Abstract
Lung cancer is the leading cause of cancer mortality in women worldwide. Although the rise and growing epidemic status of this disease is overwhelmingly attributed to tobacco use, the rank of lung cancer in nonsmokers as the seventh most common cause of cancer worldwide suggests that other factors contribute to this disease. The majority of lung cancers among nonsmokers occur in women. Aside from geographic, cultural and genetic differences, hormonal and possibly infectious factors may also play etiologic roles. This review aims to discuss the epidemiology of lung cancer in women as well as the incidence of second primaries, and presents current opinions on the myriad of causes.
INTRODUCTION
Steady increases in the incidence of lung cancer during the past century have led to its dubious position as the leading cause of cancer-related death in both men and women in the United States and the most frequent cause of cancer mortality worldwide. While lung cancer has historically affected primarily men, the gap between genders is narrowing quickly. Lung cancer deaths in women in the U.S. began rising in 1960 and reached a critical juncture in 1987 when the number of female deaths from lung cancer exceeded those from breast cancer.1 Ironically, this was only 7 years after the Surgeon General issued a report on the health consequences of smoking in women, confirming that smoking caused lung cancer in women as well as men. The number of female deaths attributed to lung cancer currently far exceeds that from all gynecological cancers combined; a disease of epidemic proportions. The American Cancer Society (ACS) estimates that in 2008, females contributed 47% of the 215,020 new cases of lung/bronchus cancer in the U.S. and 44% of the 161,840 deaths associated with this disease. A woman’s lifetime risk of developing lung cancer is high (1 in 16 women) but lower than that of a man (1 in 13 men).2 Although the survival rates of women with lung cancer are usually higher than those of men,3,4 estimated 5-year survival for both sexes from 1990–2005 is very poor (15% in women versus 11% in men).5
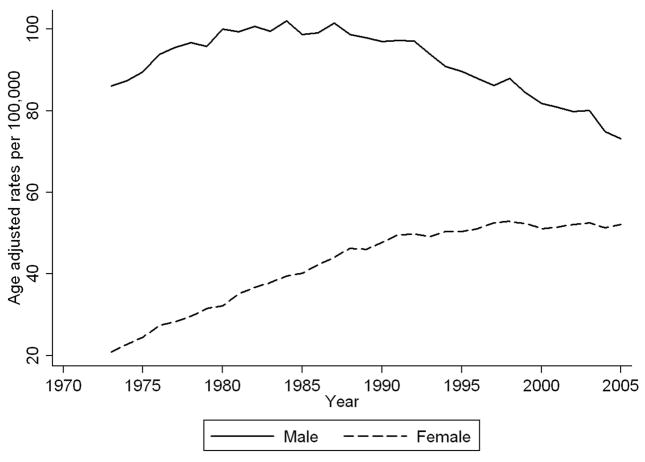
Surveillance Epidemiology and End Results (SEER) were used to examine trends in lung cancer rates from 1973–2005.5 In Figure 1, we present rates of lung cancer in men and women age-adjusted to the 2000 U.S. population as implemented in the SEER*Stat software.6 As illustrated, the incidence of lung cancer in men increased until the mid-1980s, at which point the rate leveled off and began to decline. In contrast, rates for women started to increase dramatically in 1973 and did not begin to level off until the mid-1990s. As demonstrated in Figure 1, the difference in the incidence of lung cancer in women and men has narrowed over time.
Fig. 1.
Age-adjusted rates of lung cancer in men and women over time (Source: SEER 9-registry data)
Geographic Differences in Lung Cancer Incidence in Women
Although lung cancer remains the most common cause of cancer mortality worldwide, age-standardized incidence rates for women vary 30-fold (<1 to <36.1/100,000) among geographic regions.7 Rates of female lung cancer are highest in North America and Northern Europe, followed by Micronesia, Eastern Asia, Australia/New Zealand.7 According to Alberg and colleagues,8 intercontinental variability in lung cancer incidence cannot be attributed solely to differences in diagnostic practices and data quality. An analysis of cancer mortality data from the World Health Organization for 1970–2003 revealed a steady increase in the mortality of females with lung cancer over the past three decades, with an annual percent change of 4.6% between 2001 and 2003.9 Further analysis of the age-standardized trends in lung cancer mortality among women 20–44 years of age in six European countries demonstrated significant increases in recent years in France and Spain.10 France exhibited both the highest lung cancer rate during the past 30 years and the largest increase in the past 20 years of the six European countries evaluated. Based on these data, Levi and colleagues10 estimate that within the next two to three decades, the “female lung cancer epidemic” will expand from its current rates of 5/100,000 in Spain and 7.7/100,000 in France to a staggering 20/100,000 per year in younger women.
As the incidence of lung cancer begins to decline in the Western world, higher rates are emerging in developing countries. Toh et al.11 estimates that by the middle of this century, more than half of all lung cancer cases will be diagnosed in developing countries. Unfortunately, the rates reported to date are most likely an underestimate, particularly in developing countries where a percentage remains undiagnosed due to limited financial and technological resources. Most notable is the current high incidence of lung cancer among nonsmoking women from Asian countries. Although the basis for this geographic “hot spot” remains unknown, exposure to fumes generated by heating cooking oils and burning coal in poorly ventilated households have been implicated (see Sun et al. for review).12
Lung Cancer in Smokers
As in men, exposure to tobacco smoke remains the primary risk factor for the development of lung cancer in women, with approximately 90% of all female lung cancer deaths attributed to smoking. U.S. Surgeon General Dr. David Satcher indicated in his report in 2001 that “there is no better word than epidemic to describe the 600-percent increase since 1950 in women’s death rates for lung cancer, a disease primarily caused by cigarette smoking. Clearly, smoking-related disease among women is a full-blown epidemic.”1 An in-depth analysis of prospective data from the Nurses’ Health Study and the men’s Health Professionals Follow-up Study yielded incidence rates (per 100,000 person years) of lung cancer of 253 and 232 for female and male current smokers and 81 and 73 for female and male former smokers, respectively.13 The hazard ratio for women ever smokers as compared to men was 1.1 (confidence interval 0.95 – 1.31).
