Abstract
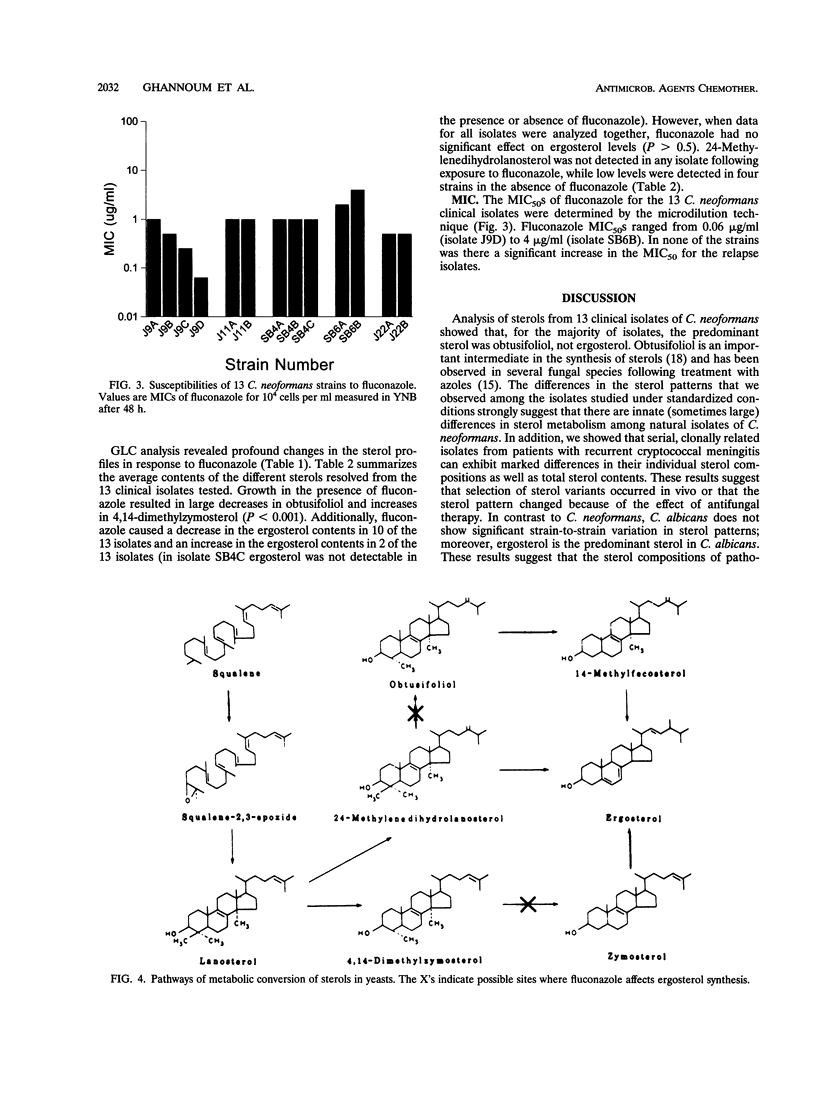
Analysis of the sterol compositions of 13 clinical isolates of the pathogenic yeast Cryptococcus neoformans obtained from five patients with recurring cryptococcal meningitis showed that, unlike Candida albicans, the major sterols synthesized by this yeast were obtusifoliol (range, 21.1 to 68.2%) and ergosterol (range, 0.0 to 46.5%). There was considerable variation in the sterol contents among the 13 isolates, with total sterol contents ranging from 0.31 to 5.9% of dry weight. The isolates from the five patients who had relapses had different total sterol contents and compositions in comparison with those of the pretreatment isolates, indicating either that the sterols had been changed by therapy or that the patients were infected with new isolates with different sterol compositions. Growth of the cryptococcal isolates in the presence of subinhibitory concentrations of fluconazole (0.25x the MIC) significantly altered the sterol content and pattern. The total sterol content decreased in nine isolates and increased in four isolates in response to pretreatment with fluconazole. Fluconazole had no consistent effect on ergosterol levels. In contrast, fluconazole caused a decrease in obtusifoliol levels and an increase in 4,14-dimethylzymosterol levels in all isolates. These results indicate extensive diversity in sterol content, sterol composition, and sterol synthesis in response to subinhibitory concentrations of fluconazole in C. neoformans strains. We propose that fluconazole inhibits the sterol synthesis of C. neoformans by interfering with both 14 alpha-demethylase-dependent and -independent pathways. No correlation between the sterol compositions of C. neoformans isolates and their susceptibilities to fluconazole was found.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P. Azole antifungal agents. Clin Infect Dis. 1992 Mar;14 (Suppl 1):S161–S169. doi: 10.1093/clinids/14.supplement_1.s161. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Freundlich L. F., Marsh L., Scharff M. D. Extensive allelic variation in Cryptococcus neoformans. J Clin Microbiol. 1992 May;30(5):1080–1084. doi: 10.1128/jcm.30.5.1080-1084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Spitzer E. D., Webb D., Rinaldi M. G. Susceptibilities of serial Cryptococcus neoformans isolates from patients with recurrent cryptococcal meningitis to amphotericin B and fluconazole. Antimicrob Agents Chemother. 1993 Jun;37(6):1383–1386. doi: 10.1128/aac.37.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
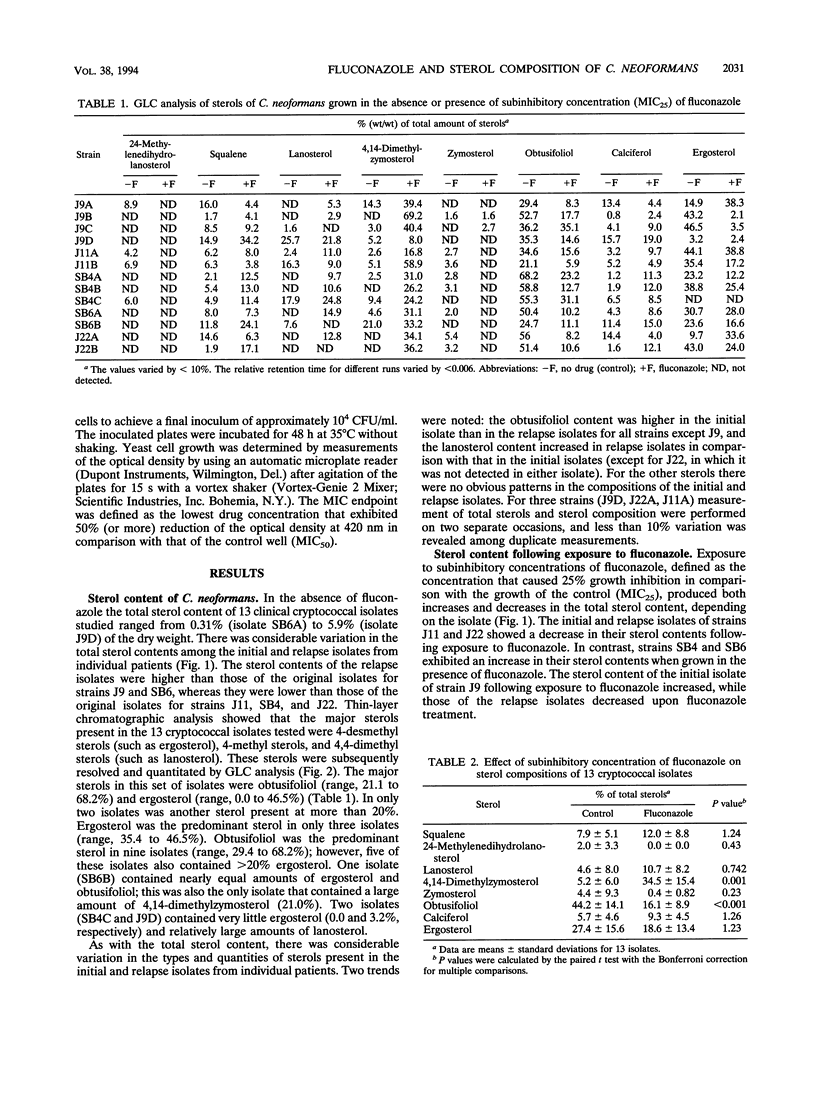
