SYNOPSIS
Public health laboratories (PHLs) are critical components of the nation's health-care system, serving as stewards of valuable specimens, delivering important diagnostic results to support clinical and public health programs, supporting public health policy, and conducting research. This article discusses the need for and challenges of creating standards-based data-sharing networks across the PHL community, which led to the development of the PHL Interoperability Project (PHLIP). Launched by the Association of Public Health Laboratories and the Centers for Disease Control and Prevention in September 2006, PHLIP has leveraged a unique community-based collaborative process, catalyzing national capabilities to more effectively share electronic laboratory-generated diagnostic information and bolster the nation's health security. PHLIP is emerging as a model of accelerated innovation for the fields of laboratory science, technology, and public health.
Public health laboratories (PHLs) in the United States play a key role in promoting the health and protecting the safety of the nation's communities. PHLs are integral to the health system through their provision of a wide range of essential services, including disease surveillance and the timely, accurate identification of infectious organisms associated with outbreaks of disease. These laboratories are a critical component of the disease response and readiness infrastructure and are depended upon to identify and respond to newly emerging diseases and threats associated with bioterrorism or natural disasters. PHLs are the foundation for state-based efforts to protect the population's health and safety through the testing of human specimens, food, air, water, and soil. They also provide other essential services, including newborn screening and routine, clinical diagnostic testing.
THE PHL COMMUNITY
There are more than 600 PHLs in the U.S. operating at the state, county, or city level. Each state and territory, as well as the District of Columbia, has its own PHL, and many large cities or municipalities have sizable laboratory operations. Approximately 100 PHLs in the U.S. provide comprehensive, high-complexity services; however, virtually every PHL differs in the type of tests performed and organizational structure. Some states, such as Texas and Florida, operate multiple branch facilities that are coordinated by a central office. Other PHLs, such as those in Wisconsin and Nebraska, are affiliated with the state's university system, whether they are university based or simply contract with the university. Still other PHLs consolidate statewide programs for laboratory services to provide more efficient and cost-effective testing services, such as the Virginia Division of Consolidated Laboratory Services.
In many respects, PHLs are in the business of providing data on human and environmental specimens; whether to identify the scientific name of an organism, the number of tests that are positive, or the site from which specimens are collected. A PHL can have up to 10 different recipients of similar or identical datasets, such as primary care physicians, hospital infection-control practitioners, intermediate health program directors, the state public health department, the state chief medical/health officer, city or county chief medical/health officers, state epidemiologists, and national experts at the Centers for Disease Control and Prevention (CDC) or the Environmental Protection Agency (EPA), in the case of environmental testing data. At the time of the 9/11 terror attacks, most PHLs reported data to these recipients via U.S. mail, fax, or telephone. Since 2001, much progress has been made in the implementation of a laboratory information management system (LIMS) for the collection and processing of laboratory data; however, there is still a long way to go.
Data from a survey conducted in 2007 by the Association of Public Health Laboratories (APHL) show that approximately 90% of PHLs have a LIMS; however, many of these states are in various stages of implementing their systems, and some do not have the necessary funding to complete implementation.1 The surge of federal funding to states as a result of 9/11 made acquiring updated information technology (IT) infrastructure a short-term possibility, but it has not answered the long-term need. In addition, many PHLs are facing the centralization of IT services within their states' health departments, which is making it much more difficult to obtain IT staff assistance for LIMS implementation, as these needs must compete with other important state IT projects. The current dire fiscal situation all PHLs are facing in the wake of the U.S. economic downturn will make continuing LIMS implementation and maintenance practically impossible.
The IT infrastructure challenges extend beyond funding to software or operating systems for laboratory computers. PHLs have had to purchase existing commercial LIMSs, such as ChemWare, Mysis, and STARLiMS®, which are not created with the specialized functions of a PHL in mind. A second major problem is that many health programs want PHLs to collect data beyond the usual information, such as vaccination history, hospitalization records, and detailed racial/ethnic origin categories. Without a basic IT infrastructure, PHLs will be unable to effectively and efficiently manage their laboratory data or electronically message critical laboratory test results.
