Abstract
Objective
Transient receptor potential vanilloid 4 (TRPV4) is a Ca2+ permeable channel that can be gated by tonicity (osmolarity) and mechanical stimuli. Chondrocytes, the cells in cartilage, respond to their osmotic and mechanical environments; however, the molecular basis of this signal transduction is not fully understood. The objective of this study was to demonstrate the presence and functionality of TRPV4 in chondrocytes.
Methods
TRPV4 protein expression was measured by immunolabeling and Western blotting. In response to TRPV4 agonist/antagonists, osmotic stress, and interleukin-1 (IL-1), changes in Ca2+ signaling, cell volume, and prostaglandin E2 (PGE2) production were measured in porcine chondrocytes using fluorescence microscopy, light microscopy, or immunoassay, respectively.
Results
TRPV4 was expressed abundantly at the RNA and protein level. Exposure to 4αPDD, a TRPV4 activator, caused Ca2+ signaling in chondrocytes, which was blocked by the selective TRPV4 antagonist, GSK205. Blocking TRPV4 diminished the chondrocytes' response to hypo-osmotic stress, reducing the fraction of Ca2+ responsive cells, regulatory volume decrease (RVD), and PGE2 production. Ca2+ signaling was inhibited by removal of extracellular Ca2+ or depletion of intracellular stores. Specific activation of TRPV4 restored defective RVD caused by IL-1. Chemical disruption of the primary cilium eliminated Ca2+ signaling in response to either 4αPDD or hypo-osmotic stress.
Conclusion
TRPV4 is present in articular chondrocytes, and chondrocyte response to hypo-osmotic stress is mediated by this channel, which involves both an extracellular Ca2+ and intracellular Ca2+ release. TRPV4 may also be involved in modulating the production or influence of pro-inflammatory molecules in response to osmotic stress.
Articular cartilage, the avascular connective tissue that covers diarthrodial joint surfaces, provides a low-friction surface that supports and distributes mechanical loads. Cartilage comprises a hydrated extracellular matrix (ECM) of proteoglycans and collagen fibrils, as well as chondrocytes, the cells responsible for maintaining the ECM. The chondrocytes' metabolic function is influenced by a number of factors in the microenvironment, including soluble mediators, ECM composition, and mechanical loading (1, 2). The transduction of biomechanical factors to intracellular signals appears to involve changes in other biophysical parameters secondary to compression of the ECM (3), but such pathways have not been fully elucidated.
The ECM of cartilage is inherently negatively charged due to the large concentration of the anionic proteoglycan aggrecan which attracts cations to counterbalance the charge. The resulting increase in interstitial osmolarity causes the tissue to imbibe water (4). Upon joint loading, water is exuded from the tissue but is reabsorbed when the tissue is no longer compressed (5, 6). Thus, chondrocytes experience large fluctuations in their osmotic environment as a result of normal joint loading, in addition to changes in other biophysical parameters, such as cell deformation, fluid flow, pH, and fluid pressure (1, 7).
Chondrocytes respond to osmotic stress with the initiation of intracellular signaling cascades and acute volume change (8-10), followed by volume regulation involving cytoskeletal F-actin restructuring (9) and/or solute transport (10, 11). Osmotic stimulation of chondrocytes also elicits a cytoplasmic Ca2+-signal originating from both extracellular Ca2+-influx as well as intracellular Ca2+ release from stores (8, 9). This Ca2+-signal may play a role in volume regulation (12), cell metabolism, gene expression (13), and restructuring of the F-actin cytoskeleton (9). While the response of chondrocytes to mechanical and osmotic stresses has been extensively characterized, the molecular mechanisms by which these cells sense changes in the biophysical environment are not well understood (1, 2).
Osteoarthritis, the primary disease afflicting cartilage, is characterized by the gradual breakdown of the cartilage ECM. In this disease, damage to the collagen network results in swelling and increased water content of the tissue, thus decreasing the interstitial osmotic pressure (1). Osteoarthritis is also characterized by a significant inflammatory component at the molecular level. In particular, the pro-inflammatory cytokine, interleukin 1 (IL-1) has been shown to elicit Ca2+ transients in chondrocytes and to affect the response to hypo-osmotic stress by preventing volume regulation (14). Furthermore, IL-1 is a potent activator of cyclo-oxygenase 2 (COX2) and associated pro-inflammatory autacoids such as prostaglandin-E2 (PGE2) (15), which serve as the primary pharmacologic targets for combating pain and inflammation in osteoarthritis (16).
One potential candidate involved in chondrocyte osmo-sensation, and thus potentially mechanotransduction, is the Ca2+-permeable, nonspecific cation channel Transient Receptor Potential Vanilloid 4 (TRPV4). Although the permeability of TRPV4 is relatively similar for Ca2+, Sr2+, Ba2+, Mg2+ (17, 18), under physiologic conditions calcium is the primary ion traversing the channel. TRP channels act as cellular sensors in a variety of settings (19). TRPV4 was the first osmotically sensitive vertebrate ion channel to be discovered and subsequently has been found expressed in a variety of tissues (17, 19-23). TRPV4 is activated by hypo-osmotic cell swelling (17, 20, 23), warmth (>27°C) (24-26), phorbol esters (e.g., 4α-phorbol 12,13-didecanoate, 4αPDD, a specific synthetic activator of TRPV4) (27), and arachidonic acid and its metabolites (28, 29). TRPV4 activation has also been shown to promote chondrogenesis by inducing SOX9 transcription through a Ca2+/calmodulin pathway (22). Furthermore, a TRPV4 gain-of-function mutation has recently been shown to underlie a familial form of brachyolmia, a developmental skeletal dysplasia (30), which suggests a potentially critical role for TRPV4 in skeletal development.
The goal of this study was to characterize the expression and function of TRPV4 in porcine articular cartilage. As an osmotically gated ion channel, we investigated whether TRPV4 would conduct extracellular Ca2+ in response to osmotic stress, and whether it would participate in osmotically-induced volume regulation. We also addressed whether TRPV4 plays a role in chondrocytes' response to IL-1, and in the release of PGE2, which is a pathogenic, pro-nociceptive downstream effect of IL-1-receptor signaling. Finally, because ciliary expression of TRP channels has been implicated in signal transduction in a variety of cell types and organisms (31-33), we examined the role of chondrocytic cilia in TRPV4 function.