Lung cancer incidence traditionally reflects smoking patterns with a lag of 2–3 decades. Data on smoking rates from 1965–2005 from the National Health Interview Survey, as accessed on the CDC website, are presented in Figure 2. In 1965, the rate of smoking among men was much higher than that of women. The rapid decline in smoking rates since that time most likely explains why the rate of lung cancer in men leveled off in the late 1980s and started to decline subsequently. The decline in smoking rates among adult women plateaued in the 1990s and was paralleled by a steep increase in smoking rates among teenaged girls, in particular those with less than a high school education. It will likely be several more years before rates of lung cancer in women demonstrate a decline similar to that of men.
Fig. 2.
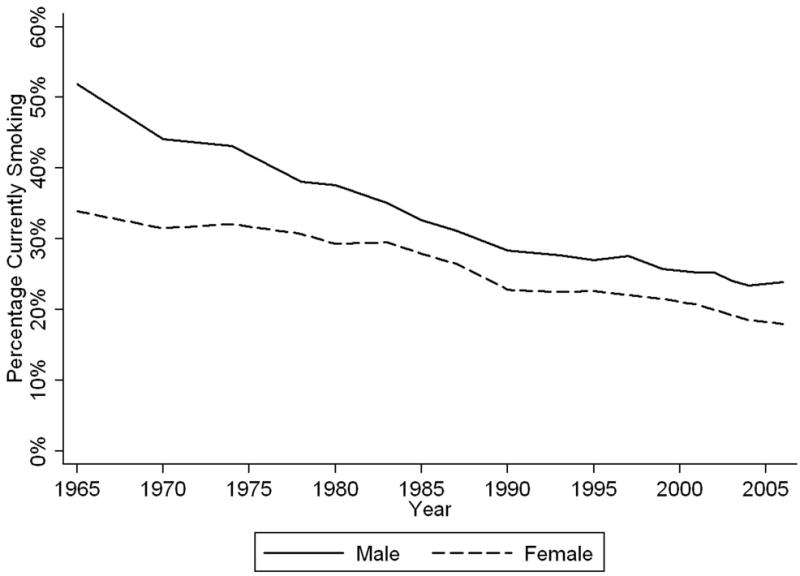
Percentage of men and women who report being current smokers each year (Source: National Health Interview Survey as accessed on CDC website http://www.cdc.gov/tobacco/data_statistics/tables/adult/table_2.htm, Feb. 26, 2009)
Female smokers have historically had a lower risk of developing lung cancer than men.1,14,15 Over time, however, the relative risk of lung cancer in female smokers compared to male smokers has increased.1,15 Possible temporal explanations include changes in the number of cigarettes smoked by female smokers,16–19 changes in the way that female smokers inhale smoke,19 changes in the types of cigarettes smoked by women,20,21 and earlier age of initiation of smoking among women over time.1,16,20 Other differences between the sexes might also play a role since female lung cancer patients have been found to have a reduced pack-year smoking history as compared to men.20,22,23 Some have hypothesized that women are at increased susceptibility for lung cancer as compared to men with a similar level of exposure to tobacco smoke.14,24,25 For example, results from a meta-analysis indicate that among current smokers, the combined odds ratio for squamous cell carcinoma (SCC) and small cell carcinoma was higher in women than in men, with less of a gender difference observed for adenocarcinoma.26 However, findings that women are at an increased risk of lung cancer after controlling for smoking exposure have not been confirmed in prospective cohorts. Comparison of the susceptibility of men and women for lung cancer in six prospective cohort studies failed to provide evidence that women are at increased risk for lung cancer when smoke exposure is equivalent.13 Freedman and colleagues found that overall, the adjusted risk of lung cancer among women was lower than among men.27 Hence, there are only limited data to suggest that women are at increased susceptibility for lung cancer at the population level as compared to men with an equivalent smoke exposure.
Lung Cancer in Nonsmokers
Lung cancer in nonsmokers is the seventh leading cause of cancer deaths worldwide, killing more than cancers of the cervix, pancreas and prostate.12 A significant fraction of lung cancer cases in the U.S. (10–15%) occur in nonsmokers, with a higher incidence reported for females.12,28,29 A review of data collected over the past 25 years indicates that the proportion of lung cancer cases that occur among nonsmokers is higher in females than males in Europe (2% of European lung cancers in men are among nonsmokers versus 21% in women), the U.S. (6% in men are among nonsmokers versus 15% in women), and both East and South Asia (11% of East Asian lung cancers in men are among nonsmokers versus 61% in women; similarly, 15% of South Asian lung cancers in men are among nonsmokers versus 83% in women).12 Thun and colleagues30 recently examined the incidence of lung cancer among nonsmokers (376,600 women and 253,600 men) using data pooled from eight large cohort studies conducted in the U.S. and Europe. This study evaluated participants in the Black Women’s Health Study (BWHS), the Cancer Prevention Study II Nutrition Cohort (CPS-II Nutrition), the European Prospective Investigation into Cancer and Nutrition (EPIC), the Health Professionals’ Follow-up Study (HPFS), the Multiethnic Cohort (MEC), the Nurses’ Health Study (NHS), the Swedish Construction Worker cohort (SCW), and the Women’s Health Study (WHS).30 The most striking finding was the lack of a gender difference in the rate of lung cancer. Lung cancer incidence, standardized to ≥40 years, was similar among nonsmoking men of European descent and nonsmoking women (14.0 and 13.8 per 100,000). However, a difference was observed when the data were stratified by age. For subjects 40–59 years of age, the incidence was higher in women as compared to men. For subjects 60–79 years of age, the incidence rates were similar in both genders, while incidence rates were lower in women than in men over age 80.