DATA-SHARING INFRASTRUCTURE HISTORICALLY PROGRAM SPECIFIC
Federal and state programs for collecting data from PHLs have historically been vertical in nature. Software was created to collect data specific to and exclusive for one (and only one) public health program, such as tuberculosis treatment, human immunodeficiency virus prevention, hepatitis surveillance, or sexually transmitted disease monitoring efforts. This data-collection strategy was aligned with the manner in which programs were administered and funded, with CDC funding the testing program with the expectation that test data from the PHL would be forwarded by either the state epidemiology program or the local board of health. In many cases, the software would allow direct transfer of data to CDC. One such application was the Public Health Laboratory Information System.2 It was capable of collecting data for conditions such as rabies, influenza, and certain enteric diseases. Another example, PulseNet, was established in 1996 to share pulsed-field gel electrophoresis data on enteric pathogens, including hemorrhagic Escherichia coli (E. coli).3
IMPORTANCE AND COMPLEXITY OF DATA SHARING ACROSS BORDERS
Public health legal jurisdictions typically end at state borders, yet infectious diseases easily cross such boundaries. The importance of sharing public health data is illustrated by the detection of E. coli O157:H7 in hamburgers in multiple states and the use of PulseNet to fingerprint the pathogen and then epidemiologically link outbreaks. The current decade has reinforced this experience numerous times with the appearance of diseases as diverse as West Nile virus, monkeypox virus, and sudden acute respiratory syndrome, as well as the intentional spread of anthrax through the U.S. postal system. These public health risks accelerated the urgency of sharing laboratory data beyond state borders to support effective public health response at the national and international levels.
National interoperability and data-sharing standards, policies, and practices need to accommodate each stakeholder's specific needs with regard to the variable levels of data required. Stakeholders such as clinicians and epidemiologists require different types of laboratory data to support their business needs. The laboratorian generates data that are required to confirm diagnoses or a suspected outbreak. A clinician is most interested in the laboratory test result, as well as the normal ranges for a defined population, for the purposes of clinical decision-making. The epidemiologist must assimilate and analyze the specimen-based results to create an environmental and population-based perspective for effective assessment and response. Public health laboratorians use LIMS to link the test to a specific patient to support clinical diagnoses, prospective patient management, and historical testing.
DATA TIMELINESS, ACCURACY, AND COMPLETENESS
While the type of data is important and varies by stakeholder, the method of distribution has a significant impact on the efficiency and effectiveness of data sharing as measured by such factors as timeliness, accuracy, and completeness.4,5 Whereas paper reports may be available within days, the development of the fax machine made them available within minutes of testing. However, entering or copying results from one document to another or into an electronic record is not an efficient process and often results in the corruption of data. The discourse and promise around electronic health records (EHRs) and more general electronic data sharing has been in process for decades; however, there has been increased activity and encouraging progress in critical arenas in the past five years.6–8
THE FEDERAL AGENDA
In 2004, President George W. Bush issued an executive order that called for the establishment of a national health IT coordinator within the Office of the Secretary of Health and Human Services, with the goal that Americans have access to an interoperable electronic medical record by 2014.9 The Office of the National Coordinator for Health Information Technology was established in 2005 and has served as a focal point for diverse stakeholders to convene and collaborate on data-sharing issues.10 It has propagated the development of the National Health Information Network, which has provided a context for the development of diverse data-sharing use cases that cross public and private sectors, as well as many domains.
President Barack Obama also has made health IT a top priority for the nation. The new administration allocated $2 billion to health IT through the American Recovery and Reinvestment Act of 2009.11 These funds will go toward the adoption of health IT within all levels of government and across all sectors of health care, and also include incentives for adoption in private industry.
PHL COLLABORATIVE BUSINESS REQUIREMENTS PROJECT
One of the first comprehensive assessments of the information needs of the PHL community was conducted collaboratively by APHL and the Public Health Informatics Institute.12 This initiative provided a detailed enumeration of the 16 business functions of a typical PHL and has helped PHL experts across the country appreciate the commonality of their workflow, information systems, and data requirements, setting the stage for a new level of community-based collaboration. In addition, it has helped to illuminate the different data needs of the laboratory community vs. the epidemiology community. Integrated data-sharing networks must exist for each community, and, subsequently, these networks should share data based on a clearly defined set of public health business cases. This collaboration also demonstrated how a community effort could accelerate the development of a national laboratory data-sharing network.