- Ghannoum M. A., Ibrahim A. S., Fu Y., Shafiq M. C., Edwards J. E., Jr, Criddle R. S. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol. 1992 Nov;30(11):2881–2886. doi: 10.1128/jcm.30.11.2881-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum M. A., Moussa N. M., Whittaker P., Swairjo I., Abu-Elteen K. H. Subinhibitory concentration of octenidine and pirtenidine: influence on the lipid and sterol contents of Candida albicans. Chemotherapy. 1992;38(1):46–56. doi: 10.1159/000238941. [DOI] [PubMed] [Google Scholar]
- Hitchcock C. A., Russell N. J., Barrett-Bee K. J. Sterols in Candida albicans mutants resistant to polyene or azole antifungals, and of a double mutant C. albicans 6.4. Crit Rev Microbiol. 1987;15(1):111–115. doi: 10.3109/10408418709104454. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kwon-Chung J., Milne G. W., Hill W. B., Patterson G. Relationship between polyene resistance and sterol compositions in Cryptococcus neoformans. Antimicrob Agents Chemother. 1975 Jan;7(1):99–106. doi: 10.1128/aac.7.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R. Cryptococcosis. Infect Dis Clin North Am. 1989 Mar;3(1):77–102. [PubMed] [Google Scholar]
- Saag M. S., Dismukes W. E. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother. 1988 Jan;32(1):1–8. doi: 10.1128/aac.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M. S., Powderly W. G., Cloud G. A., Robinson P., Grieco M. H., Sharkey P. K., Thompson S. E., Sugar A. M., Tuazon C. U., Fisher J. F. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med. 1992 Jan 9;326(2):83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- Spitzer E. D., Spitzer S. G., Freundlich L. F., Casadevall A. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet. 1993 Mar 6;341(8845):595–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H., Marichal P., Le Jeune L., Coene M. C., Gorrens J., Cools W. Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethylation and reduction of 3-ketosteroids in Cryptococcus neoformans. Antimicrob Agents Chemother. 1993 Oct;37(10):2101–2105. doi: 10.1128/aac.37.10.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Bossche H., Marichal P., Willemsens G., Bellens D., Gorrens J., Roels I., Coene M. C., Le Jeune L., Janssen P. A. Saperconazole: a selective inhibitor of the cytochrome P-450-dependent ergosterol synthesis in Candida albicans, Aspergillus fumigatus and Trichophyton mentagrophytes. Mycoses. 1990 Jul-Aug;33(7-8):335–352. doi: 10.1111/myc.1990.33.7-8.335. [DOI] [PubMed] [Google Scholar]
- Vandenheuvel F. A., Court A. S. Reference high-efficiency nonpolar packed columns for the gas-liquid chromatography of nanogram amounts of steroids. I. Retention time data. J Chromatogr. 1968 Dec 17;38(4):439–459. doi: 10.1016/0021-9673(68)85073-3. [DOI] [PubMed] [Google Scholar]
- Varma A., Kwon-Chung K. J. Restriction fragment polymorphism in mitochondrial DNA of Cryptococcus neoformans. J Gen Microbiol. 1989 Dec;135(12):3353–3362. doi: 10.1099/00221287-135-12-3353. [DOI] [PubMed] [Google Scholar]


