Abstract
The tricyclic core of the cylindricine or lepadiformine families of alkaloid natural products was assembled via a Prins addition/intramolecular Schmidt rearrangement under Lewis acid conditions. Both single-pot and two-stage variations of this process were examined, with particular attention to the stereochemical outcome of the processes. This technology has been applied to a formal total synthesis of lepadiformine A and a total synthesis of lepadiformine C.
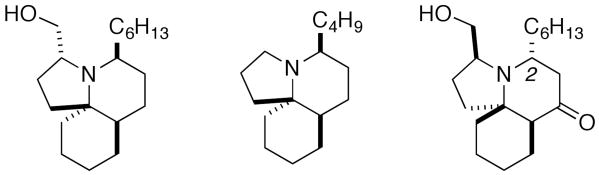
The ocean is a fertile source of natural products, many of which are valuable due to their biological properties. The genus Clavelina has proven no exception, yielding a number of alkaloids that have attracted substantial synthetic interest over the past decade. Two such families are the lepadiformines and the cylindrines (Figure 1). The cyclindricines are a large family of tricyclic compounds that were isolated by the Blackman group from Clavelina cylindrica found off of the coast of Tasmania.1 At about the same time, the structurally related lepadiformines were isolated from several different species by Biard and coworkers.2 Despite only a few reports of biological activity (e.g., lepadiformines A and B are inwardly rectifiying K+ channel blockers2,3 and some of the cyclindricines have modest cytotoxicity1) the members of both families have attracted a great deal of attention due to their intricate tricyclic structures.4 In this letter, we describe a synthetic method that allows rapid access to the tricyclic ring systems embodied by these alkaloids, a formal total synthesis of lepadiformine A,5 and the first total synthesis of lepadiformine C.3
Figure 1.
Representative Structures from the Genus Clavelina
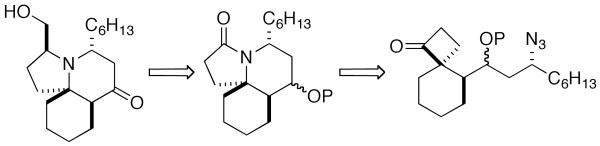
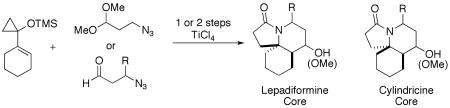
One retrosynthetic analysis of cyclindricine C would entail the late-stage installation of the hydroxymethyl substituent via a lactam intermediate, a maneuver that had been utilized by the Renaud group's total synthesis of lepadiformine A (Scheme 1).6 Going further, one could gain access to this lactam via an intramolecular Schmidt reaction on a [5.3]spirocyclic ketone such as that shown. Although the lepadiformines lack the oxygenation associated with cyclindricine C (Figure 1), incorporating an appropriately protected alcohol at this stage of the synthesis would enable, in principle, access to analogs of both classes by deoxygenation or oxidation as appropriate. However, the preparation of the necessary [5.3]spirocyclic keto azide precursor still constitutes a considerable synthetic challenge.
Scheme 1.