PREPARING FOR SEASONAL AND PANDEMIC INFLUENZA
The public health community was recently challenged to conduct extensive planning in anticipation of the outbreak of pandemic influenza. A primary goal was to develop surge-capacity plans because the inability of laboratories to manage surge was identified as a problem during the anthrax incident and West Nile virus outbreak. A second goal of this effort was to develop continuity-of-operations plans for PHLs—a critical need that received national attention following the destruction of the PHL infrastructure in Louisiana by Hurricane Katrina. In response to these challenges, it was determined that, in most cases, state PHLs would look to a neighboring or even distant state PHL to provide backup laboratory testing services—a plan that proved effective for Louisiana in 2005, when the University of Iowa Hygienic Laboratory answered the call to provide newborn screening assays. The need for interoperability has been well established, and PHLs are developing capabilities that will enable laboratories across jurisdictions to provide mutual multidirectional support.
CREATING PHL INTEROPERABILITY
In 2004, APHL and CDC conducted a survey (Unpublished data, State Public Health Laboratory Participation in Health Information Exchanges) to better understand the impact of evolving health-care interoperability standards on the ability of laboratories to share influenza data. The survey found that PHLs were aware of relevant standards, such as the Logical Observation Identifiers Names and Codes database and the Systematized Nomenclature of Medicine—Clinical Terms, and that the standards were being deployed appropriately. However, the survey revealed tremendous variability in the implementation of the standards, which rendered data sharing impossible without an additional step to map the data across the laboratories to a standard data dictionary. This was the result of having multiple options for messaging structure, security protocol, and network infrastructure in the U.S. Members of the APHL Informatics Committee met in 2006 to review the need for and obstacles to building national interoperability. In their effort to address effective data sharing, they were driven by the following:
The need to harmonize the adoption of technical interoperability standards to support PHL electronic data exchange;
The need to reduce the overhead or expense of transmitting laboratory test orders and results;
The need to provide continuity of operations and surge capacity among PHLs;
The desire to share best practices in the adoption of LIMSs;
The desire to work more effectively with vendors of public health LIMS products; and
The need to increase the effectiveness of identifying and propagating the adoption of new methodologies and technologies.
PHL INTEROPERABILITY PROJECT (PHLIP)
Collaborative structure and processes to promote data interoperability
In 2005, APHL collaborated with CDC to launch PHLIP to support and accelerate the development of a national laboratory standards-based electronic data-sharing network. Recognizing the excellent work referenced in the aforementioned historical perspective, PHLIP provides a more comprehensive approach, which is gaining traction where previous efforts have failed. PHLIP leverages an open-innovation approach (that is, opening up to others outside the organization) to deliver a suite of services that start with the development of a common understanding of laboratory workflow, progress through technical data-sharing capabilities, and support those capabilities for improved public health impact. Specifically, PHLIP provides these eight key products and services to catalyze the development and support the maintenance of a community-wide standards-based electronic information exchange:
Management of an innovative community that leverages laboratorians, technical experts, informaticians, and public health experts to advance standards-based electronic data sharing for public health;
Support for a PHL's selection, implementation, and management of an internal electronic data-management capability (e.g., LIMS);
Development of use cases and workflows for each of the nationally notifiable diseases (NNDs);
Development of vocabulary coding schema and messaging to support use cases and workflows (PHLIP creates mapping workbooks and encoding guidelines to document the data-exchange schema);
Provision of a forum and working groups to support PHLs in their implementation of data-exchange standards;
Validation of data-exchange capabilities to identify any issues with the data and initiate performance improvement activities, if necessary;
Provision of a forum between states and CDC to determine opportunities and methodologies that enable the emerging data-sharing network to improve the performance of public health programs and their outcomes (e.g., food safety, water safety, and influenza); and
Leverage of an open-innovation network to accelerate progress in scientific discovery, technology adoption, and health-care transformation.
Approaches to community policy
This new approach was experimental in the sense that it sought to determine whether a select panel of PHL and informatics experts could come to consensus on a process that would address the needs and uses for all laboratory test ordering and reporting. The traditional way to address these types of problems has been to either generate high-level rulings from one overarching administrative entity or create a drawn-out process in which as many participants as possible discuss the various alternatives without a defined end point. A long history in the PHL community with minimal progress reveals that neither of the latter two approaches is effective.
Understanding data sharing among stakeholders
PHLIP is documenting the very specific needs of the PHL community through its development of clear use-case definitions. While PHLIP is beginning to reach out to the clinical and epidemiologic communities to ensure data sharing across the use cases for these three communities, it is careful to maintain its focus on the PHL community and not attempt to also serve domains that support direct patient care, case reporting, or epidemiologic investigations.
