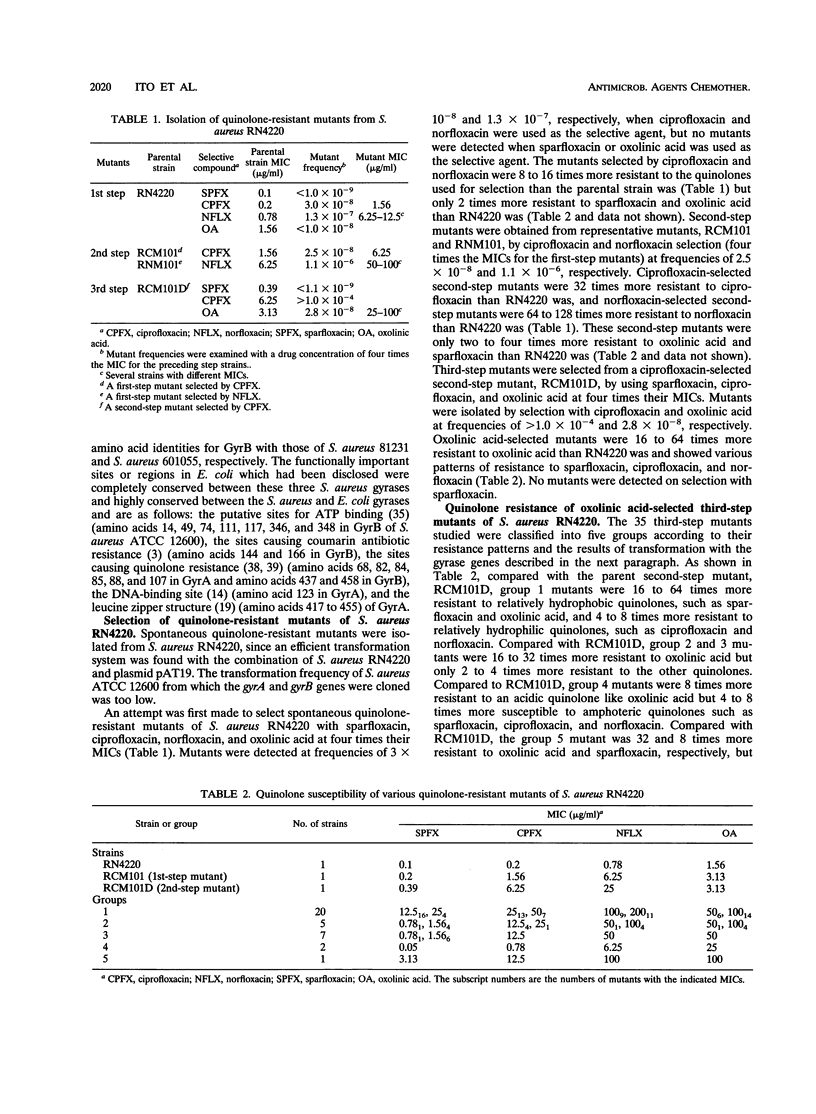
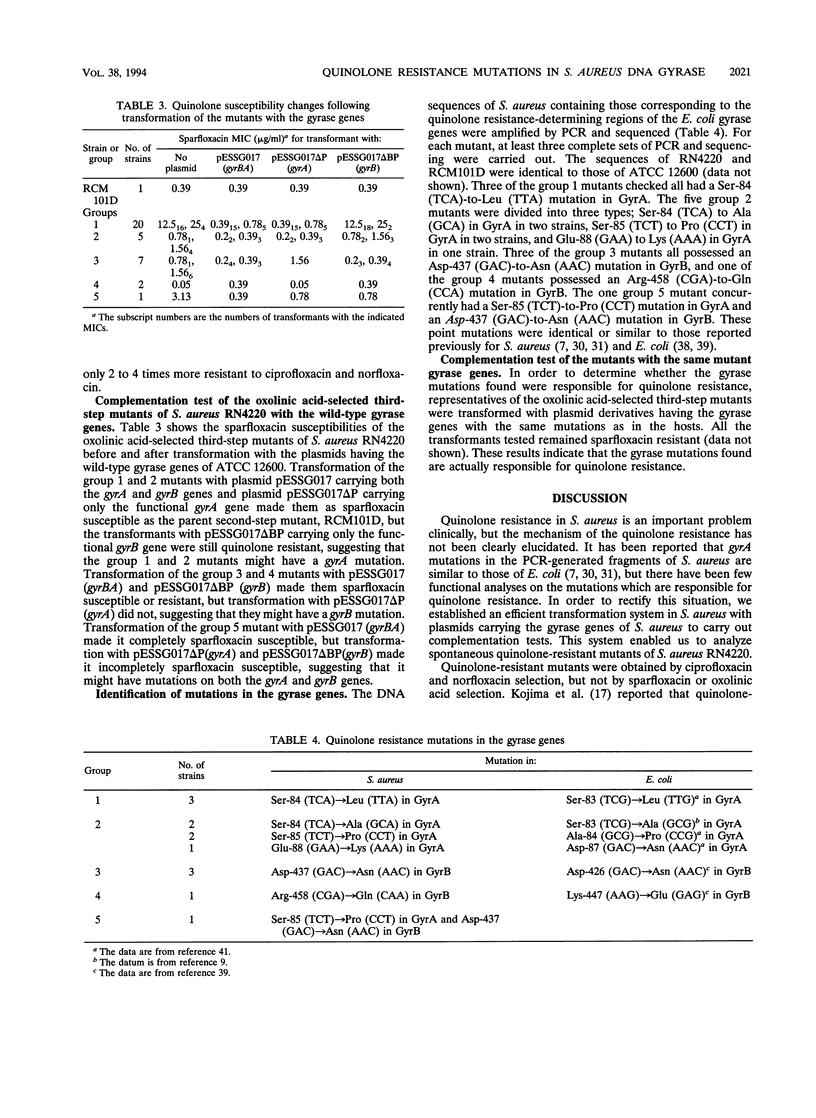
Abstract
A 6.4-kb DNA fragment containing the DNA gyrase gyrA and gyrB genes was cloned and sequenced from the quinolone-susceptible Staphylococcus aureus type strain ATCC 12600. An expression plasmid was constructed by inserting the cloned genes into the Escherichia coli-S. aureus shuttle vector pAT19, and deletion plasmids carrying only functional gyrA and gyrB genes were derived from this plasmid. An efficient transformation system for S. aureus RN4220 was established by using these plasmids. Quinolone-resistant mutants of S. aureus RN4220 were isolated by three-step selection with quinolones. The first- and second-step mutants were considered to be transport mutants, and the third-step mutants were divided into five groups with respect to their resistance patterns and transformation results with gyrA and gyrB genes. Sequencing analysis of the resulting mutant gyrase genes showed that they had the following point mutations: group 1, Ser-84 (TCA) to Leu (TTA) in GyrA; group 2, Ser-84 (TCA) to Ala (GCA), Ser-85 (TCT) to Pro (CCT), or Glu-88 (GAA) to Lys (AAA) in GyrA; group 3, Asp-437 (GAC) to Asn (AAC) in GyrB; group 4, Arg-458 (CGA) to Gln (CAA) in GyrB; and group 5, Ser-85 (TCT) to Pro (CCT) in GyrA and Asp-437 (GAC) to Asn (AAC) in GyrB. When the gyrA and/or gyrB mutants were transformed with the wild-type gyrA and/or gyrB plasmids, they became quinolone susceptible, but transformants with the plasmids having the same mutations on the gyrA and/or gyrB genes did not confer susceptibility. These results indicate that mutations in both gyrA and gyrB can be responsible for quinolone resistance in S. aureus.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockbank S. M., Barth P. T. Cloning, sequencing, and expression of the DNA gyrase genes from Staphylococcus aureus. J Bacteriol. 1993 Jun;175(11):3269–3277. doi: 10.1128/jb.175.11.3269-3277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras A., Maxwell A. gyrB mutations which confer coumarin resistance also affect DNA supercoiling and ATP hydrolysis by Escherichia coli DNA gyrase. Mol Microbiol. 1992 Jun;6(12):1617–1624. doi: 10.1111/j.1365-2958.1992.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. The nature of the transformation process in Escherichia coli K12. Mol Gen Genet. 1973 Jul 31;124(1):1–10. doi: 10.1007/BF00267159. [DOI] [PubMed] [Google Scholar]
- Fasching C. E., Tenover F. C., Slama T. G., Fisher L. M., Sreedharan S., Oram M., Willard K., Sinn L. M., Gerding D. N., Peterson L. R. gyrA mutations in ciprofloxacin-resistant, methicillin-resistant Staphylococcus aureus from Indiana, Minnesota, and Tennessee. J Infect Dis. 1991 Nov;164(5):976–979. doi: 10.1093/infdis/164.5.976. [DOI] [PubMed] [Google Scholar]
