimal residual disease. Leukemia. 2004;18(4):703–708. doi: 10.1038/sj.leu.2403303. [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Benda PM, Wood BL, et al. Lineage-specific identification of nonhematopoietic neoplasms by flow cytometry. Am J Clin Pathol. 2003;119(5):643–655. doi: 10.1309/FU3F-DKYN-8AU0-891N. [DOI] [PubMed] [Google Scholar]
- 14.Leon ME, Hou JS, Galindo LM, et al. Fine-needle aspiration of adult small-round-cell tumors studied with flow cytometry. Diagn Cytopathol. 2004;31(3):147–154. doi: 10.1002/dc.20074. [DOI] [PubMed] [Google Scholar]
- 15.Kreppel M, Aryee DN, Schaefer KL, et al. Suppression of KCMF1 by constitutive high CD99 expression is involved in the migratory ability of Ewing's sarcoma cells. Oncogene. 2006;25(19):2795–2800. doi: 10.1038/sj.onc.1209300. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Ramirez A, Rodriguez-Perales S, Melendez B, et al. Characterization of the A673 cell line (Ewing tumor) by molecular cytogenetic techniques. Cancer Genet Cytogenet. 2003;141(2):138–142. doi: 10.1016/s0165-4608(02)00670-2. [DOI] [PubMed] [Google Scholar]
- 17.Scotlandi K, Baldini N, Cerisano V, et al. CD99 engagement: an effective therapeutic strategy for Ewing tumors. Cancer Res. 2000;60(18):5134–5142. [PubMed] [Google Scholar]
- 18.Donnenberg AD, Donnenberg VS. Rare-event analysis in flow cytometry. Clin Lab Med. 2007;27(3):627–652. viii. doi: 10.1016/j.cll.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Yaniv I, Stein J, Luria D, et al. Ewing Sarcoma tumor cells express CD34: implications for autologous stem cell transplantation. Bone Marrow Transplant. 2007;39(10):589–594. doi: 10.1038/sj.bmt.1705640. [DOI] [PubMed] [Google Scholar]
- 20.Shah VO, Civin CI, Loken MR. Flow cytometric analysis of human bone marrow. IV. Differential quantitative expression of T-200 common leukocyte antigen during normal hemopoiesis. J Immunol. 1988;140(6):1861–1867. [PubMed] [Google Scholar]
- 21.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 22.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23(7):1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 23.Kollermann J, Weikert S, Schostak M, et al. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J Clin Oncol. 2008;26(30):4928–4933. doi: 10.1200/JCO.2007.15.0441. [DOI] [PubMed] [Google Scholar]
- 24.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pachmann K, Camara O, Kavallaris A, et al. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol. 2008;26(8):1208–1215. doi: 10.1200/JCO.2007.13.6523. [DOI] [PubMed] [Google Scholar]