Abstract
Fate maps are generated by marking and tracking cells in vivo to determine how progenitors contribute to specific structures and cell types in developing and adult tissue. An advance in this concept is Genetic Inducible Fate Mapping (GIFM), linking gene expression, cell fate, and cell behaviors in vivo, to create fate maps based on genetic lineage.
GIFM exploits X-CreER lines where X is a gene or set of gene regulatory elements that confers spatial expression of a modified bacteriophage protein, Cre recombinase (CreERT). CreERT contains a modified estrogen receptor ligand binding domain which renders CreERT sequestered in the cytoplasm in the absence of the drug tamoxifen. The binding of tamoxifen releases CreERT, which translocates to the nucleus and mediates recombination between DNA sequences flanked by loxP sites. In GIFM, recombination typically occurs between a loxP flanked Stop cassette preceding a reporter gene such as GFP.
Mice are bred to contain either a region- or cell type-specific CreER and a conditional reporter allele. Untreated mice will not have marking because the Stop cassette in the reporter prevents further transcription of the reporter gene. We administer tamoxifen by oral gavage to timed-pregnant females, which provides temporal control of CreERT release and subsequent translocation to the nucleus removing the Stop cassette from the reporter. Following recombination, the reporter allele is constitutively and heritably expressed. This series of events marks cells such that their genetic history is indelibly recorded. The recombined reporter thus serves as a high fidelity genetic lineage tracer that, once on, is uncoupled from the gene expression initially used to drive CreERT.
We apply GIFM in mouse to study normal development and ascertain the contribution of genetic lineages to adult cell types and tissues. We also use GIFM to follow cells on mutant genetic backgrounds to better understand complex phenotypes that mimic salient features of human genetic disorders.
This video article guides researchers through experimental methods to successfully apply GIFM. We demonstrate the method using our well characterized Wnt1-CreERT;mGFP mice by administering tamoxifen at embryonic day (E)8.5 via oral gavage followed by dissection at E12.5 and analysis by epifluorescence stereomicroscopy. We also demonstrate how to micro-dissect fate mapped domains for explant preparation or FACS analysis and dissect adult fate-mapped brains for whole mount fluorescent imaging. Collectively, these procedures allow researchers to address critical questions in developmental biology and disease models.
Protocol
Tamoxifen Preparation and Oral Gavage Procedure (E8.5)
Prepare a 20 mg/ml stock solution of tamoxifen by dissolving tamoxifen in pre-warmed corn oil.
Incubate the solution at 37° C for 2 hours on a nutator and vortex intermittently.
Protect the tamoxifen stock from light, store at 4°C, and use for up to one month.
For fate mapping experiments, establish a breeding pair consisting of a Swiss Webster female (wildtype; purchased from Taconic) and a male carrying both a gene specific CreERT allele and a reporter allele. For demonstration purposes, we are using Wnt1-CreERT;mGFP males (Ellisor 2009).
Check Swiss Webster females each morning for the appearance of a vaginal plug. Designate the morning (0900) of the day a vaginal plug is seen as 0.5 days post-coitus and calculate the date of tamoxifen administration based on this starting point. (For this experiment, embryonic day 8.5 was used).
Use a 1 ml syringe with an animal feeding needle (20G x 1-1/2) to draw up 200 μl of the tamoxifen stock solution (4 mg tamoxifen).
Firmly restrain the time-pregnant Swiss Webster female by grasping the nape of the neck and back to immobilize the head and turn over so the ventral side is facing up.
Hold the tail between free fingers to keep the body in a straight line.
Place the feeding needle into the corner of the mouth and gently guide the needle along the roof of the mouth.
Rotate the needle so that it is parallel to the body, while simultaneously tilting the head back to keep the neck straight.
Guide the needle down the esophagus, toward the stomach. Be careful not to enter the trachea. If there is resistance on the needle, the animal's gag reflex engages, or if the mouse struggles, immediately remove the needle and try the gavage again.
Once the feeding needle is in the stomach, administer the tamoxifen into the stomach and return the female to her home cage until the date of dissection.
Craniotomy:
Intracardially perfuse the adult fate mapped mouse with 4% PFA and remove the head with scissors by cutting through the spinal column just above the shoulders.
Run a scalpel along the dorsal midline of the head (rostral to caudal) to cut through the scalp and expose the scull.
Using the scalpel, scrape away any excess tissue or muscle from along the side and posterior of the cranium.
With forceps, puncture the skull at the midline just rostral to the olfactory bulbs and create a small hole to accommodate the tips of fine scissors.
Insert the fine scissors into this hole and make an incision medial to lateral approximately the length of the olfactory bulbs. This cut will break the skull at the intersection of the nasal bone and frontal bone and provide good access for the scissors.
Cut along the sagittal sutures in the skull (dorsal midline) making sure to keep the scissor tips angled away from the brain to avoid damaging the underlying tissue.
Gently grasp the skull with forceps and peel away the bone along the medial incision to expose the brain. The skull may chip off in small pieces or break away in larger sections. Continue using the forceps to remove all of the frontal, parietal, interparietal, and occipital bones.