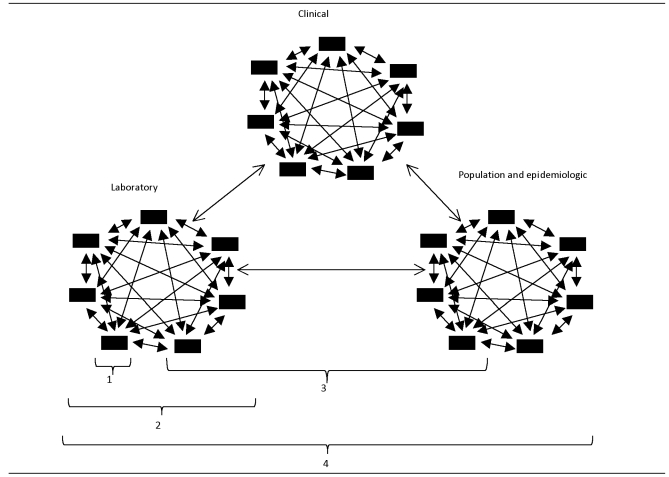
The PHL community engages in at least four types of data management and exchange activities (Figure 1). Firstly, each PHL engages in activities to manage the data it generates internally; this is the role of the LIMS (Figure 1, bracket 1). Secondly, laboratories have reason to share data with other laboratories, including surge capacity, continuity of operations, and access to analytic capability lacking in one's own laboratory (Figure 1, bracket 2). Thirdly, to increase efficiency and decrease the amount of time to register patients and release test results, PHLs will exchange data with their clinical submitters for patient-based test-order and result information (Figure 1, bracket 3). Finally, the laboratory will share data with health-care stakeholders including population health-oriented offices that are responsible for epidemiology services, such as departments of health.
Figure 1.
The four typesa of data-management and network activities expected of PHLs
a(1) management of PHL-generated internal data, (2) PHL data exchange with partner laboratories, (3) PHL data exchange with clinical submitters for patient-based test-order and result information, and (4) PHL data exchange with health-care stakeholders (i.e., population health and epidemiology services)
PHL = public health laboratory
PHLIP structure and methodology
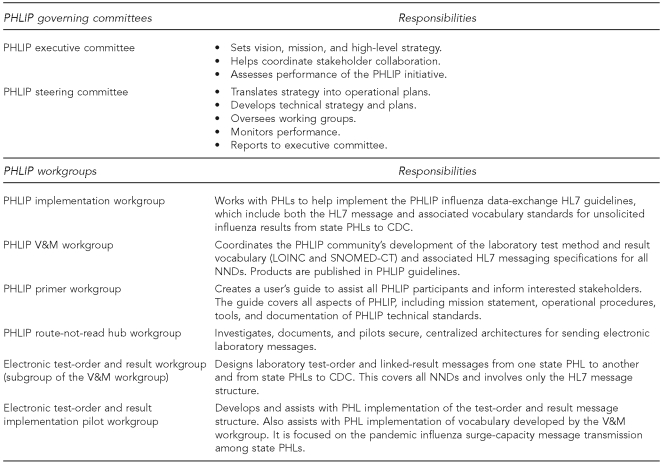
The overall strategic direction of PHLIP is set by the executive committee, which comprises representatives from the state PHLs, APHL, and CDC. The PHLIP steering committee, with representation from the same organizations, provides direction and oversight for the six PHLIP technical working groups that provide the collaborative framework and processes that enable PHLIP to progress interoperability and data exchange via use cases developed by the steering committee and approved by the executive committee. A complete listing of PHLIP committees and workgroups and their respective tasks is provided in Figure 2.
Figure 2.
PHLIP governance structure and working groups and their responsibilities
PHLIP = Public Health Laboratory Interoperability Project
PHL = public health laboratory
HL7 = Health Level Seven
CDC = Centers for Disease Control and Prevention
V&M = vocabulary and messaging
LOINC = Logical Observation Identifiers Names and Codes
SNOMED-CT = Systematized Nomenclature of Medicine—Clinical Terms
NND = nationally notifiable disease
PHLIP work to date
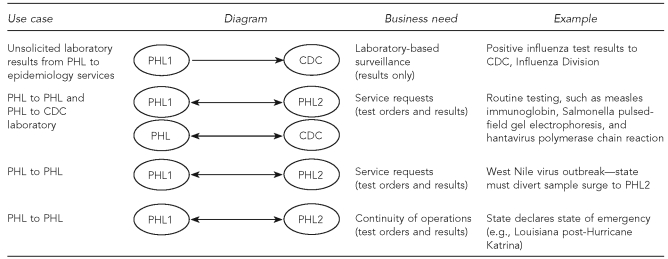
Use case development: four prototypes.