Materials and Methods
Porcine cell culture
All cell culture reagents were obtained from Gibco (Invitrogen Corp., Grand Island, NY), unless otherwise stated. Cartilage was harvested from the femoral condyles of skeletally mature pigs (Sus scrofa), and chondrocytes were isolated from the tissue in a sequential pronase (Calbiochem, San Diego, CA) and collagenase (Worthington Biochemical Corp., Lakewood, NJ) digestion as previously described (34). Chondrocytes were cultured overnight at 37°C in 5% CO2 on glass coverslips (1.0-1.5E6 cells/mL) in DMEM, 10% FBS, 15 mM HEPES, 0.1mM non-essential amino acids. Measurements of intracellular Ca2+, volume, and PGE2 experiments were performed at 37°C in medium containing only DMEM and 15 mM HEPES.
Immunohistochemistry
Samples of porcine articular cartilage were fixed in paraformaldehyde and embedded in paraffin (35). An anti-TRPV4 rabbit antibody (33) was applied to 5 μm sections and detected using a fluorescent secondary antibody (Alexa Fluor 555; Molecular Probes, Invitrogen, Eugene, OR). A negative control and a pre-immune control using serum from the antibody-producing rabbit were also run.
Western blotting
We followed standard methods described previously (33). Briefly, cells were lysed with RIPA buffer and separated on an 8% SDS polyacrylamide gel, then transferred to PVDF filters (0.45 mm pore; Millipore, Amherst MA) by semi-dry blot using Tris-glycine/20% methanol transfer buffer. Blotted proteins were immuno-detected using a two-step antibody-mediated chemoluminescence assay, employing a specific antibody against the TRPV4 (36), and secondary peroxidase-coupled antibodies (Jackson ImmunoResearch, West Grove, PA). As a control to assure specificity of detected bands, the antibodies were pre-incubated with the immunogenic peptide (17 C-terminal amino acids of rat TRPV4).
RT-PCR and Sequencing
A BLAST (http://www.ncbi.nlm.nih.gov/) search among porcine expressed sequence tags (ESTs) was done to search for sequences homologous to the human TRPV4 gene (NCBI Accession # NM_021625.1). Two sets of custom primers (Integrated DNA Technologies, Coralville, IA) based on ESTs similar to the TRPV4 gene were developed. RNA was extracted from porcine chondrocytes and reverse-transcribed using commercial kits (RNeasy Mini Kit; Qiagen, Valencia, CA, and SuperScript™ First-Strand Synthesis System for RT-PCR; Invitrogen, Carlsbad, CA). PCR was performed using reagents from Qiagen, and porcine GAPDH (33, 37) was used as a housekeeping gene. The PCR products were submitted to a core facility (Duke University DNA Analysis Facility, Durham, NC) for sequencing.
Sequencing porcine TRPV4
The 5′ and 3′ sequence ends of the porcine TRPV4 gene were determined via RACE PCR and cloning from porcine brain and spleen SMART cDNA (Clontech Mountain View, CA). Primers were designed based on an alignment between the human (NM_021625), and mouse (AF208026) sequences and RACE PCR was performed to amplify these ends. Once the 5′ and 3′ sequence ends were confirmed between the 2 different tissue sources, primers were then designed to amplify the full length cDNA ORF. The full length ORF was obtained by PCR from porcine brain SMART cDNA. This sequence was confirmed by comparison with cDNA clones obtained from multiple tissue sources. The final clone was flanked with a 5′ CACC for directional cloning into the pcDNA3.1_V5-His-TOPO vector. The sequence was deposited in GenBank.
Ligand-gated porcineTRPV4 activation
Porcine articular chondrocytes were loaded with Fura-2-AM and the intracellular concentration of Ca2+ ([Ca2+]i) was measured in a multiwell format using a FlexStation (Molecular Devices) following exposure to TRPV4 agonist (4αPDD). Inhibition of ligand-gated (4αPDD) activation by Ruthenium Red or GSK-205 was also demonstrated.
Osmotic stress
Media osmolarity was determined by a freezing point osmometer (Osmette 2007; Precision Systems, Inc., Natick, MA), using 380 mOsm as the iso-osmotic point for chondrocytes (2). Hypo-osmotic (280 mOsm) and hyper-osmotic media (480 mOsm) were prepared by adding distilled water or sucrose (EMD Chemicals, Gibbstown, NJ) to iso-osmotic experimental medium (DMEM/HEPES).
Calcium Measurements
Changes in [Ca2+]i were measured by previously described fluorescence ratio imaging of Fura Red and fluo-3 using a laser scanning microscopy (8, 38). Changes in [Ca2+]i at 37°C were measured in response to: 1 μM 4αPDD (MP Biomedicals, Inc., Aurora, OH, and Sigma, St. Louis, MO), a TRPV4 agonist. The Ca2+ response to 1 μM 4αPDD was also evaluated in chondrocytes pre-stimulated with the following compounds: 1 μM 4αPDD with a 30 minute pre-incubation in 1 μM Ruthenium Red (RR; non-selective TRPV4 inhibitor, Sigma); 10 μM GSK205 (selective TRPV4 inhibitor, GlaxoSmithKline); 3 μM thapsigargin (thaps, inhibitor of intracellular Ca2+ release; Calbiochem); 10 μM gadolinium (Gd3+, non-specific inhibitor of mechanosensitive ion channels, Sigma); Ca2+-free medium containing EGTA (10 mM, Calbiochem); and 1 μM capsazepine (selective TRPV1 inhibitor, Sigma). Cells were exposed to all of the aforementioned compounds, except for 4αPDD, for a pre-imaging incubation period ranging from a few minutes to an hour. Cells were also exposed to 480 mOsm media and 280 mOsm media alone or with 1 μM RR or 10 μM GSK205. After the iso-osmotic medium was withdrawn from the perfusion chamber, 1 mL of experimental solution was perfused on to the cells by a syringe pump.
[Ca2+]i, as measured by the intensity ratio of the two Ca2+ indicators, was normalized to baseline levels, and a positive response was defined as an increase of [Ca2+]i greater than three standard deviations over the average response to control. Vehicular controls, ethanol in 380 mOsm DMEM/HEPES, were also run to confirm a lack of response. The time to the first peak [Ca2+]i and the normalized height of this peak were also measured using a custom-written Matlab (The MathWorks, Natick, MA) program.