Retrosynthetic Analysis of Cylindricine C
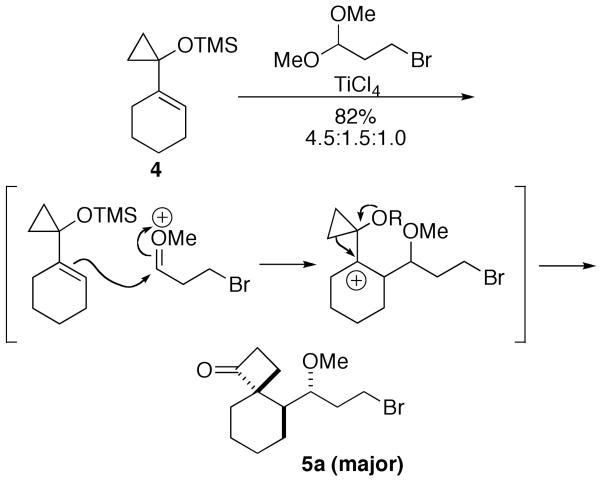
One attractive solution would be to employ the Prins-like reactivity of 1-silyloxy-1-alkylcyclopropanes introduced by Trost7 and later exploited by Cha's group8 as shown in Scheme 2. In this chemistry, cyclopropane 48,9 was treated with an acetal in the presence of Lewis acid. Conversion of the acetal to a reactive oxonium ion and attachment of 4 yields a cyclopropyl cation that undergoes ring expansion to afford a mixture of three spirocyclobutanone diastereomers, of which 5a was predominant. Not only would this approach afford a concise entry into the appropriately functionalized ring system, but the use of Lewis acid in the key step suggested that incorporation of an azide into the starting acetal (or its equivalent) might lead directly into a subsequent Schmidt-styled ring expansion of the cyclobutanone adduct to achieve the 5,6,6-tricyclic lactam.10,11
Scheme 2.
Prins Precedent8b
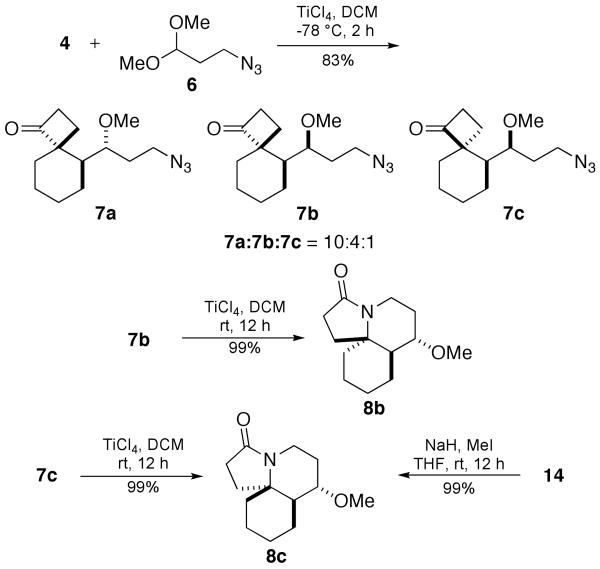
The concept was initially tested by treating 4 with azide-containing acetal 6, which yielded three spirocyclobutanone diastereomers 7a, 7b, and 7c in high combined yield (Scheme 3). The structure of the major product 7a was based on the structure of 5a as determined by Cha and via X-ray crystallography.8b The structures of the minor products 7b and 7c were determined by subsequent conversion of the ketones to the corresponding lactams via intramolecular Schmidt rearrangement. Thus, treatment of 7b with TiCl4 cleanly afforded lactam 8b in excellent yield, the stereochemistry of which was determined by NMR spectroscopy (see Supporting Information for details of stereochemical assignments). The stereochemistry of 7c was obtained by chemical correlation of 8c with alcohol 14 as prepared below. We also sought conditions by which reaction of 4 and 6 would lead directly to 8 in a single pot, but the best overall yield obtained was considerably less than that of the two-step version (35% for 4 + 6 → 8 (mixture)).
Scheme 3.