In contrast to the findings from the study conducted by Thun and colleagues,30 a thorough analysis of data from six large cohorts yielded age-adjusted incidence rates of lung cancer among female never smokers of 14.4 to 20.8 per 100,000 person-years, higher than that for male never smokers (4.8 to 13.7 per 100,000 person-years).31 This observation was based on individuals 40–79 years of age from three of the cohorts listed above (NHS, HPFS and MEC) plus the California Teachers Study (CTS), Swedish Uppsala/Örebro Lung Cancer Registry (U/OLCR), and First National Health and Nutrition Examination Survey Epidemiologic Follow-Up Study (NHEFS).31
It is debatable whether the incidence of lung cancer in nonsmokers is increasing.32,33 The proportion of U.S. adults (age ≥18 years) reported never having smoked 100 or more cigarettes increased from 44% in 1965 to 59% in 2006.30 Growth in the percentage of nonsmokers in economically developed countries is anticipated to lead to an absolute increase in the number of cases of lung cancer among nonsmokers in the population. While clinical data to document temporal differences in lung cancer incidence among nonsmokers may be confounded by the changing composition of nonsmokers over time, the incidence among a large cohort of nonsmoking male construction workers in Sweden increased from 1.5 per 100,000 from 1976 to 1980 to 5.4 per 100,000 from 1991 to 1995.32
The extent to which the rate of lung cancer mortality among nonsmokers may be increasing remains controversial. A study from many decades ago reports that the mortality rate among nonsmokers with lung cancer between 1914 and 1968 had a relative increase of 15-fold and 7-fold for white men and women, respectively.33 Comparison of lung cancer death rates among female nonsmokers in two American Cancer Society cohorts, CPS-I (1959–1972) and CPS-II (1982–2000), revealed a higher rate of mortality in the most recent study among white women (HR = 1.25, 95% CI = 1.12 – 1.41) and black women (HR = 1.22, 95% CI = 0.64 – 2.33) but not white men (HR = 0.89, 95% CI = 0.74 – 1.08).34 However, this trend of increased mortality in women was significant only in women 70–84 years of age (P < 0.001). Among white men, the age-specific lung cancer death rates decreased from CPS-I to CPS-II among never smokers 50–69 years of age, suggesting that the temporal association of lung cancer mortality with gender is influenced by age.34 On the other hand, when the data from the CPS-II cohort were extended four additional years, no statistically significant difference in lung cancer mortality between CPS-I and CPS-II was observed for women of European descent (RR = 1.11, 95% CI = 0.98 –1.25), African American women (RR = 1.15, 95% CI = 0.62 – 2.13), or men of European descent (RR = 0.83, 95% CI = 0.66 – 1.05).30
Lung Tumor Histology
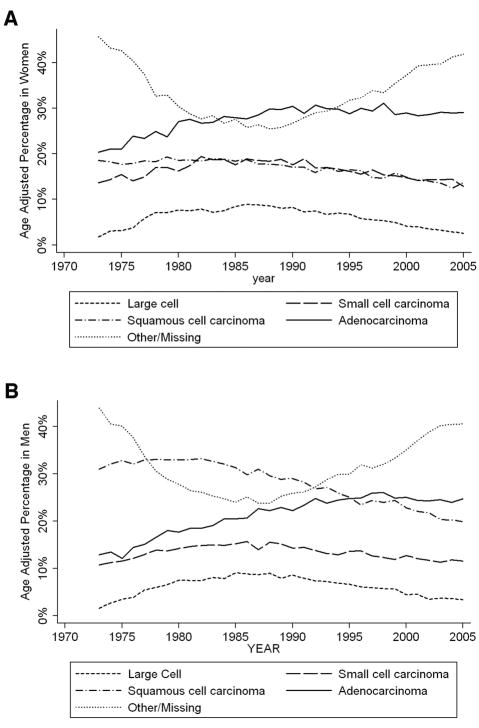
Figure 3 depicts the proportion of the four common histological types of lung cancer in female and male incident cases over the past 35 years using SEER 9-registry data. Note that the proportions at each year within each gender sum to 100%. Adenocarcinoma has remained the most prevalent tumor histology among women over the past three decades, with incidence rates increasing slowly over time. In contrast, squamous cell carcinoma has historically been the predominant tumor type in men, with rates declining and hence converging with the rates in women, which have been fairly stable over time. The profile of large-cell carcinoma in women parallels that of men, with rates decreasing slightly over time. The rate of small-cell carcinoma has remained relatively constant in both genders. It was not until the mid 1990s that adenocarcinoma surpassed squamous cell carcinoma as the leading pathological subtype of lung tumor in men (Figure 3b). The striking change in the incidence of adenocarcinoma in both genders has been attributed to changes in the composition of cigarettes and the implementation of filters.11,35,36 It is postulated that use of filtered cigarettes has caused an increase in the incidence of adenocarcinomas, located in the peripheral lung. Because smokers now need to inhale more deeply to achieve a comparable effect, the exposure of the lung tissue to tobacco smoke is more extensive. In their review of the literature, Stellman et al.35 suggest that filters increase exposure to tobacco-specific nitrosamines, which may be risk factors for the development of adenocarcinoma. One caveat in any presentation of population level data is that there is often a great deal of missing and incorrectly recorded data on histology in observational and administrative datasets such as SEER and as noted in the other/missing category in Figure 3. While the observed changes in histology could be impacted by revisions in the World Health Organization’s classification of lung tumors (1981, 1999 and 2004), the magnitude of the changes in the figures suggests that the changes are not simply due to changes in classification.
Fig. 3.
Fig. 3a. Age-adjusted percentages of common histological subtypes in women
Fig. 3b. Age-adjusted percentages of common histological subtypes in men
Specific molecular alterations such as the presence of activating mutations in the Epidermal Growth Factor Receptor (EGFR) have been associated with adenocarcinoma histology. In addition, mutations in EGFR are more frequently found in women, nonsmokers and those of East Asian ethnicity and have been associated with differential response of lung tumors to EGFR inhibitors.37,38 The putative favorable response of nonsmokers with lung cancer, in particular women, to treatment with EGFR inhibitors has drawn attention to the possibility that lung tumors in nonsmokers are different from those of smokers.37,39 Other distinct molecular features of lung tumors in never smokers include the lower frequency of mutations in the KRAS and TP53 genes as compared to lung tumors from smokers.12
Effect of Hormones on the Incidence of Lung Cancer in Women
The contribution of menstrual and reproductive factors to lung cancer risk has been evaluated prospectively in the Shanghai Women’s Health Study. The risk of lung cancer among 71,314 lifetime nonsmoking females was decreased significantly among those with increased parity, later age of menopause and a longer reproductive period.40 This finding is contrary to prospective studies from other geographical regions. Early age at menarche and late age at menopause conferred an increased risk of lung cancer among Japanese female nonsmokers.41 Kabat et al.42 observed an increased risk of lung cancer among both female smokers and nonsmokers with higher parity and a younger age at first live birth.