Cell volume measurements
Cell surface area was measured using a custom-written digital image analysis program (39). Eight baseline images were obtained prior to infusing experimental medium. Experimental media tested were: 280 mOsm, 280 with 1 μM RR, 280 with 10 μM GSK205, 280 with 10 ng/mL IL-1α (R&D Systems, Minneapolis, MN), 280 with IL-1 and 1 μM 4αPDD, 380 mOsm, 380 with 4αPDD, 380 with 4αPDD and RR, 380 with 4αPDD and GSK205, and 480 mOsm. Cell volumes were derived from these data and normalized to baseline levels. Maximum volume change within the first 200 s, final volume (average of the last 20 images) and recovered volume (maximum-final) were computed.
PGE2 Assay
Isolated chondrocytes were plated directly onto tissue culture-treated 12-well plates (1E6 cells per well) and incubated overnight. The cells were treated with 280 mOsm media, 280 plus RR (1 μm), 280 plus GSK205 (10 μm), 380 mOsm media, 380 plus 4αPDD (1 μM), 380 plus 4αPDD plus RR, 380 plus 4αPDD plus GSK205, or 480 mOsm media. Samples of media were taken from the wells at 16 and 30 min. for the PGE2 assay, which was used to the manufacturer's specifications (PGE2 Parameter Immunoassay; R&D Systems). The assay sample absorbances were evaluated in a GENios plate reader (Tecan US, Inc., Research Triangle Park, NC).
Cilia and TRPV4
Freshly isolated porcine chondrocytes were fixed with cold methanol and labeled with the following antibodies: α-tubulin (Sigma) to detect primary cilia and TRPV4. To determine whether cilia affected the function of TRPV4, freshly isolated porcine chondrocytes were cultured for 24 hours in either normal feed media or chemical deciliation media (380 mOsm feed media plus 4 mM chloral hydrate) (31), which acts by disrupting ciliary microtubules (40). Changes in [Ca2+]i were measured (as described above) in response to 380 mOsm, 280 mOsm or 380 mOsm plus 1μM 4αPDD. Positive controls with 380 mOsm plus 10 μM ionomycin were also run.
Statistical analysis
Comparisons of continuous values among treatment groups were made using a one-factor ANOVA and Fisher's PLSD post-hoc analysis. Nominal data were compared between treatment groups using the chi-square test. PGE2 data were analyzed with a repeated measures ANOVA with time as the repeated measure. All data reported are mean ± standard error with statistical significance at the 95% confidence level. Statistical analysis was performed with StatView (SAS Institute, Cary, NC) or Statistica (Statsoft, Tulsa, OK)
Results
Chondrocyte TRPV4 Expression
Immunohistochemistry using TRPV4 antibodies clearly demonstrated localization of the channel to the cell membrane with some intracellular labeling of in situ porcine chondrocytes (Fig. 1a), while the negative and pre-immune controls exhibited little to no fluorescence. Western blotting confirmed the presence of TRPV4 in protein extracts from chondrocytes (Fig. 1b). The porcine sequence (genbank accession# EU784669) is 97% similar to human TRPV4 at the amino acid level. RT-PCR from porcine chondrocyte RNA yielded two DNA fragments that showed 94.4% homology to the human TRPV4 cDNA (Fig. 1c).
Figure 1.
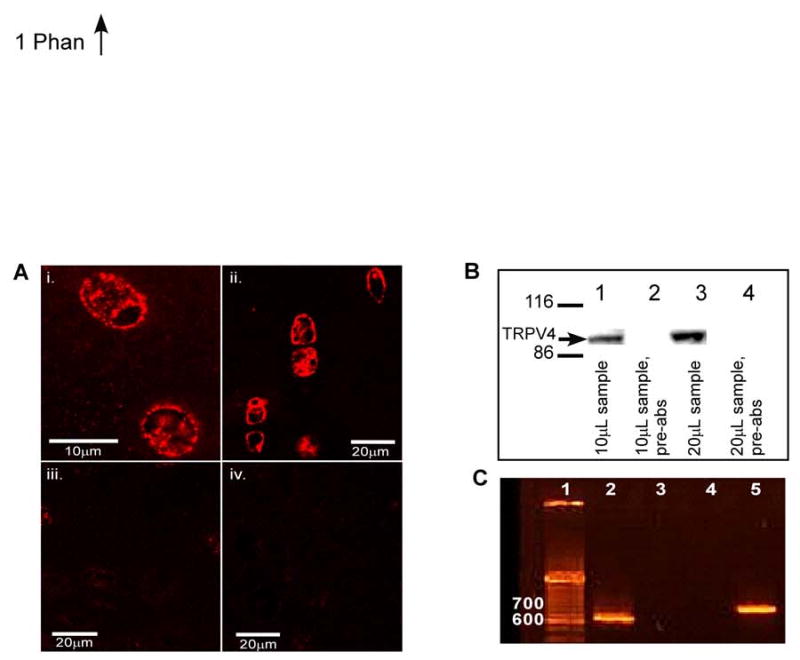
A. Micrographs showing binding of the anti-TRPV4 antibody to chondrocytes in porcine tissue. Surface zone cells labeling positive for TRPV4 (i); deep zone cells showing positive labeling for TRPV4 primarily on the cell membrane (ii); pre-immune control shows negligible labeling (iii); negative control shows little labeling (iv). B. Western blot showing binding of the anti-TRPV4 antibody to protein extracts from porcine chondrocytes. Lanes 1 and 3 show 10 μl and 20 μl of protein extract respectively; lanes 2 and 4 show the TRPV4 peptide pre-absorbed antibody controls for lanes 1 and 3. All lanes were run on the same gel. C. Agarose gel electrophoresis of RT-PCR products from porcine articular cartilage showing amplification of TRPV4. Lane 1 contains a DNA ladder, lane 2 is product amplified with primers to TRPV4 Segment 1 (620 bp), lanes 3 and 4 are empty, and lane 5 is product amplified with primers to TRPV4 Segment 2 (740 bp).
Chemical activation of TRPV4
In isolated porcine chondrocytes the TRPV4 agonist, 4αPDD induced an influx of Ca2+ (EC50∼2 μM; Fig. 2). The Ca2+ influx induced by 10 μM 4αPDD was inhibited by the TRP-blocker Ruthenium Red (RR, IC50∼5 nM). A selective TRPV4 channel blocker, GSK-205 (N-(4-{2 [methyl(phenylmethyl)amino]ethyl}phenyl)-5-(3-pyridinyl)-1,3-thiazol-2-amine hydrobromide), which was identified using a FLIPR-based high throughput screen and has been shown by electrophysiology and Ca2+ imaging studies to block both ligand-gated and hypo-osmotic activation of endogenous and transiently expressed human TRPV4 channels, was found to inhibit Ca2+ influx (IC50 ∼ 600 nM; Fig. 2).