Crack the bones encasing the paraflocculi (located along the lateral edges of the brain at the level of the cerebellum) by pinching the spherical bones on each side. Gently pull away the bones.
Turn the head upside down (dorsal side down) and use the forceps to sever the cranial nerves and release the brain from the skull.
Whole Mount Microscopy: Adult Brain
Assess adult brain for GFP marking using a fluorescent dissecting scope.
Transfer brain to a Petri dish containing PBS and photograph using PictureFrame or other appropriate software.
Whole Mount Microscopy: E12.5 Embryos
Assess whole mount embryos for GFP marking using a fluorescent dissecting scope.
Transfer those that are GFP positive by whole mount to a Petri dish containing PBS and photograph using PictureFrame or other appropriate software.
Use forceps to pinch off a small piece of tail from each embryo, place each piece in a PCR tube and genotype the tissue using PCR (Ellisor 2009) for both alleles to confirm results seen via whole mount analysis.
Micro-dissection and explant preparation of the ventral mesencephalon from E12.5 embryos
Following dissection from the uterine chain and identification of fate-mapped embryos by whole-mount fluorescence, transfer embryos to ice-cold sterile PBS in a cell culture dish.
Using fine scissors, remove the head portion of the embryo cutting transversely, caudal to rhombomere 1.
Next, remove the rostral portion of the head with a coronal cut through the diencephalon. This will expose a tube-like structure in which the 4th ventricle through the mesencephalic vesicle form a conduit between dorsal and ventral tissues.
Carefully insert the scissor tips into the 4th ventricle, and snip along the dorsal midline, caudal to rostral, fully opening the tube, creating an "open-book" preparation. The ventral mesencephalon will now be exposed medially, while the two dorsal halves of the mesencephalon will reside laterally.
Remove any remaining non-neural tissue underneath the ventral mesencephalon in order for the tissue to lay more flatly. If need be, make notches in the rostral and caudal aspects such that the dorsal "wings" of the explant will lay flat as well. The explant should now resemble a "butterfly, in which the dorsal mesencephalon represents the wings, and rhombomere 1 the tail.
To further isolate the ventral mesencephalon for FACS analysis or cell culture experiments, remove the lateral (dorsal aspects) of the tissue.
Representative Results:
In this GIFM experiment, Wnt1-expressing cells are permanently and heritably marked during embryogenesis by administering tamoxifen at E8.5 to Wnt1-CreERT;mGFP embryos. Subsequently, these marked cells are visualized via whole mount fluorescence and section immunohistochemistry to determine how Wnt1-derived cells contribute to neural structures across development. Whole mount fluorescence relies on the membrane bound GFP component of the mGFP reporter and therefore provides cellular information as well as an overarching view of Wnt1-derived neural projections. For example, with tamoxifen administration at E8.5, Wnt1-CreERTmGFP embryos at E12.5 exhibit GFP fluorescence primarily in the midbrain, posterior hindbrain and spinal cord (Figure 1A). At high magnification, fine neuronal projections are seen innervating the midbrain, body wall, limb, and craniofacial region. At this developmental stage, the embryo is translucent and internal GFP labeling is easily discerned. However, as the brain develops, the density of mature tissue obscures GFP fluorescence emanating from internal brain structures. Therefore, with tamoxifen administration at E8.5, only minor GFP labeling is observed in the superior colliculi of the adult by whole mount fluorescence (Figure 1C, inset). In addition, some axonal projections are seen coursing along the ventral surface of the hindbrain (not shown). In contrast, when tamoxifen is administered at E9.5, substantial GFP labeling occurs throughout the entire inferior colliculi (Figure 1B, inset). This increase in whole mount fluorescence may indicate that Wnt1-expressing cells at E9.5 contribute more significantly to superficial regions of the dorsal midbrain or extend more processes within this region than Wnt1 expressing cells from E8.5. Regardless, this difference in whole mount fluorescence reflects the dynamic nature of Wnt1 expression during development and demonstrates how GIFM can be used to follow distinct cell populations from the embryo into the mature adult.
With immunohistochemistry, fate mapped tissue sections from Wnt1-CreERT;mGFP animals are analyzed at the cellular level. Antibodies against GFP and β-galactosidase (β-gal) are used in conjunction with a variety of tissue specific biomarkers to determine the fine scale cellular contribution of the Wnt1 lineage to neural structures and cell types. With tamoxifen administration at E8.5 and tissue analysis at E12.5, double positive cells (GFP+ and β-gal+) are observed throughout the midbrain and hindbrain with projections coursing through the ventral midbrain (Figure 1B). In the adult brain, the Wnt1-lineage marked at E8.5 gives rise to many midbrain structures including the superior and inferior colliculi as well as in the vicinity of dopaminergic cell populations of the ventral tegmental area and substantia nigra (Figure 1D) (see also Zervas 2004). In contrast, the Wnt1-lineage marked at E9.5 gives rise to inferior colliculi as well as to neurons in the vicinity of dopaminergic cell populations (Figure 1F).