The potential impact of widespread hormone use in peri- and postmenopausal women on the changing epidemiology of lung cancer in women is uncertain. The frequency of hormonal therapy use fluctuated dramatically in the 1970s and 1980s in the U.S., first rising as new formulas evolved and then declining by 50% in response to reports that exposure to unopposed estrogens increased women’s risk of developing endometrial cancer.43–45 Prescriptions for hormonal therapies increased 57% between 1995 and 2002, with the introduction of combined progestin/estrogen therapy accounting for 70% of this growth. Use of hormonal therapies plummeted in 2002 after data from the Women’s Health Initiative (WHI) indicated that administration of progestin in combination with estrogen conferred an increased risk of breast cancer and cardiovascular disease relative to non-users.46 Several studies have failed to detect an association between use of hormonal therapies and risk for lung cancer.47,48 However, results from an early study indicated that women who smoked cigarettes and took hormone replacement therapy (HRT) were at a 2.5-fold increased risk of developing adenocarcinoma of the lung as compared to female smokers who did not take HRT.49 A significant association between HRT use and both a lower age of lung cancer diagnosis and decreased median survival time has been observed recently.50 Other epidemiological studies suggest that HRT use may provide female smokers with protection against lung cancer.51,52 Kreuzer et al.51 reported a reduction in lung cancer risk among German women who smoked during their lifetime and used oral contraceptives (OR = 0.69; 95% CI: 0.51–0.92). However, no association was observed between risk and duration of use, age at first use, or calendar year of first use. A decreased risk for lung cancer was also observed among women who took HRT, (OR = 0.83; 95% CI: 0.64–1.09), in particular after 7 years of use (OR = 0.59; 95% CI: 0.37–0.93).51 If the effect of hormonal exposures on lung tissue requires a two- to three-decade lag period for observation, as with tobacco smoke, the full impact of hormone use on the incidence of lung cancer in women may not be evident for some time.
Effect of Human Papilloma Viruses on the Incidence of Lung Cancer in Women
The potential contribution of human papillomaviruses (HPVs) to bronchial squamous cell lesions was first suggested 30 years ago.53 More recently, the presence of both HPV oncoproteins and E6 and E7 transcripts in lung tissue has been reported (for review, see Guiliani et al.).54 Since HPV-induced lesions occur at squamocolumnar mucosal junctions, and smokers often have foci of metaplastic squamous mucosa, it is not unreasonable to postulate a role for HPV in the development of SCC. The detection rate of HPV (mostly high-risk subtypes 16 and 18) by a variety of methods in de novo SCC ranges from 0–80%.55–63 Calculation of the incidence of HPV in lung cancer based on data from 53 published studies yielded a mean incidence of 15% in America and 24.5% worldwide.64 A literature review concluded that HPV DNA was detected in approximately 22% of bronchial carcinomas.65 While geographic, racial and seasonal variation may impact HPV data, the generation of opposite results in similar locations points to methodological flaws in viral detection.66–70 A recent analysis of 217 stage I NSCLC patients revealed high expression of HPV-16 and -18, classified as the most carcinogenic types of HPV in humans,71 in female patients, nonsmokers and, surprisingly, patients with adenocarcinoma.72 Lung cancer patients who expressed HPV-16 and HPV-18 had a significantly higher survival rate (72%) than those who did not express both oncoproteins (48%). The biological significance of these observations remains to be determined. Hematogenous spread of virus from cervical infections (subtypes 16 and 18) rather than inhaled virus (subtypes 6 and 11) is also postulated, with additional studies necessary.
Risk of Developing Second Primary Tumors Following Lung Cancer
If use of hormonal therapies is associated with increased risk for lung cancer, as well as breast cancer, one might expect an increased risk of breast cancer among women who develop lung cancer. Likewise, if HPV infection increases the risk for cervical cancer, lung cancer and certain types of cancers of the head and neck (i.e., carcinomas of the oropharynx and tonsils), an association between the development of these primary tumors in HPV-infected individuals would be anticipated. Table 1 provides SEER data (9-registries) on the risk of detecting second primaries in patients at least 3 months subsequent to a diagnosis of lung cancer. The person-years at risk of developing cancer in the late 1970s, 1980s, 1990s, and early 2000s, the observed rate of cancers at each time point and the observed/expected ratio, also known as the standardized incidence ratio (SIR), are presented. The SIR estimates reflect the increase in the incidence of tumors over what we would expect after adjusting for age, sex, race, and year of diagnosis. The SIR estimates were obtained using the MP-SIR macro in SEER*Stat. A SIR greater than 1 suggests that individuals who have lung cancer are at increased risk of developing another type of cancer as compared to the general population, while a SIR less than 1 suggests that individuals with lung cancer are at a decreased risk of developing another type of cancer as compared to the general population. Any inferences based on the SIR estimates need to be made cautiously. Because the overall 5-year survival rate for lung cancer is approximately 15%, these results might be influenced by a healthy survivor effect in which the women who lived long enough to develop a second tumor are very different than those who did not live long enough.
Table 1.
Increased risk of cancers over time that occur three or more months following a lung cancer diagnosis in SEER. Year refers to the year of diagnosis of the secondary tumor.
| Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Upper GI | Lower GI | Lung | Urinary | ||||||
| Year | Person Years at Risk | Observed | SIR | Observed | SIR | Observed | SIR | Observed | SIR |
| 1975–1979 | 25,486.34 | 33 | 0.87 | 63 | 1.04 | 76 | 0.9 | 46 | 1.51* |
| 1980–1989 | 94,999.43 | 241 | 1.60* | 263 | 0.98 | 581 | 1.57* | 199 | 1.48* |
| 1990–1999 | 116,760.71 | 281 | 1.37* | 382 | 1.13* | 1,025 | 2.17* | 286 | 1.48* |
| 2000–2005 | 70,844.35 | 190 | 1.49* | 232 | 1.27* | 655 | 2.56* | 173 | 1.39* |
| Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Upper GI | Lower GI | Lung | Urinary | ||||||
| Year | Person Years at Risk | Observed | SIR | Observed | SIR | Observed | SIR | Observed | SIR |
| 1975–1979 | 11,479.51 | 12 | 1.52 | 24 | 1.34 | 21 | 2.18* | 8 | 2.44* |
| 1980–1989 | 58,649.03 | 75 | 1.55* | 122 | 1.09 | 269 | 3.16* | 42 | 2.01* |
| 1990–1999 | 99,281.37 | 133 | 1.37* | 235 | 1.15* | 748 | 3.58* | 73 | 1.66* |
| 2000–2005 | 74,522.38 | 86 | 1.1 | 184 | 1.23* | 681 | 4.01* | 71 | 1.98* |
| Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lymphoma/Leukemia | Kidney/Renal Pelvis | Oral Cavity without Oropharynx/Tonsil | Oropharynx/Tonsil | ||||||
| Year | Person Years at Risk | Observed | SIR | Observed | SIR | Observed | SIR | Observed | SIR |
| 1975–1979 | 25,486.34 | 19 | 0.69 | 25 | 2.87* | 38 | 1.59* | 2 | 1 |
| 1980–1989 | 94,999.43 | 128 | 1 | 58 | 1.36* | 214 | 2.34* | 19 | 2.52* |
| 1990–1999 | 116,760.71 | 195 | 0.99 | 63 | 0.96* | 246 | 2.40* | 28 | 3.08* |
| 2000–2005 | 70,844.35 | 111 | 0.86 | 78 | 1.69* | 124 | 2.45* | 22 | 4.05* |
| Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphoma/Leukemia | Kidney/Renal Pelvis | Oral Cavity without Oropharynx/Tonsil | Oropharynx/Tonsil | Müllerian | |||||||
| Year | Person Years at Risk | Observed | SIR | Observed | SIR | Observed | SIR | Observed | SIR | Observed | SIR |
| 1975–1979 | 11,479.51 | 7 | 0.95 | 4 | 2.6 | 9 | 3.36* | 3 | 9.66* | 51 | 1.07 |
| 1980–1989 | 58,649.03 | 51 | 1.02 | 27 | 2.36* | 37 | 2.23* | 10 | 5.61* | 271 | 0.94 |
| 1990–1999 | 99,281.37 | 111 | 1.01 | 41 | 1.52* | 86 | 2.99* | 5 | 1.95 | 495 | 0.88* |
| 2000–2005 | 74,522.38 | 89 | 1 | 39 | 1.62* | 46 | 2.35* | 4 | 2.45 | 345 | 0.85* |
p < 0.05
Table 1 provides clear evidence that, in general, lung cancer patients develop more second primary carcinomas of the oral cavity (excluding tonsilar and oropharyngeal sites), lung, upper gastrointestinal tract (GI), urinary tract and kidney relative to the number expected in the general population. This increased risk is likely related to tobacco smoke exposure being a risk factor for both lung cancer and these types of cancer.73 Increased risk for second primaries of the lower GI is confined to lung cancer patients diagnosed after 1990. The risk of lymphomas or leukemias does not appear to be increased in patients diagnosed with lung cancer.
When comparing SIRs over time for a particular target organ, it is important to acknowledge the potential contribution of changes in exposure to various environmental agents, other than tobacco smoke, to the observed trends. First, while SEER data suggest that the increased risk of urinary cancer following a diagnosis of lung cancer has declined over time, this could reflect changes in the composition or use of industrial chemicals and dyes. For example, Czene et al.74 found an increased risk of bladder cancer and lung cancer among hairdressers, particularly in the 1960s. Such evidence is consistent with the hypothesis that exposure to hair dyes increased one’s risk of bladder cancer. Second, a link between inhalation of occupational forms of dust or exhaust fumes and gastrointestinal cancer has been suggested.75 Presumably, patterns of exposure to occupational dust and exhaust fumes have changed over time as better safety procedures were implemented and fuel formulations refined. Third, the suggested contribution of organic solvents and polycyclic aromatic hydrocarbons to kidney cancer risk76 could impact trends of increased risk of kidney cancer after a diagnosis of lung cancer. In all cases, synergistic effects between smoking and such time-varying exposures could further confound the analysis of such time dependent trends.
Interestingly, the risk for cancers of the oropharynx/tonsil has decreased in women over time, while increasing steadily in men. Because few second events have been reported in SEER for female lung cancer patients in recent years, it is unclear to what degree the trend represents a true decline. For men, in whom there are more oropharynx/tonsil events, the increase in risk over time, from a SIR of 2.52 in the 1980s to 4.05 in the 2000s, might reflect changes in the incidence of HPV infection, as Laukkanen found in Finland,77 and in the use of chewing tobacco, the prevalence of which quadrupled between 1970 and 1986 among 17–19-year-olds.78 However, the fact that the risk of other types of oral cancers did not similarly increase over the same time period argues against chewing tobacco being the sole cause of the increase in cancers for which HPV infection has been implicated.
The SIR for a müllerian cancer (breast, cervical, uterine) following a diagnosis of lung cancer has decreased over time. While this may argue against hormonal therapies having a strong role in lung cancer, it is too early to judge. A similar analysis of women who were diagnosed with primary breast cancer between 1973 and 2000 and registered in the SEER database revealed an increased risk of developing a second primary of the lung, with the highest SIR (6.7) observed in women between the ages of 30 and 39.79 It should be noted that for decades, use of radiation for the treatment of breast cancer led to excess exposure of the upper body, including the lungs. The ability of radiation exposure to increase the risk of lung cancer has been suggested.80,81 An association between lung cancer and mutation of BRCA1 and 2 has also been suggested.82
The negative correlation observed between lung cancer and müllerian cancers in the present analysis could be related to selection bias among women who used hormonal therapies. Observational data used to justify randomized trials of HRT in the prevention of cardiovascular disease found a protective effect of HRT against cardiovascular events. The finding that HRT was related to an increased risk of cardiovascular disease and breast cancer in randomized trials83,84 suggests that women who had historically used HRT in the community had healthier behaviors than those who did not. As such, one might hypothesize that there was an inverse historical correlation between smoking, a negative health behavior, and HRT use, considered a positive health behavior previously. If HRT use did drive increases in breast cancers among nonsmokers, then lower rates of HRT use among smokers might have caused the protective SIRs (SIR < 1) for müllerian cancers (which include breast cancer) among those who developed lung cancer in the 1990s and 2000s.