Figure 2.
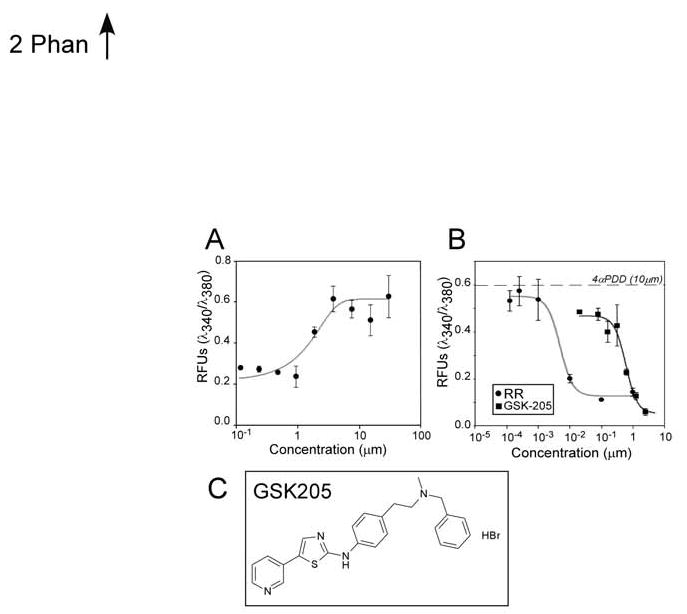
Ca2+ response of porcine articular chondrocytes to increasing concentrations of TRPV4 agonist, 4αPDD, (A) and antagonists, RR and GSK205 (B). Lines show best fit curve (Logistic 4-parameter). Data points represent the mean (±SD) from triplicate determinations in 2 independent experiments. Structure of GSK205 (C).
Ca2+ signaling in chondrocytes
Control experiments demonstrated that iso-osmotic medium or vehicular controls did not result in significant increases in [Ca2+]i. Cell stimulation with 4αPDD caused either sharp increases (often with subsequent oscillations) or sustained elevations in intracellular Ca2 in 49% of the cells (Fig. 3). Treatment with RR prior to and during 4αPDD stimulation significantly decreased the percentage of cells signaling to only 29%. Treatment with the TRPV4 antagonist, GSK205, decreased Ca2+ signaling in response to 4αPDD even further with 26% of cells responding. Thirty minute pre-incubation with Gd3+ (10 μM), a purported stretch-activated channel blocker (41), significantly attenuated the response to 4αPDD with 30% of cells responding. The response to 4αPDD was clearly dependent upon extracellular Ca2+, as only 6% of the cells exposed to 4αPDD in Ca2+-free medium signaled. Moreover, 4αPDD-induced elevations in [Ca2+]i were facilitated by Ca2+ release from intracellular stores because only 3% of the cells treated with thapsigargin showed increased [Ca2+]i. Capsazepine, a TRPV1 channel antagonist, did not significantly block the 4αPDD response, as 43% of the cells signaled in its presence.
Figure 3.
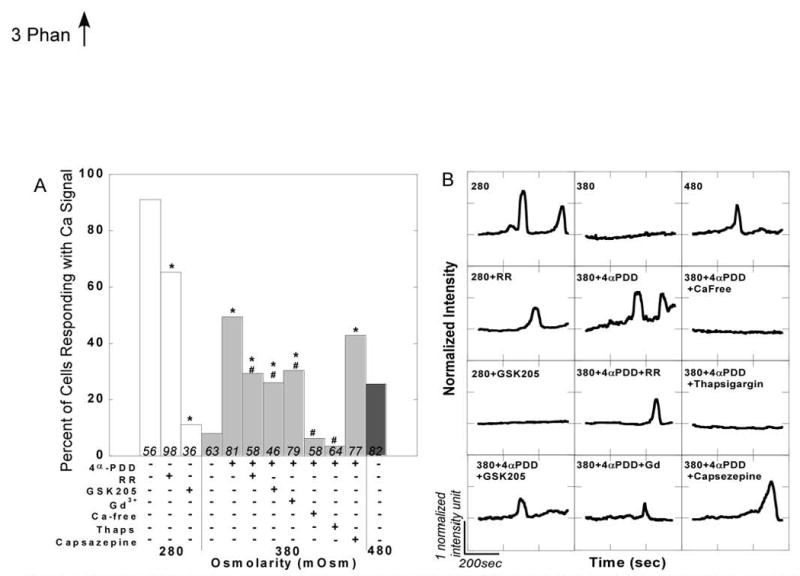
A. Percentage of cells responding to osmotic and chemical stimuli with increases in intracellular Ca2+. Asterisks denote experimental groups that are significantly different from control within a given osmolarity (chi-squared test, p<0.05); number signs specify bars within the 380 mOsm group that are significantly different from 380+4αPDD (chi-squared test, p<0.05). Both the 280 and 480 mOsm controls are significantly different from the 380 mOsm control (chi-squared, p<0.05). Numbers in bars show n; bars do not have error bars because the percent responding metric does not have an error associated with it. B. Representative Ca2+ traces for each condition showing normalized intensity versus time.
Ca2+ signaling in chondrocytes was also induced by osmotic stress (Fig. 3). Hypo-osmotic stress (280mOsm) invoked increases in [Ca2+]i in 91% of the cells, whereas hyper-osmotic stress (480mOsm) elicited responses in only 26% of the cells. Both hypo- or hyper-osmotic stress caused significantly more Ca2+ signaling than iso-osmotic control. In the presence of GSK205, the hypo-osmotic response was strikingly attenuated, with only 11% of cells signaling respectively (RR: 65%).
Changes in cell volume
Joint loading can cause changes in the osmotic environment of the chondrocytes as fluid is forced out and reabsorbed into the cartilage. These fluctuations can cause regulatory volume changes as well as Ca2+ signaling (8, 9), thus we further examined whether TRPV4 plays a role in volume regulation. Both osmotic and chemical stimuli altered the volume of the chondrocytes (Fig. 4). Chondrocyte maximum volume increased in hypotonic (280mOsm) medium, decreased in hypertonic (480mOsm) medium, and remained constant in isotonic (380mOsm) medium. Cells exposed to 4αPDD in iso-osmotic conditions underwent a more modest, but nonetheless significant volume increase to a maximum volume that was significantly diminished by RR and GSK205. IL-1 receptor signaling significantly increased the maximum volume in response to hypo-osmotic stress, and that increase was inhibited by stimulating TRPV4 channels with 4αPDD. The addition of RR or GSK205 decreased the maximum volume in response to hypo-osmotic stress.