Two-Step Prins/Schmidt Reaction Sequence
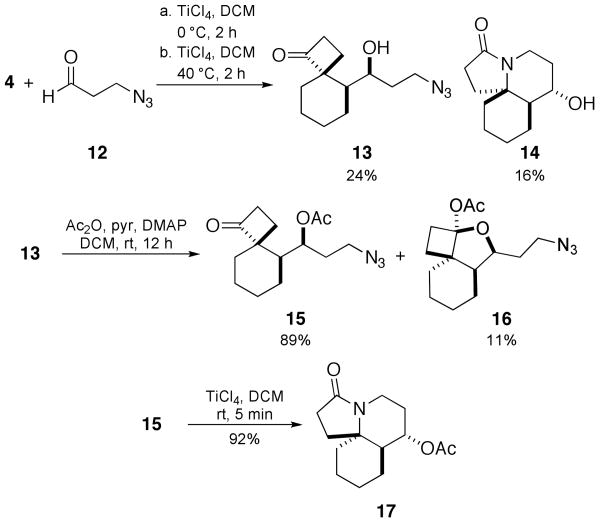
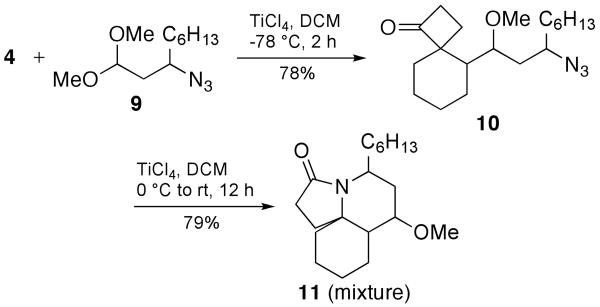
Although aldehydes were not previously reported as partners in this Prins reaction variant, we chose to examine them in the present instance, in part to avoid using a methyl ether as a protecting group.12 Thus, reacting aldehyde 12 with 4 under optimized conditions for a one-pot sequence yielded two products, cyclobutanone 13 and lactam 14, as single diastereomers (Scheme 5). Separate submission of 13 to a variety of Lewis acid conditions did not result in lactam formation. However, acetylation of the free hydroxyl group of 13 and subsequent treatment with TiCl4 did generate the desired lactam 17 in high yield over the two steps. Compound 16 was also isolated from the acetylation procedure.
Scheme 5.
Tandem Prins/Schmidt Reaction with 3-Azidopropanal
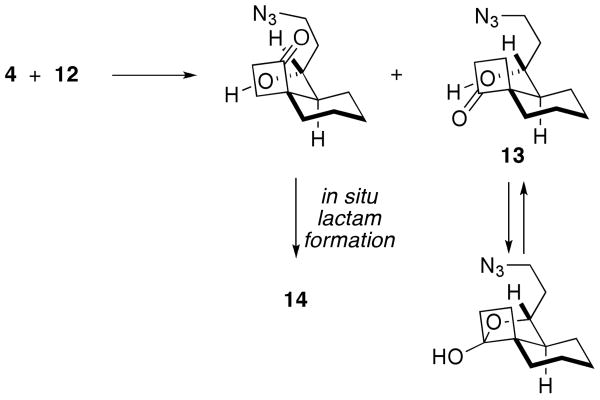
A crystal structure of 14 confirmed the relative stereochemistry shown, which maps onto that of the cylindricines (i.e., containing a cis-azadecalin ring system). The relative stereochemistry for 17 was determined by NMR and matched the core of the lepadiformines with a trans-azadecalin ring structure. It is interesting that the reaction pathways seem to diverge depending on the relative stereochemistry between the azide-containing side chain and the emerging cyclobutanone ring (Scheme 6). Thus, the intermediate in which the cyclobutanone carbonyl group is cis to the azide connector readily affords lactam 14 under these reaction conditions. In contrast, the recovery of the ketone 13 from the reaction suggests that this isomeric species is unable to undergo an intramolecular Schmidt reaction in the presence of the free alcohol group (in contrast to the successful reaction with acetylated 15). We suggest that in situ hemiketal formation – the likelihood of which is supported by the isolation of 16 from the acetylation reaction shown in Scheme 5 – as one possible explanation for this divergent behavior.
Scheme 6.