In any event, while the data supplied in Table 1 suggest interesting trends over time in the incidence of second primaries among lung cancer patients, it is not possible to make firm conclusions related to causality using these data. The mortality rate among those with lung cancer remains high. Hence, it is likely that those who experience long-term survival following a diagnosis of lung cancer are quite different from the general population. As the long-term survivors contribute more person-years of data to the calculation of the SIRs, a healthy survivor effect might be influencing the SIR estimates.
CONCLUSION
In summary, the worldwide epidemic of lung cancer in women continues to trouble clinicians and researchers alike. Of greatest concern is the increasing number of lung cancer deaths among nonsmokers. We can only hope that an in-depth understanding of the detrimental effects of various environmental exposures when combined with a comprehensive analysis of the molecular basis of lung cancer will lead to the establishment of new and efficacious strategies for the prevention and treatment of this devastating disease.
Acknowledgments
We wish to thank Maureen Climaldi for her excellent assistance in preparing this manuscript for publication.
This work was supported by USPHS grant CA-06927 from the National Cancer Institute, the Kitty Jackson Fund, the Estate of Jane Villon, the Jerome M. Spencer and Arnold Zaslow Family Foundation, the Fox Chase Cancer Center Keystone Program in Personalized Risk and Prevention, and by an appropriation from the Commonwealth of Pennsylvania. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Public Health Service, Office of the Surgeon General; Washington, D.C: 2001. [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. 2008 [Google Scholar]
- 3.Adebonojo SA, Bowser AN, Moritz DM, Corcoran PC. Impact of revised stage classification of lung cancer on survival: a military experience. Chest. 1999;115:1507–13. doi: 10.1378/chest.115.6.1507. [DOI] [PubMed] [Google Scholar]
- 4.Minami H, Yoshimura M, Miyamoto Y, Matsuoka H, Tsubota N. Lung cancer in women: sex-associated differences in survival of patients undergoing resection for lung cancer. Chest. 2000;118:1603–9. doi: 10.1378/chest.118.6.1603. [DOI] [PubMed] [Google Scholar]
- 5.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database. Incidence - SEER 9 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission.
- 6.Surveillance Research Program. National Cancer Institute SEER*Stat software. ( www.seer.cancer.gov/seerstat) version 6.4.4.
- 7.International Agency for Research on Cancer. GLOBOCAN 2002. Lyon, France: International Agency for Research on Cancer; 2002. [Google Scholar]
- 8.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 9.Bosetti C, Bertuccio P, Levi F, Lucchini F, Negri E, La Vecchia C. Cancer mortality in the European Union, 1970–2003, with a joinpoint analysis. Ann Oncol. 2008;19:631–40. doi: 10.1093/annonc/mdm597. [DOI] [PubMed] [Google Scholar]
- 10.Levi F, Bosetti C, Fernandez E, Hill C, Lucchini F, Negri E, et al. Trends in lung cancer among young European women: the rising epidemic in France and Spain. Int J Cancer. 2007;121:462–5. doi: 10.1002/ijc.22694. [DOI] [PubMed] [Google Scholar]
- 11.Toh C-K. The changing epidemiology of lung cancer. Methods Mol Biol. 2009;472:397–411. doi: 10.1007/978-1-60327-492-0_19. [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 13.Bain C, Feskanich D, Speizer FE, Thun M, Hertzmark E, Rosner BA, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst. 2004;96:826–34. doi: 10.1093/jnci/djh143. [DOI] [PubMed] [Google Scholar]
- 14.Ernster VL. Female lung cancer. Annu Rev Public Health. 1996;17:97–114. doi: 10.1146/annurev.pu.17.050196.000525. [DOI] [PubMed] [Google Scholar]
- 15.Thun MJ, Heath CW., Jr Changes in mortality from smoking in two American Cancer Society prospective studies since 1959. Prev Med. 1997;26:422–6. doi: 10.1006/pmed.1997.0182. [DOI] [PubMed] [Google Scholar]
- 16.Morabia A, Costanza MC, Bernstein MS, Rielle JC. Ages at initiation of cigarette smoking and quit attempts among women: a generation effect. Am J Public Health. 2002;92:71–4. doi: 10.2105/ajph.92.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davy M. Time and generational trends in smoking among men and women in Great Britain, 1972–2004/05. Health Stat Q. 2006;32:35–43. [PubMed] [Google Scholar]
- 18.Sarna L, Bialous SA, Jun HJ, Wewers ME, Cooley ME, Feskanich D. Smoking trends in the Nurses’ Health Study (1976–2003) Nurs Res. 2008;57:374–82. doi: 10.1097/NNR.0b013e31818bf38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doll R, Gray R, Hafner B, Peto R. Mortality in relation to smoking: 22 years’ observations on female British doctors. Br Med J. 1980;280:967–71. doi: 10.1136/bmj.280.6219.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stellman SD, Garfinkel L. Smoking habits and tar levels in a new American Cancer Society prospective study of 1.2 million men and women. J Natl Cancer Inst. 1986;76:1057–63. [PubMed] [Google Scholar]
- 21.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50:307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 22.Dresler C, Fratelli C, Babb J, Everley LC, Evans AA, Clapper ML. Gender differences in genetic susceptibility to lung cancer. Lung Cancer. 2000;30:153–60. doi: 10.1016/s0169-5002(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 23.McDuffie HH, Klaassen DJ, Dosman JA. Female-male differences in patients with primary lung cancer. Cancer. 1987;59:1825–30. doi: 10.1002/1097-0142(19870515)59:10<1825::aid-cncr2820591024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.International Early Lung Cancer Action Program Investigators. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296:180–4. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 25.Belani CP, Marts S, Schiller J, Socinski MA. Women and lung cancer: epidemiology, tumor biology, and emerging trends in clinical research. Lung Cancer. 2007;55:15–23. doi: 10.1016/j.lungcan.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer. 2001;31:139–48. doi: 10.1016/s0169-5002(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 27.Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abnet CC. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol. 2008;9:649–56. doi: 10.1016/S1470-2045(08)70154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sridhar KS, Thurer RJ, Markoe AM, Chatoor HT, Fountzilas G, Raub WJ, et al. Multidisciplinary approach to the treatment of locally and regionally advanced non-small cell lung cancer: University of Miami experience. Semin Surg Oncol. 1993;9:114–9. doi: 10.1002/ssu.2980090209. [DOI] [PubMed] [Google Scholar]