Figure 4.
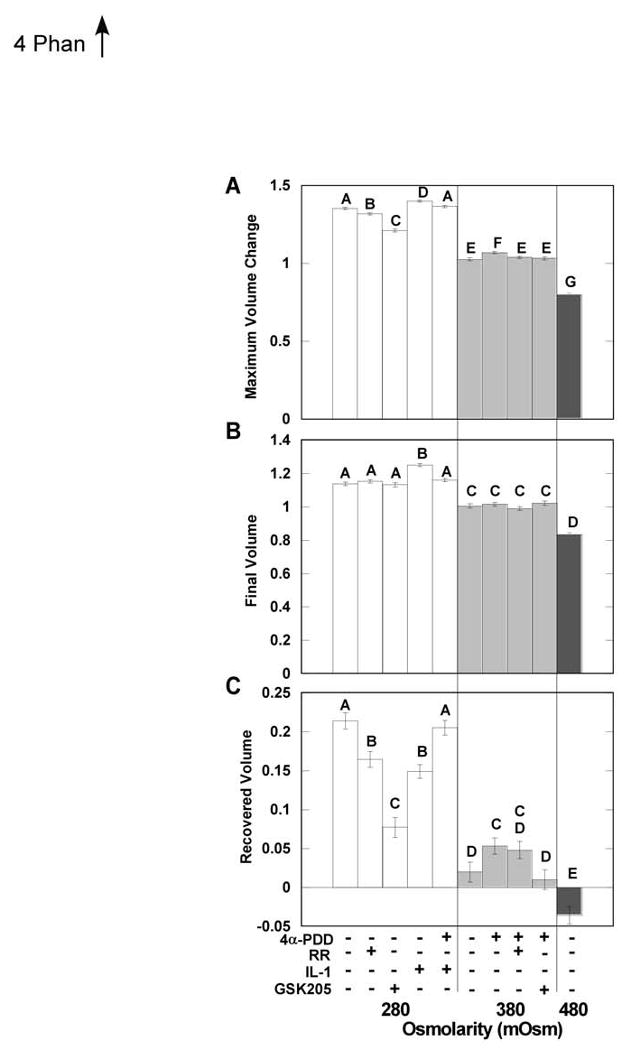
Mean (±sem) maximum volume change (swelling or shrinking) (A), final volume (average of the last 117 seconds of the experiment) (B), and recovered (maximum-final) (C) volume after stimulation with different osmotic and chemical stimuli. Bars with different letters are significantly different from one another (ANOVA, p<0.05).
The chondrocytes exhibited regulatory volume decrease (RVD) in response to hypo-osmotic stimuli, and that RVD was significantly inhibited by blocking TRPV4 activation with RR or GSK205 (Fig. 4). The IL-1-treated chondrocytes showed greatly impaired RVD which was eliminated when TRPV4 channels were stimulated with 4αPDD.
PGE2 Release
We monitored stimulus-induced release of PGE2 because of the important role of this pro-inflammatory autacoid in joint inflammation and pain. Hypo-osmotic stimulation significantly increased the short-term (15-30minutes) release of PGE2 in a TRPV4-dependent manner, as indicated by the strong inhibitory effect of GSK205 (also by RR; Fig. 5). Specific chemical stimulation of TRPV4 with 4αPDD, under isotonic conditions, did not induce significant PGE2 release.
Figure 5.
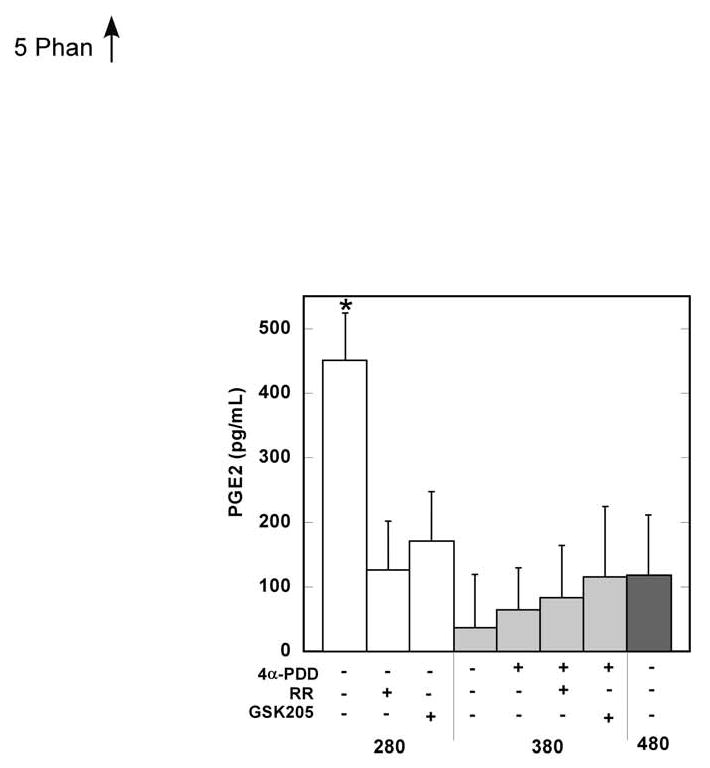
Mean (+sem) levels of PGE2 in culture medium after treatment with different osmolarities and TRPV4 agonists/antagonists. Measurements at 16 and 30 minutes of exposure were combined as there was no significant effect of time. Bars with different letters are significantly different from one another (Repeated Measures ANOVA, p>0.05).
TRPV4 and Cilia
Because TRP channel-mediated signal transduction has been linked to primary cilia in a variety of cell types and organisms (31-33), we examined co-localization of cilia and TRPV4 in articular chondrocytes and asked whether TRPV4 activation required intact cilia. Tubulin (α and γ) labeling clearly showed the presence of a primary cilium on all cells (Fig. 6a; data not shown). TRPV4 labeling was relatively uniform on the periphery of the cells. Careful inspection demonstrated TRPV4 decorating the cilium itself. Therefore, TRPV4 labeling in chondrocytes, while co-localizing with cilia, was not restricted to cilia.
Figure 6.