PHLIP has documented four use cases to date (Figure 3). The first use case was designed to address common surveillance needs, where the PHL sends unsolicited results for a specific NND to the respective epidemiology program. The results are unsolicited in that they are sent at an agreed-upon frequency, without a specific request from the epidemiology program. PHLIP's pilot projects are based on the flow of influenza data between state PHLs and CDC.
Figure 3.
PHLIP data-exchange use cases by diagram, covered business need, and example
PHLIP = Public Health Laboratory Interoperability Project
PHL = public health laboratory
CDC = Centers for Disease Control and Prevention
The other three use cases address laboratory-to-laboratory data-sharing needs in different circumstances. In each of these uses, a test request originates from one laboratory and is sent to another laboratory, with the expectation of receiving linked results back. These are summarized in Figure 3 and include:
Routine reference testing use case, where one laboratory lacks the analytic capability for a specific test and, therefore, sends the specimen and test request to another laboratory (usually another state PHL or the PHL at CDC);
Surge capacity use case where, due to a disease outbreak situation, one PHL reaches its capacity to perform requested tests and will ship the additional workload to another PHL; and
Continuity of operations requirement use case, in which one PHL's business function is completely interrupted and all essential services need to be outsourced to another PHL until the emergency situation is resolved.
Data-sharing schema and its implementation.
PHLIP has established six working groups that are dedicated to the development of data interoperability specifications to support the four use cases. The six working groups and their scopes of work are briefly described in Figure 2. PHLIP, using influenza as a prototype, has developed a detailed workbook and guideline format for the development and documentation of data-sharing schema for the NNDs. To date, PHLIP has developed data-sharing schema for 18 NNDs. Standards-based electronic data exchange is operational among five states and CDC, with plans to implement this capability across 15 additional state PHLs in 2010. PHLIP is accelerating toward its 50/60 goal—all 50 states harmonized on the data exchange of the 60 NNDs.
PHLIP collaborative method.
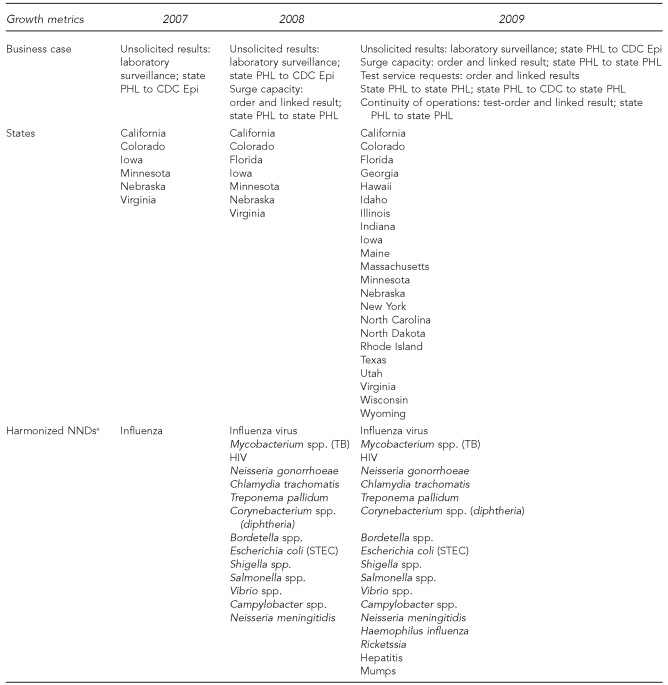
Figure 4 illustrates the collaborative's successful growth. During a three-year period, the number of states grew from six to 22, working groups and committees from six to eight, and the number of individual collaborators continues to grow from 15 in year one to 70 in year three. These data strongly suggest that the community is increasingly invested in PHLIP and recognizes its value and success.
Figure 4.
PHLIP developments by use case and state participation, 2007–2009
aShows all NNDs harmonized during the last three years. Influenza is the only NND currently in production (unsolicited results messaging; state PHL to CDC). Salmonella is slated for production for the service request use case by June 2010.