- 29.Zell JA, Ou SH, Ziogas A, Anton-Culver H. Epidemiology of bronchioloalveolar carcinoma: improvement in survival after release of the 1999 WHO classification of lung tumors. J Clin Oncol. 2005;23:8396–405. doi: 10.1200/JCO.2005.03.0312. [DOI] [PubMed] [Google Scholar]
- 30.Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, et al. Lung cancer occurrence in never-smokers: An analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5:e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25:472–8. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boffetta P, Jarvholm B, Brennan P, Nyren O. Incidence of lung cancer in a large cohort of non-smoking men from Sweden. Int J Cancer. 2001;94:591–3. doi: 10.1002/ijc.1507. [DOI] [PubMed] [Google Scholar]
- 33.Enstrom JE. Rising lung cancer mortality among nonsmokers. J Natl Cancer Inst. 1979;62:755–60. [PubMed] [Google Scholar]
- 34.Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst. 2006;98:691–9. doi: 10.1093/jnci/djj187. [DOI] [PubMed] [Google Scholar]
- 35.Stellman SD, Muscat JE, Thompson S, Hoffmann D, Wynder EL. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer. 1997;80:382–8. doi: 10.1002/(sici)1097-0142(19970801)80:3<382::aid-cncr5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89:1580–6. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 37.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 38.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 40.Weiss JM, Lacey JV, Jr, Shu XO, Ji BT, Hou L, Yang G, et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am J Epidemiol. 2008;168:1319–25. doi: 10.1093/aje/kwn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Inoue M, Sobue T, Tsugane S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. Int J Cancer. 2005;117:662–6. doi: 10.1002/ijc.21229. [DOI] [PubMed] [Google Scholar]
- 42.Kabat GC, Miller AB, Rohan TE. Reproductive and hormonal factors and risk of lung cancer in women: a prospective cohort study. Int J Cancer. 2007;120:2214–20. doi: 10.1002/ijc.22543. [DOI] [PubMed] [Google Scholar]
- 43.Wysowski DK, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982–1992. Obstet Gynecol. 1995;85:6–10. doi: 10.1016/0029-7844(94)00339-f. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy DL, Baum C, Forbes MB. Noncontraceptive estrogens and progestins: use patterns over time. Obstet Gynecol. 1985;65:441–6. [PubMed] [Google Scholar]
- 45.Hemminki E, Kennedy DL, Baum C, McKinlay SM. Prescribing of noncontraceptive estrogens and progestins in the United States, 1974–86. Am J Public Health. 1988;78:1479–81. doi: 10.2105/ajph.78.11.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 47.Beral V, Hermon C, Kay C, Hannaford P, Darby S, Reeves G. Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from Royal College of General Practitioners’ oral contraception study. BMJ. 1999;318:96–100. doi: 10.1136/bmj.318.7176.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet. 2003;362:185–91. doi: 10.1016/S0140-6736(03)13907-4. [DOI] [PubMed] [Google Scholar]
- 49.Taioli E, Wynder EL. Endocrine factors and adenocarcinoma of the lung in women. (Letter) J Natl Cancer Inst. 1994;86:869–70. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 50.Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 51.Kreuzer M, Gerken M, Heinrich J, Kreienbrock L, Wichmann HE. Hormonal factors and risk of lung cancer among women? Int J Epidemiol. 2003;32:263–71. doi: 10.1093/ije/dyg064. [DOI] [PubMed] [Google Scholar]
- 52.Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res. 2004;10:113–23. doi: 10.1158/1078-0432.ccr-0911-3. [DOI] [PubMed] [Google Scholar]
- 53.Syrjanen KJ. Condylomatous changes in neoplastic bronchial epithelium. Report of a case. Respiration. 1979;38:299–304. doi: 10.1159/000194095. [DOI] [PubMed] [Google Scholar]
- 54.Giuliani L, Favalli C, Syrjanen K, Ciotti M. Human papillomavirus infections in lung cancer. Detection of E6 and E7 transcripts and review of the literature. Anticancer Res. 2007;27:2697–704. [PubMed] [Google Scholar]
- 55.Bohlmeyer T, Le TN, Shroyer AL, Markham N, Shroyer KR. Detection of human papillomavirus in squamous cell carcinomas of the lung by polymerase chain reaction. Am J Respir Cell Mol Biol. 1998;18:265–9. doi: 10.1165/ajrcmb.18.2.3033. [DOI] [PubMed] [Google Scholar]
- 56.Brouchet L, Valmary S, Dahan M, Didier A, Galateau-Salle F, Brousset P, et al. Detection of oncogenic virus genomes and gene products in lung carcinoma. Br J Cancer. 2005;92:743–6. doi: 10.1038/sj.bjc.6602409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clavel CE, Nawrocki B, Bosseaux B, Poitevin G, Putaud IC, Mangeonjean CC, et al. Detection of human papillomavirus DNA in bronchopulmonary carcinomas by hybrid capture II: a study of 185 tumors. Cancer. 2000;88:1347–52. doi: 10.1002/(sici)1097-0142(20000315)88:6<1347::aid-cncr10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 58.Cook JR, Hill DA, Humphrey PA, Pfeifer JD, El-Mofty SK. Squamous cell carcinoma arising in recurrent respiratory papillomatosis with pulmonary involvement: emerging common pattern of clinical features and human papillomavirus serotype association. Mod Pathol. 2000;13:914–8. doi: 10.1038/modpathol.3880164. [DOI] [PubMed] [Google Scholar]