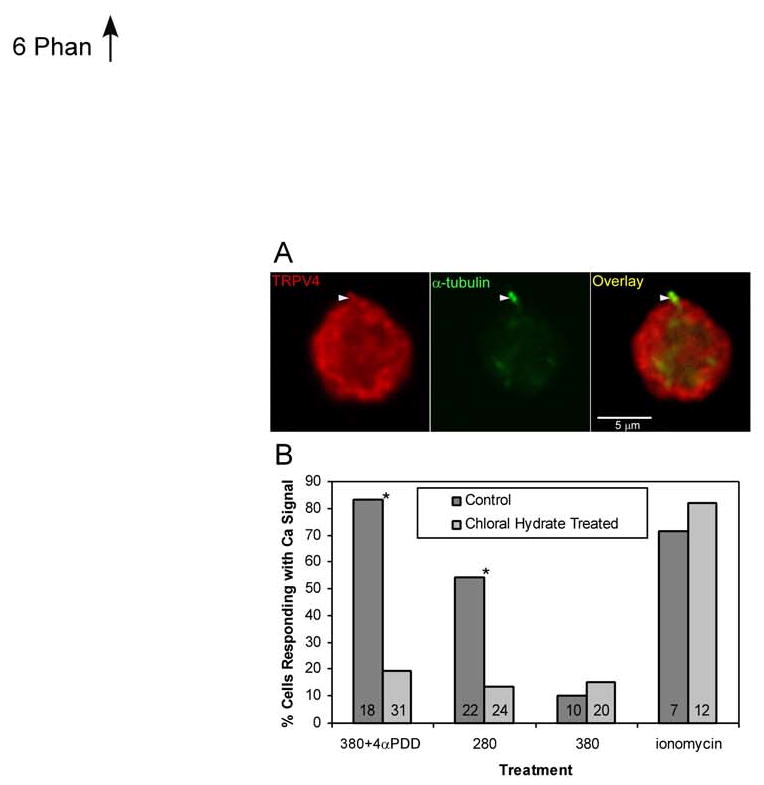
A: Micrograph of porcine chondrocytes labeled for TRPV4 and α-tubulin (to show primary cilia). Arrowhead shows primary cilium with both tubulin and TRPV4 labeling. B: Percent of cells responding with a Ca2+ signal to 4αPDD or hypo-osmotic stress significantly decreases when the cilia are disrupted with chloral hydrate treatment. Bars show means, numbers in bars show n, asterisks indicate significant difference between chloral hydrate treated and controls (chi-squared, p<0.05).
In order to examine the function of ciliary TRPV4 in chondrocytes, they were subjected to treatment with chloral hydrate, which de-ciliates cells. This treatment almost fully eliminated Ca2+ signaling in response to TRPV4-activating stimuli, exposure to 4αPDD and hypo-osmotic stress (Fig. 6b). Control experiments validated this provocative result, inasmuch that iso-osmotic media caused negligible Ca2+ signaling. Chondrocytic Ca2+ signaling appeared basically unaffected, since intracellular store depletion using ionomycin was fully functional.
Discussion
The goal of this study was to demonstrate and characterize the functional expression of the TRPV4 channel in primary articular chondrocytes, the cell population responsible for maintenance of joint function and disease. Using a reductionist cellular model, we demonstrated that TRPV4 is expressed robustly in porcine articular chondrocytes at both the RNA and protein levels. Channel function was confirmed using both chemical and physical activators of TRPV4. For example, 4αPDD, a known TRPV4 agonist, induced Ca2+ transients, which were blocked by a novel TRPV4-selective small molecule inhibitor, GSK205. We also established that specific blocking of TRPV4 inhibits the volume-regulatory and Ca2+ signaling responses to hypo-osmotic stress, suggesting a pivotal role for TRPV4 in osmotically-mediated signal transduction in chondrocytes. Our data also reveal a potentially central role for TRPV4 in mediating the inflammatory response of chondrocytes; activation of TRPV4 counteracts several tested effects of IL-1, while TRPV4 block decreases short-term release of PGE2. Finally, our data suggest that chondrocytic TRPV4 activation is critically dependent on the integrity of the primary cilium.
These experiments were conducted in primary cells isolated from tissue expressing normal levels of TPRV4. Previous studies have suggested that cell-ECM interactions may be important for proper signal transduction (14, 42), but chondrocytes appear to exhibit similar signaling responses to osmotic stress whether isolated or in situ (43). Furthermore, isolated chondrocytes represent a heterogeneous population, as studies have shown that cells from the different zones of cartilage may respond to stimuli differently (14, 44). Thus, further in situ studies will be important in developing a full understanding of the potentially site-specific role of TRPV4 in articular cartilage.
TRPV4 was localized to the cell membrane, and pharmacological activation of TRPV4 with 4αPDD caused a significant Ca2+ influx, which is consistent with the reported role of TRPV4 as a Ca2+ permeable channel (45). Our data also show that stimulation of Ca2+ signals with 4αPDD is blocked by both RR and by the TRPV4-selective compound GSK205 (Fig. 2). The effects of selective inhibition of TRPV4 using GSK205 suggest that it is TRPV4, and not other possible RR-targets, that is necessary for osmotically-mediated signal transduction and volume regulation in chondrocytes.
Specifically activating the TRPV4 channel with 4αPDD in Ca2+-free medium did not elicit Ca2+ signaling, implicating extracellular influx as an initiating signal in the Ca2+ response (Fig. 3). Furthermore, a novel finding of this study was that thapsigargin, an inhibitor of Ca2+-ATPases on the endoplasmic reticulum, strongly diminished Ca2+ signaling in response to 4αPDD. Taken together, these results suggest that the TRPV4 response is dependent upon both Ca2+ influx from the extracellular milieu and subsequent Ca2+ release from intracellular stores suggesting a form of “Ca2+-induced Ca2+ release”. Previous studies have shown that the chondrocytes' response to hypotonicity also occurs through this mechanism, consistent with the hypothesis that the effects of hypotonicity are mediated by TRPV4 (8, 9).
Chondrocyte volume regulation in response to osmotic stress appears to be mediated by TRPV4, since specifically inhibiting TRPV4 with GSK205, to a lesser degree also with the less specific RR, prevented normal RVD in response to hypo-osmotic stress (Fig. 4). The mechanism is likely a block of TRPV4-mediated Ca2+ influx in response to hypotonicity (Fig. 3). According to previous results, the chondrocytic RVD response to hypotonic stress depends not only on Ca2+, but also on actin remodeling which in turn is evoked by influx of extracellular Ca2+ (9, 14, 46). Therefore, it is plausible that Ca2+ influx mediated by TRPV4 may influence cytoskeletal reorganization following an osmotic stimulus (9). Furthermore, other studies have also posited a link between the cytoskeleton and TRPV4 (21, 47).