PHLIP = Public Health Laboratory Interoperability Project
PHL = public health laboratory
CDC = Centers for Disease Control and Prevention
Epi = epidemiology
NND = nationally notifiable disease
TB = tuberculosis
HIV = human immunodeficiency virus
STEC = shiga toxin-producing Escherichia coli
OTHER ELECTRONIC LABORATORY DATA-EXCHANGE EFFORTS
Other efforts are currently underway to support standards-based data exchange in the laboratory community, but they are quite different from PHLIP. The EHR-Lab Interoperability and Connectivity Specification (ELINCS), now part of Health Level Seven (HL7), is an effort that focuses on the standardization of laboratory data between a diagnostic laboratory and a clinical setting. As discussed previously, the data specifications for a laboratory, a clinical or provider location, and an epidemiology disease-control program are quite different. The use cases that PHLIP has developed to support laboratory business functions and the subsequent sharing of data across laboratories are different than the use cases that exist for sharing data between a laboratory and a provider site. Recognizing this distinction, PHLIP is in the process of reaching out to ELINCS/HL7 to support progress in the harmonization between the laboratory and clinical stakeholder communities.
The Integrated Consortium of Laboratory Networks, established in 2005, is another important player in promoting interoperability across laboratories. Sponsored by the Department of Homeland Security, this effort focuses on data sharing across laboratories associated with federal agencies of agriculture, commerce, defense, energy, health and human services, homeland security, interior, justice, state, and environmental protection.
THE FUTURE
Program priorities
PHLIP supports the evolution of the PHL community through the establishment of an open-innovation-initiative. This approach has paved the way for accelerated progress in the development of a national electronic data-sharing network. In the past four years, a robust collaborative infrastructure has developed to allow more than 20 additional laboratories to join the project, thus incorporating one-third of the nation's largest laboratories into a collaborative effort to create the technical specifications that will make a national standards-based electronic data-sharing network a reality. Future work and areas of focus will include:
Completion of harmonized data elements for the remaining NNDs.
Closer collaboration with the epidemiology and clinical communities to ensure effective data exchange for all programs, including improved coordination with ELINCS and HL7, the Healthcare IT Standards Panel, and other data standards efforts. This also will include collaboration with clinical laboratories and public health partners, such as the Council of State and Territorial Epidemiologists, the Association of State and Territorial Health Officials, and the National Association of City and County Health Officials.
The leveraging of the open-innovation PHL network to accelerate and improve public health programs, including the national or local response to emerging infections or acute threats. This will include drawing on the evolving national standards-based electronic laboratory data-sharing network and its harmonized data-exchange schema to more effectively support food safety, multidrug-resistant tuberculosis control, influenza control, and other public health challenges.
The establishment of new open-innovation initiatives that will leverage the PHL community in such promising areas as a clinical trial network for diagnostics.
Challenges
While PHLIP has an increasingly recognized track record and well-defined future goals, the community will need to recognize and prepare for certain challenges. Because the engine for a successful open-innovation effort is the community, any change in the environment that compromises the stability and efficiency of the community-based collaboration represents a significant threat. In the public health domain, several common challenges are notable.
Sustainability of program support.
There is significant historical precedent for short-term program perspectives where momentum gained early on subsequently stalls and leads to premature termination of program support. It is imperative to secure sustainable funding sources for these projects so the infrastructure and processes created will be lasting. In addition, state resource constraints threaten the stability of the laboratories to engage in this work. Savings down the road with regard to function and efficiency require system investments now to sustain health IT, just as investments are being made for health reform.
Sustainability of community unity.
The maintenance of a large, complex collaborative requires a long-term commitment on the part of many stakeholders. For example, programs that are similar in function and purpose may receive funding, but there is a large amount of work that is redundant. While there are times when competition promotes innovation, in the context of community-based open innovation, fractioning of the finite PHL community will rapidly diminish any initiative's effectiveness and productivity. Unlike a vastly populated community, such as the hundreds of thousands of chemists in the world, the PHL community is measured in the hundreds. The implication is that even a few competing initiatives can adversely impact and even undermine the productivity and value proposition of a community-based open-innovation network.
Program ownership and governance.