- Goswitz J. J., Willard K. E., Fasching C. E., Peterson L. R. Detection of gyrA gene mutations associated with ciprofloxacin resistance in methicillin-resistant Staphylococcus aureus: analysis by polymerase chain reaction and automated direct DNA sequencing. Antimicrob Agents Chemother. 1992 May;36(5):1166–1169. doi: 10.1128/aac.36.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P., Maxwell A. Novel quinolone resistance mutations of the Escherichia coli DNA gyrase A protein: enzymatic analysis of the mutant proteins. Antimicrob Agents Chemother. 1991 Feb;35(2):335–340. doi: 10.1128/aac.35.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hopewell R., Oram M., Briesewitz R., Fisher L. M. DNA cloning and organization of the Staphylococcus aureus gyrA and gyrB genes: close homology among gyrase proteins and implications for 4-quinolone action and resistance. J Bacteriol. 1990 Jun;172(6):3481–3484. doi: 10.1128/jb.172.6.3481-3484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S., Ohshita Y., Utsui Y., Hiramatsu K. Sequential acquisition of norfloxacin and ofloxacin resistance by methicillin-resistant and -susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1993 Nov;37(11):2278–2284. doi: 10.1128/aac.37.11.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987 Apr 15;262(11):5339–5344. [PubMed] [Google Scholar]
- Kaminsky D., Meltzer R. I. Quinolone antibacterial agents. Oxolinic acid and related compounds. J Med Chem. 1968 Jan;11(1):160–163. doi: 10.1021/jm00307a041. [DOI] [PubMed] [Google Scholar]
- Koga H., Itoh A., Murayama S., Suzue S., Irikura T. Structure-activity relationships of antibacterial 6,7- and 7,8-disubstituted 1-alkyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acids. J Med Chem. 1980 Dec;23(12):1358–1363. doi: 10.1021/jm00186a014. [DOI] [PubMed] [Google Scholar]
- Kojima T., Inoue M., Mitsuhashi S. In vitro activity of AT-4140 against quinolone- and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Jun;34(6):1123–1127. doi: 10.1128/aac.34.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Margerrison E. E., Hopewell R., Fisher L. M. Nucleotide sequence of the Staphylococcus aureus gyrB-gyrA locus encoding the DNA gyrase A and B proteins. J Bacteriol. 1992 Mar;174(5):1596–1603. doi: 10.1128/jb.174.5.1596-1603.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Matsumoto J., Chiba K., Egawa H., Shibamori K., Minamida A., Nishimura Y., Okada H., Kataoka M., Fujita M. Synthesis and structure-activity relationships of 5-substituted 6,8-difluoroquinolones, including sparfloxacin, a new quinolone antibacterial agent with improved potency. J Med Chem. 1990 Jun;33(6):1645–1656. doi: 10.1021/jm00168a018. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Yoshida S., Wakebe H., Inoue M., Yamaguchi T., Mitsuhashi S. Mechanisms of clinical resistance to fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Dec;35(12):2562–2567. doi: 10.1128/aac.35.12.