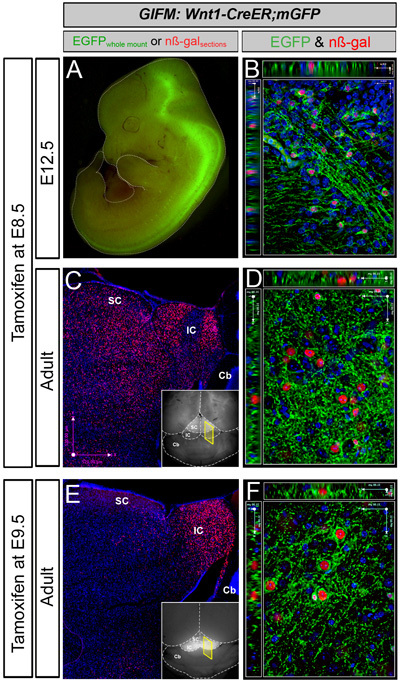 Figure 1. Representative Results for Wnt1-fate mapping:
Figure 1. Representative Results for Wnt1-fate mapping:
Examples of whole-mount and immunocytochemistry results in Wnt1-CreERT;mGFP embryos and adult brains fate mapped (marked) at E8.5 (A-D) and E9.5 (E-F). A. Wnt1-CreER;mGFP embryos at E12.5 exhibit GFP fluorescence primarily in the midbrain (Mb), posterior hindbrain (Hb) and spinal cord (Sp). B. Marked cells visualized and analyzed at the cellular level by immunohistochemistry as shown on this 1-μm thick optical section (40x objective, z-series acquisition). Nuclear β-galactosidase (nβ-gal) antibody labeling (red) and GFP antibody labeling (green) indicates fate-mapped cells shown in the ventral midbrain. Here, recombined cells are double positive (nβ-gal+/GFP+) because of the nature of the conditional mGFP reporter. C. Whole-mount florescence microscopy reveals faint GFP fluorescence in the superior colliculi (SC) of the adult (inset). Assessing the Wnt1 lineage marked at E8.5 on adult sections with low magnification (5X) microscopy reveals that the Wnt1 lineage gives rise to midbrain structures including the superior (SC) and inferior (IC) colliculi (C). D. A 40x, 1 mm optical section taken from the ventral midbrain in the vicinity of dopaminergic neurons with nβ-gal+ cells and a rich GFP+ axonal plexus. E. Marking at E9.5 results in substantial GFP labeling that is readily observed in the inferior colliculus of the midbrain by whole mount fluorescence (inset). The Wnt1 lineage marked at E9.5 on adult sections (β-gal+, red) is concentrated in the inferior colliculus (dorsal-posterior midbrain) as seen with low magnification (5X) microscopy (E). F. A 40x, 1 mm optical section taken from the ventral midbrain in the vicinity of dopaminergic neurons with nβ-gal+ cells and a rich GFP+ axonal plexus. Wnt1-derived neurons marked at E8.5 versus E9.5 are progressively restricted from contributing to dorsal midbrain, while the Wnt1 lineage persists in contributing to ventral midbrain.
Discussion
The GIFM system that we have demonstrated can be used to answer a wide range of questions in a variety of cellular processes. For example, mice engineered with different Cre drivers can be used to target any cell type, tissue or genetic lineage of interest. In addition, because tamoxifen can be administered at any embryonic or postnatal time point, recombination can be targeted to any relevant developmental stage. The spatial and temporal control of this system are ideal for investigating how cells expressing particular genes contribute to a structure or region of interest as well as cell migration and patterning events during development.
In addition to simply marking and visualizing cells by immunohistochemistry (Figure 1), GIFM methodology can be used for a variety of applications. By combining GIFM with a conditional knock-out allele, genetic loss-of-function can be temporally and spatially targeted to relevant cell populations, which are simultaneously marked with a reporter allele. Alternatively, tissue collected from fate mapped animals can be used in combination with Fluorescence-Activated Cell Sorting (FACS) to physically isolate cells expressing reporter genes or particular markers into distinct populations. Marked tissues isolated during embryogenesis or from adult animals can be cultured, allowing the delivery of drugs or genetic constructs to defined lineages in vitro.
Our lab uses the results obtained from GIFM studies to help answer complex questions posed by neurological diseases, such as Parkinson s disease, schizophrenia, autism and tuberous sclerosis. By understanding which populations of cells are affected in each of these diseases and how these populations change over time in mouse models, we hope to better understand the pathology observed and even pose possible means of treatment in clinical settings.
Acknowledgments
We are grateful to S. Arber for the mGFP mice and to members of the Zervas lab who critically read the paper and provided technical assistance. A. Brown was supported by a Brain Science Kaplan Summer Graduate Research Award. E. Normand was supported by a Brain Science Program Graduate Research Award. This research was also funded by startup research funds (MZ).
References
- Ellisor D, Koveal D, Hagan N, Brown A, Zervas M. Comparative analysis of conditional reporter alleles in the developing embryo and embryonic nervous system. Gene Expr Patterns. 2009;9:475–489. doi: 10.1016/j.gep.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas M, Millet S, Ahn S, Joyner AL. Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron. 2004;43:345–357. doi: 10.1016/j.neuron.2004.07.010. [DOI] [PubMed] [Google Scholar]