Stereochemical Effect on Reaction Products From the Tandem Reaction of 4 and 12
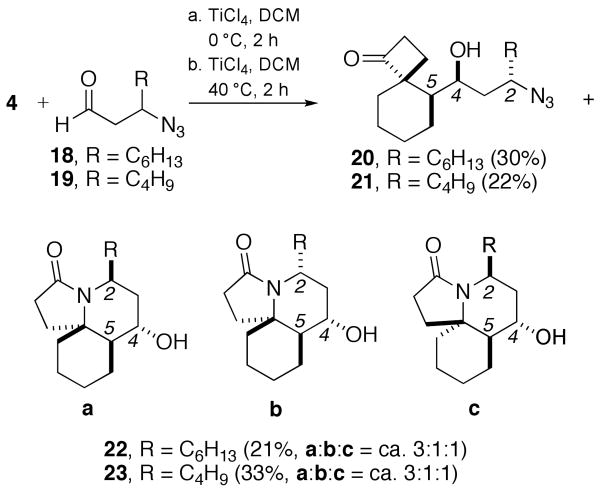
The reactions of β-substituted azidoaldehydes 18 and 19 behaved analogously to 12 (Scheme 7). Thus, these reactions afforded a single diastereomer of cyclobutanone (20 or 21) along with three diastereomers of lactams 22a–c or 23a–c, respectively (ratios estimated by 13C NMR of the crude reaction mixture). In all isomers, the relative stereochemistry of the emerging alcohol from the Prins addition is syn (C-4 and C-5, cylindricine numbering imposed1). Interestingly, unexpectedly high 1,3-syn ratios were observed between C-2 and C-4 (ca. 11:1 for (20 + 22a + 22c)/22b and 7:1 for (21 + 23a + 23c)/23b)), suggesting a reasonable level of diastereofacial selectivity in additions to aldehydes 18 and 19.
Scheme 7.
Tandem Prins/Schmidt Reaction with β-Substituted Azidoaldehydes
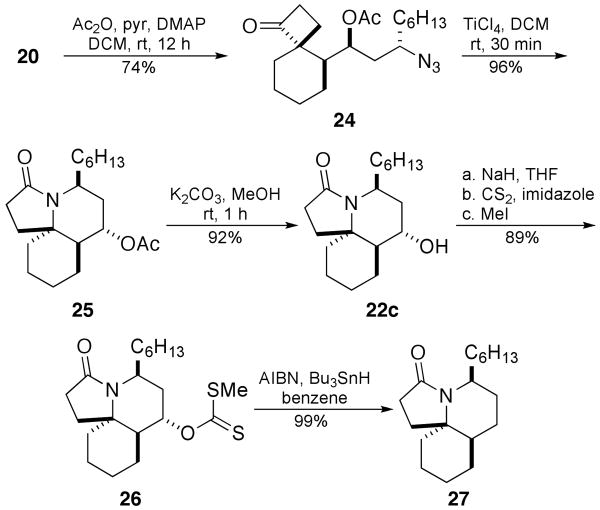
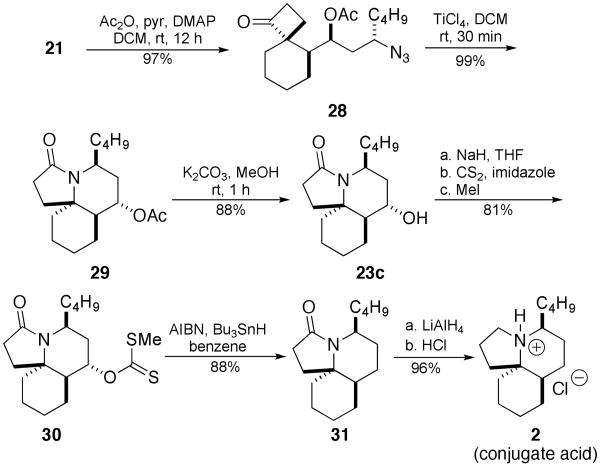
Several of the compounds so prepared were used to gain entry into the alkaloids that inspired these studies in the first place, beginning with a formal synthesis of lepadiformine A. Thus spirocyclobutanone 20 was acetylated and then treated with TiCl4 to afford lactam 25 in excellent yield (Scheme 8). Deprotection of the hydroxyl group, xanthate formation, and Barton–McCombie dehydroxylation13 furnished lactam 28, which is a common intermediate to Renaud's synthesis of lepadiformine A.6
Scheme 8.