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J., Okamoto S., Takahata M., Nishino T. Inhibitory effects of ciprofloxacin and sparfloxacin on DNA gyrase purified from fluoroquinolone-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Nov;35(11):2288–2293. doi: 10.1128/aac.35.11.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Hoshino K., Tanaka M., Hayakawa I., Osada Y. Antimicrobial activity of DU-6859, a new potent fluoroquinolone, against clinical isolates. Antimicrob Agents Chemother. 1992 Jul;36(7):1491–1498. doi: 10.1128/aac.36.7.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S. Methicillin-resistant strains of Staphylococcus aureus resistant to quinolones. J Clin Microbiol. 1989 Feb;27(2):335–336. doi: 10.1128/jcm.27.2.335-336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit I., Berger S. A., Gorea A., Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989 Apr;33(4):593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussy C. J., Wolfson J. S., Ng E. Y., Hooper D. C. Limitations of plasmid complementation test for determination of quinolone resistance due to changes in the gyrase A protein and identification of conditional quinolone resistance locus. Antimicrob Agents Chemother. 1993 Dec;37(12):2588–2592. doi: 10.1128/aac.37.12.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan S., Oram M., Jensen B., Peterson L. R., Fisher L. M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan S., Peterson L. R., Fisher L. M. Ciprofloxacin resistance in coagulase-positive and -negative staphylococci: role of mutations at serine 84 in the DNA gyrase A protein of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1991 Oct;35(10):2151–2154. doi: 10.1128/aac.35.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Carlier C., Poyart-Salmeron C., Courvalin P. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene. 1991 Jun 15;102(1):99–104. doi: 10.1016/0378-1119(91)90546-n. [DOI] [PubMed] [Google Scholar]
- Trucksis M., Wolfson J. S., Hooper D. C. A novel locus conferring fluoroquinolone resistance in Staphylococcus aureus. J Bacteriol. 1991 Sep;173(18):5854–5860. doi: 10.1128/jb.173.18.5854-5860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wigley D. B., Davies G. J., Dodson E. J., Maxwell A., Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991 Jun 20;351(6328):624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Yamanaka L. M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991 Aug;35(8):1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura S., Ubukata K., Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990 Dec;172(12):6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kojima T., Yamagishi J., Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Nakamura M., Bogaki M., Ito H., Kojima T., Hattori H., Nakamura S. Mechanism of action of quinolones against Escherichia coli DNA gyrase. Antimicrob Agents Chemother. 1993 Apr;37(4):839–845. doi: 10.1128/aac.37.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]