Osteoarthritis is increasingly recognized as a human disease with a significant inflammatory component at the molecular level, involving the action of cytokines such as IL-1 (15). In the present study, hypotonic exposure in the presence of IL-1 was shown to result in a significant impairment of physiological RVD, consistent with previous studies (48). However, specific chemical activation of TRPV4, under the same conditions, was able to fully compensate for this deficit. This suggests that TRPV4-mediated Ca2+ influx is down-stream of IL-1 receptor signaling and that IL-1 receptor mediated changes are capable of disabling osmotic TRPV4 activation. In chondrocytes, IL-1 has been shown to induce stabilization of cortical F-actin (14). Presumably, this increased stabilization of the actin cytoskeleton inhibits normal RVD, and thus the specific chemical activation of TRPV4 induces Ca2+ transients which might be necessary for F-actin remodeling, which in turn will counteract the F-actin stabilization evoked by IL-1 receptor signaling (9).
TRPV4 may also be involved in release of pathogenic autacoid mediators, such as prostaglandins, a final effector pathway leading to tissue damage and stimulation of nociceptor neurons' peripherals. Hypo-osmotic stress was found to increase PGE2 release through a TRPV4-dependent mechanism, since it was blocked by GSK205. However, specific chemical activation of TRPV4 in isotonicity had no effect on PGE2 production. Similar to our results, Le et al. have also shown little to no PGE2 production in response to iso- or hyper-osmotic stimuli (49). The observed PGE2 release in response to hypo-osmotic stress may be related to the RVD cascade because taurine efflux mediates RVD in response to hypotonicity (50), and taurine efflux is down-stream of cAMP, which is known to be produced in response to PGE2 in chondrocytes (51). The mechanism of TRPV4 activation by hypo-osmotic stimulation is not fully understood but could possibly involve the activation of PLA2 by cell swelling, which then releases arachidonic acid (AA) from membrane phospholipids; AA is converted by cytochrome p450 epoxygenase into EETs, which can function as endogenous activators of TRPV4 (45). AA can also be metabolized by COX enzymes to form PGE2 (15). Thus, our data demonstrating an increase in PGE2 release in response to hypo-osmotic stress in chondrocytes appear seemingly consistent with activation of an arachidonic acid pathway up-stream of TRPV4. However, specific inhibition of TRPV4 using GSK205 blocked PGE2 production in response to hypo-osmotic medium, which apparently argues against critical involvement of the PLA2-P450-epoxygenase signaling pathway.
TRP channels have been implicated in the osmo-mechanosensory functions of cilia in a variety of settings (31-33). Chondrocytes have been shown to express primary cilia (52), and we confirmed this observation for porcine cells. We also demonstrated that TRPV4 was highly expressed in the periphery of the cell and did not localize specifically the cilium. Seemingly counterintuitive to these expression data, we found that an intact cilium was required for TRPV4-mediated Ca2+ signaling in response to 4αPDD or hypo-osmotic stress. One explanation is that ciliary TRPV4 is in an active state, whereas its abundant expression in the remainder of the cell is not. Other sensory systems function optimally with only a few thousand channels for all the processes of one cell (53), which is consistent with the low level of expression of TRPV4 on the cilium. However, it also raises the question of the functional role for non-ciliary TRPV4. The primary cilium has been suggested to have a mechanical function in chondrocytes (52) as well as bone cells (54), our data suggest a role in osmotic signal transduction as an underlying mechanism.
In summary, we have demonstrated that functional TRPV4 channels are present in porcine articular cartilage and that they are involved in responding to hypo-osmotic stress and inflammation. Because of the unique structure of cartilage, mechanical loading is directly coupled to interstitial osmolarity. Thus, in acting as an osmosensor, TRPV4 may in fact be acting as a “mechanosensor” that regulates cellular response to changes in joint loading. In addition to physiological short-term changes in osmolarity with loading, the pathologic loss of proteoglycan content and increased hydration of cartilage with osteoarthritis may also result in chronic changes in the osmotic environment of the chondrocytes. Given the potential role of TRPV4 in sensing extracellular osmotic changes and in the inflammatory response of cartilage, further insight on the expression and function of this channel may provide new insights in the regulation of cartilage development, physiology, and disease.
Acknowledgments
Supported by GlaxoSmithKline, Inc., the NIH (AG15768, AI07217, AR48852, AR50245), and the Arthritis Foundation.