- 59.Guillou L, Sahli R, Chaubert P, Monnier P, Cuttat JF, Costa J. Squamous cell carcinoma of the lung in a nonsmoking, nonirradiated patient with juvenile laryngotracheal papillomatosis. Evidence of human papillomavirus-11 DNA in both carcinoma and papillomas. Am J Surg Pathol. 1991;15:891–8. doi: 10.1097/00000478-199109000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Jain N, Singh V, Hedau S, Kumar S, Daga MK, Dewan R, et al. Infection of human papillomavirus type 18 and p53 codon 72 polymorphism in lung cancer patients from India. Chest. 2005;128:3999–4007. doi: 10.1378/chest.128.6.3999. [DOI] [PubMed] [Google Scholar]
- 61.Lele SM, Pou AM, Ventura K, Gatalica Z, Payne D. Molecular events in the progression of recurrent respiratory papillomatosis to carcinoma. Arch Pathol Lab Med. 2002;126:1184–8. doi: 10.5858/2002-126-1184-MEITPO. [DOI] [PubMed] [Google Scholar]
- 62.Welt A, Hummel M, Niedobitek G, Stein H. Human papillomavirus infection is not associated with bronchial carcinoma: evaluation by in situ hybridization and the polymerase chain reaction. J Pathol. 1997;181:276–80. doi: 10.1002/(SICI)1096-9896(199703)181:3<276::AID-PATH738>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 63.Yousem SA, Ohori NP, Sonmez-Alpan E. Occurrence of human papillomavirus DNA in primary lung neoplasms. Cancer. 1992;69:693–7. doi: 10.1002/1097-0142(19920201)69:3<693::aid-cncr2820690316>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 64.Klein F, Kotb WF, Petersen I. Incidence of human papilloma virus in lung cancer. Lung Cancer. doi: 10.1016/j.lungcan.2008.10.003. Epub 2008 101016/jlungcan200810003 Available from www.elsevier.com/locate/lungcan. [DOI] [PubMed]
- 65.Syrjanen KJ. HPV infections and lung cancer. J Clin Pathol. 2002;55:885–91. doi: 10.1136/jcp.55.12.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorgoulis VG, Zacharatos P, Kotsinas A, Kyroudi A, Rassidakis AN, Ikonomopoulos JA, et al. Human papilloma virus (HPV) is possibly involved in laryngeal but not in lung carcinogenesis. Hum Pathol. 1999;30:274–83. doi: 10.1016/s0046-8177(99)90005-9. [DOI] [PubMed] [Google Scholar]
- 67.Hirayasu T, Iwamasa T, Kamada Y, Koyanagi Y, Usuda H, Genka K. Human papillomavirus DNA in squamous cell carcinoma of the lung. J Clin Pathol. 1996;49:810–7. doi: 10.1136/jcp.49.10.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyagi J, Tsuhako K, Kinjo T, Iwamasa T, Hirayasu T. Recent striking changes in histological differentiation and rate of human papillomavirus infection in squamous cell carcinoma of the lung in Okinawa, a subtropical island in southern Japan. J Clin Pathol. 2000;53:676–84. doi: 10.1136/jcp.53.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papadopoulou K, Labropoulou V, Davaris P, Mavromara P, Tsimara-Papastamatiou H. Detection of human papillomaviruses in squamous cell carcinomas of the lung. Virchows Arch. 1998;433:49–54. doi: 10.1007/s004280050215. [DOI] [PubMed] [Google Scholar]
- 70.Tsuhako K, Nakazato I, Hirayasu T, Sunakawa H, Iwamasa T. Human papillomavirus DNA in adenosquamous carcinoma of the lung. J Clin Pathol. 1998;51:741–9. doi: 10.1136/jcp.51.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.International Agency for Research on Cancer. Human Papillomaviruses. Vol. 90. Lyon: IARC; 2007. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans. [Google Scholar]
- 72.Hsu NY, Cheng YW, Chan IP, Ho HC, Chen CY, Hsu CP, et al. Association between expression of human papillomavirus 16/18 E6 oncoprotein and survival in patients with stage I non-small cell lung cancer. Oncol Rep. 2009;21:81–7. [PubMed] [Google Scholar]
- 73.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45 (Suppl 2):S3–9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 74.Czene K, Tiikkaja S, Hemminki K. Cancer risks in hairdressers: assessment of carcinogenicity of hair dyes and gels. Int J Cancer. 2003;105:108–12. doi: 10.1002/ijc.11040. [DOI] [PubMed] [Google Scholar]
- 75.Sjodahl K, Jansson C, Bergdahl IA, Adami J, Boffetta P, Lagergren J. Airborne exposures and risk of gastric cancer: a prospective cohort study. Int J Cancer. 2007;120:2013–8. doi: 10.1002/ijc.22566. [DOI] [PubMed] [Google Scholar]
- 76.Pascual D, Borque A. Epidemiology of kidney cancer. Adv Urol. doi: 10.1155/2008/782381. Epub 2008 November 4, 10.1155/2008/782381. Available from www.hindawi.com. [DOI] [PMC free article] [PubMed]
- 77.Laukkanen P, Koskela P, Pukkala E, Dillner J, Laara E, Knekt P, et al. Time trends in incidence and prevalence of human papillomavirus type 6, 11 and 16 infections in Finland. J Gen Virol. 2003;84:2105–9. doi: 10.1099/vir.0.18995-0. [DOI] [PubMed] [Google Scholar]
- 78.U.S. Department of Health and Human Services. Reducing the Health Consequences of Smoking: 25 Years of Progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. DHHS Publication No. (CDC) 89–8411. 1989.
- 79.Raymond JS, Hogue CJ. Multiple primary tumours in women following breast cancer, 1973–2000. Br J Cancer. 2006;94:1745–50. doi: 10.1038/sj.bjc.6603172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matesich SM, Shapiro CL. Second cancers after breast cancer treatment. Semin Oncol. 2003;30:740–8. doi: 10.1053/j.seminoncol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 81.Roychoudhuri R, Evans H, Robinson D, Moller H. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer. 2004;91:868–72. doi: 10.1038/sj.bjc.6602084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bergfeldt K, Silfversward C, Einhorn S, Hall P. Overestimated risk of second primary malignancies in ovarian cancer patients. Eur J Cancer. 2000;36:100–5. doi: 10.1016/s0959-8049(99)00244-0. [DOI] [PubMed] [Google Scholar]
- 83.Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 84.Beral V, Banks E, Reeves G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet. 2002;360:942–4. doi: 10.1016/S0140-6736(02)11032-4. [DOI] [PubMed] [Google Scholar]