To ensure robust and efficient community collaboration, there must be effective mechanisms in place to maintain strong program stewardship. While some forms of competition may fracture the community and have a detrimental impact on network output, it may be nonetheless reasonable, for example, to assess program effectiveness on a regular basis and even to consider competitive bids on a five-year cycle to manage the open-innovation community. This would enable a degree of healthy competition but prevent excesses that could result in community disintegration.
CONCLUSION
The primary outcome of this project has been the cost-effective acceleration of collaborative innovation to improve information sharing in the management of major public health challenges. Such innovation is imperative if our finite public health resources are to counter an ever-expanding myriad of challenges and threats to the public's well-being.
Acknowledgments
The authors thank the following for their contributions: John Carroll for facilitation and community building; Jon Lipsky and Joan Knapp for technical assistance; Mary Shaffran for program direction and support; and Gary Jones, Dariush Shirazi, Wanda “Willie” Andrews, Robin Lusk, David Butcher, and Paul Duffey for serving as the leads in the original Public Health Laboratory Interoperability Project (PHLIP) pilot states. The authors also acknowledge the contributors on the PHLIP executive committee: Scott Becker, Leslie Lenert, Janet Nicholson, Mitchell Cohen, Daniel Sosin, and Frances Downes; and the contributors on the PHLIP steering committee: Gautom “GB” Kesarinath, James Tolson, Genny Gallagher, John Carroll, Gary Jones, Jon Lipsky, and Emory Meeks. The authors also gratefully acknowledge the many individuals who comprise the PHLIP community.
Footnotes
This article was supported by cooperative agreement #CCU303019 from the Centers for Disease Control and Prevention (CDC). The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC or the Agency for Toxic Substances and Disease Registry.
REFERENCES
- 1.Association of Public Health Laboratories. 2007 State of Technology Adoption Survey. Silver Spring (MD): APHL; 2007. [Google Scholar]
- 2.Bean NH, Martin SM, Bradford H., Jr. PHLIS: an electronic system for reporting public health data from remote sites. Am J Public Health. 1992;82:1273–6. doi: 10.2105/ajph.82.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendel AM, Johnson DH, Sharapov U, Grant J, Archer JR, Monson T, et al. Multistate outbreak of Escherichia coli O157:H7 infection associated with consumption of packaged spinach, August–September 2006: the Wisconsin investigation. Clin Infect Dis. 2009;48:1079–86. doi: 10.1086/597399. [DOI] [PubMed] [Google Scholar]
- 4.Effler P, Ching-Lee M, Bogard A, Ieong MC, Nekomoto T, Jernigan D. Statewide system of electronic notifiable disease reporting from clinical laboratories: comparing automated reporting with conventional methods. JAMA. 1999;282:1845–50. doi: 10.1001/jama.282.19.1845. [DOI] [PubMed] [Google Scholar]
- 5.Overhage JM, Suico J, McDonald CJ. Electronic laboratory reporting: barriers, solutions and findings. J Public Health Manag Pract. 2001;7:60–6. doi: 10.1097/00124784-200107060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Chute CG, Koo D. Public health, data standards, and vocabulary: crucial infrastructure for reliable public health surveillance. J Public Health Manag Pract. 2002;8:11–7. doi: 10.1097/00124784-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Moore KM, Reddy V, Kapell D, Balter S. Impact of electronic laboratory reporting on hepatitis A surveillance in New York City. J Public Health Manag Pract. 2008;14:437–41. doi: 10.1097/01.PHH.0000333877.78443.f0. [DOI] [PubMed] [Google Scholar]
- 8.Overhage JM, Grannis S, McDonald CJ. A comparison of the completeness and timeliness of automated electronic laboratory reporting and spontaneous reporting of notifiable conditions. Am J Public Health. 2008;98:344–50. doi: 10.2105/AJPH.2006.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. 3 C.F.R. § 13335 (2005)
- 10.Ebeler JC, Bruno M, Schmitt T, editors. Institute of Medicine. Opportunities for coordination and clarity to advance the national health information agenda: a brief assessment of the Office of the National Coordinator for Health Information Technology—a letter report. Washington: The National Academies Press; 2007. [Google Scholar]
- 11. American Recovery and Reinvestment Act of 2009, Pub. L. No. 111-5 (2009)
- 12.Association of Public Health Laboratories; Public Health Informatics Institute. Requirements for public health laboratory information management systems: a collaboration of state public health laboratories, the Association of Public Health Laboratories and the Public Health Informatics Institute. Washington: APHL; 2003. [Google Scholar]