References
- 1.Guilak F, Hung CT. Physical Regulation of Cartilage Metabolism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechanobiology. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 259–300. [Google Scholar]
- 2.Urban JPG, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 3.Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7(1):41–58. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- 4.Maroudas A. Physicochemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Bath: Pitman Medical; 1979. pp. 215–290. [Google Scholar]
- 5.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 6.Urban JP. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994;33(10):901–8. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- 7.Guilak F, Erickson GR, Ting-Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J. 2002;82(2):720–7. doi: 10.1016/S0006-3495(02)75434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech. 2001;34(12):1527–35. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 9.Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis Cartilage. 2003;11(3):187–97. doi: 10.1053/s1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- 10.Hall AC. Volume-sensitive taurine transport in bovine articular chondrocytes. J Physiol. 1995;484(Pt 3):755–66. doi: 10.1113/jphysiol.1995.sp020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall AC, Horwitz ER, Wilkins RJ. The cellular physiology of articular cartilage. Exp Physiol. 1996;81(3):535–45. doi: 10.1113/expphysiol.1996.sp003956. [DOI] [PubMed] [Google Scholar]
- 12.McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiol Rev. 1992;72(4):1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- 13.Hardingham GE, Bading H. Calcium as a versatile second messenger in the control of gene expression. Microsc Res Tech. 1999;46(6):348–55. doi: 10.1002/(SICI)1097-0029(19990915)46:6<348::AID-JEMT3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Pritchard S, Guilak F. Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis Rheum. 2006;54(7):2164–2174. doi: 10.1002/art.21941. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg JB, Fermor B, Guilak F. Nitric oxide synthase and cyclooxygenase interactions in cartilage and meniscus: relationships to joint physiology, arthritis, and tissue repair. Subcell Biochem. 2007;42:31–62. doi: 10.1007/1-4020-5688-5_2. [DOI] [PubMed] [Google Scholar]
- 16.Rocca B, Davi G. Should patients with osteoarthritis be treated with COX2 inhibitors rather than traditional NSAIDs? Nat Clin Pract Rheumatol. 2007;3(6):316–7. doi: 10.1038/ncprheum0480. [DOI] [PubMed] [Google Scholar]
- 17.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2(10):695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 18.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278(29):26541–9. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 19.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451(1):193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 20.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103(3):525–35. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Bandyopadhyay B, Nakamoto T, Singh B, Liedtke W, Melvin JE, et al. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem. 2006;281(22):15485–95. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- 22.Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007;282(44):32158–67. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- 23.Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000;485(2-3):127–34. doi: 10.1016/s0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- 24.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22(15):6408–14. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Caterina MJ. TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch. 2005;451(1):160–7. doi: 10.1007/s00424-005-1438-y. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277(49):47044–51. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277(16):13569–77. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 28.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–15. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–8. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 30.Rock MJ, Prenen J, Funari VA, Funari TL, Merriman B, Nelson SF, et al. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet. 2008;40(8):999–1003. doi: 10.1038/ng.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, et al. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA. 2007;104(48):19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kottgen M, Buchholz B, Garcia-Gonzalez M, Kotsis F, Fu X, Doerken M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182(3):437–47. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA. 2003;100(23):13698–703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuettner KE, Pauli BU, Gall G, Memoli VA, Schenk RK. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. Isolation, culture characteristics, and morphology. J Cell Biol. 1982;93:743–750. doi: 10.1083/jcb.93.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, et al. Zonal changes in the three-dimensional morphology of the chondron in articular cartilage under compression: The relationship between cellular, pericellar, and extracellular deformation. J Biomech. 2007;40:2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, et al. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am J Physiol Renal Physiol. 2004;287(1):F17–24. doi: 10.1152/ajprenal.00397.2003. [DOI] [PubMed] [Google Scholar]
- 37.Jefferies D, Farquharson C, Thomson J, Smith W, Seawright E, McCormack H, et al. Differences in metabolic parameters and gene expression related to osteochondrosis/osteoarthrosis in pigs fed 25-hydroxyvitamin D3. Vet Res. 2002;33(4):383–96. doi: 10.1051/vetres:2002024. [DOI] [PubMed] [Google Scholar]
- 38.Lipp P, Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell calcium. 1993;14(5):359–72. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- 39.Alexopoulos LG, Erickson GR, Guilak F. A method for quantifying cell size from differential interference contrast images: validation and application to osmotically stressed chondrocytes. J Microsc. 2002;205(Pt 2):125–35. doi: 10.1046/j.0022-2720.2001.00976.x. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarti A, Schatten H, Mitchell KD, Crosser M, Taylor M. Chloral hydrate alters the organization of the ciliary basal apparatus and cell organelles in sea urchin embryos. Cell Tissue Res. 1998;293(3):453–462. doi: 10.1007/s004410051137. [DOI] [PubMed] [Google Scholar]
- 41.Berrier C, Coulombe A, Szabo I, Zoratti M, Ghazi A. Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch-activated channels in bacteria. Eur J Biochem. 1992;206(2):559–65. doi: 10.1111/j.1432-1033.1992.tb16960.x. [DOI] [PubMed] [Google Scholar]
- 42.Peterson RS, Andhare RA, Rousche KT, Knudson W, Wang W, Grossfield JB, et al. CD44 modulates Smad1 activation in the BMP-7 signaling pathway. J Cell Biol. 2004;166(7):1081–91. doi: 10.1083/jcb.200402138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erickson GR. PhD Dissertation. Duke University; 2001. Articular chondrocyte response to osmotic stress : the involvement of calcium and the cytoskeleton in cellular volume adaptation and regulation. [Google Scholar]
- 44.Eleswarapu SV, Leipzig ND, Athanasiou KA. Gene expression of single articular chondrocytes. Cell Tissue Res. 2007;327(1):43–54. doi: 10.1007/s00441-006-0258-5. [DOI] [PubMed] [Google Scholar]
- 45.Plant TD, Strotmann R. Trpv4. Handbook of experimental pharmacology. 2007;(179):189–205. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- 46.Pritchard S, Guilak F. The role of F-actin in hypo-osmotically induced cell volume change and calcium signaling in anulus fibrosus cells. Ann Biomed Eng. 2004;32(1):103–11. doi: 10.1023/b:abme.0000007795.69001.35. [DOI] [PubMed] [Google Scholar]
- 47.Ramadass R, Becker D, Jendrach M, Bereiter-Hahn J. Spectrally and spatially resolved fluorescence lifetime imaging in living cells: TRPV4-microfilament interactions. Arch Biochem Biophys. 2007;463(1):27–36. doi: 10.1016/j.abb.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 48.Pritchard S, Votta BJ, Kumar S, Guilak F. Interleukin-1 inhibits osmotically induced calcium signaling and volume regulation in articular chondrocytes. Osteoarthritis Cartilage. 2008;16(12):1466–1473. doi: 10.1016/j.joca.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le D, Hofbauer MA, Towle CA. Differential effects of hyperosmotic challenge on interleukin-1-activated pathways in bovine articular cartilage. Arch Biochem Biophys. 2006;445(1):1–8. doi: 10.1016/j.abb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Heacock AM, Foster DJ, Fisher SK. Prostanoid receptors regulate the volume-sensitive efflux of osmolytes from murine fibroblasts via a cyclic AMP-dependent mechanism. J Pharmacol Exp Ther. 2006;319(2):963–71. doi: 10.1124/jpet.106.109496. [DOI] [PubMed] [Google Scholar]
- 51.Lowe GN, Fu YH, McDougall S, Polendo R, Williams A, Benya PD, et al. Effects of prostaglandins on deoxyribonucleic acid and aggrecan synthesis in the RCJ 3.1C5.18 chondrocyte cell line: role of second messengers. Endocrinology. 1996;137(6):2208–16. doi: 10.1210/endo.137.6.8641167. [DOI] [PubMed] [Google Scholar]
- 52.Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, et al. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28(2):101–10. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Corey D. Stringing the fiddle: the inner ear's two-part invention. Nat Neurosci. 2007;10(10):1232–3. doi: 10.1038/nn1007-1232. [DOI] [PubMed] [Google Scholar]
- 54.Malone A, Anderson C, Tummala P, Kwon R, Johnston T, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci USA. 2007;104(33):13325–30. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]