Formal Synthesis of Lepadiformine A
Similarly, the total synthesis of lepadiormine C (2) was achieved from 21 as shown in Scheme 9. Analogous acetylation, intramolecular Schmidt reaction, and Barton–McCombie dehydroxylation followed by amide reduction and amine protonation yielded lepadiformine C, 2.
Scheme 9.
Total Synthesis of Lepadiformine C
In conclusion, we have developed a route to the tricyclic cores of the cylindricine and lepadiformine families using several variations of a tandem Prins/Schmidt sequence. Using this chemistry, we achieved a formal synthesis of lepadiformine A (corresponding to 12 steps and 8% overall yield) and the first total synthesis of lepadiformine C (9 total steps and 10% overall yield). Additional work on this and related domino reaction sequences involving the intramolecular Schmidt reaction is underway.
Supplementary Material
Scheme 4.
Reaction of 4 and a β-Substitued Azidoacetal
Acknowledgments
We thank the National Institutes of Health for financial support via GM-49093, Victor Day and Douglas Powell for X-ray crystallography at the University of Kansas, and Professor Jin K. Cha (Wayne State University) for helpful discussions.
Footnotes
Supporting Information Available. Experimental procedures, characterization data, and 1H and 13C NMR spectra for new compounds.
References
- 1.(a) Blackman AJ, Li C, Hockless DCR, Skelton BW, White AH. Tetrahedron. 1993;49:8645–8656. [Google Scholar]; (b) Li C, Blackman AJ. Aust J Chem. 1994;47:1355–1361. [Google Scholar]; (c) Li C, Blackman AJ. Aust J Chem. 1995;48:955–965. [Google Scholar]
- 2.Juge M, Grimaud N, Biard JF, Sauviat MP, Nabil M, Verbist JF, Petit JY. Toxicon. 2001;39:1231–1237. doi: 10.1016/s0041-0101(01)00079-4. [DOI] [PubMed] [Google Scholar]
- 3.Sauviat MP, Vercauteren J, Grimaud N, Juge M, Nabil M, Petit JY, Biard JF. J Nat Prod. 2006;69:558–562. doi: 10.1021/np050215s. [DOI] [PubMed] [Google Scholar]
- 4.For recent reviews on cylindricine and lepadiformine syntheses: Weinreb SM. Chem Rev. 2006;106:2531–2549. doi: 10.1021/cr050069v.Liu J, Hsung RP. Chemtracts. 2005;18:321–330.Kibayashi C. Chem Pharm Bull. 2005;53:1375–1386. doi: 10.1248/cpb.53.1375. For recent appraoches and syntheses of these molecules not covered in the reviews: Vital P, Hosseini M, Shanmugham MS, Gotfredsen CH, Harris P, Tanner D. Chem Commun. 2009:1888–1890. doi: 10.1039/b822955d.Zhang XM, Wang M, Tu YQ, Fan CA, Jiang YJ, Zhang SY, Zhang FM. Synlett. 2008:2831–2835.Flick AC, Caballero MJA, Padwa A. Org Lett. 2008;10:1871–1874. doi: 10.1021/ol8006056.Lygo B, Kirton EHM, Lumley C. Org Biomol Chem. 2008;6:3085–3090. doi: 10.1039/b805951a.Caldwell JJ, Craig D. Angew Chem, Int Ed. 2007;46:2631–2634. doi: 10.1002/anie.200604670.
- 5.Biard JF, Guyot S, Roussakis C, Verbist JF, Vercauteren J, Weber JF, Boukef K. Tetrahedron Lett. 1994;35:2691–2694. [Google Scholar]
- 6.Schar P, Renaud P. Org Lett. 2006;8:1569–1571. doi: 10.1021/ol060083+. [DOI] [PubMed] [Google Scholar]
- 7.Trost BM, Lee DC. J Am Chem Soc. 1988;110:6556–6558. [Google Scholar]; Trost BM, Chen DWC. J Am Chem Soc. 1996;118:12541–12554. [Google Scholar]
- 8.(a) Ethirajan M, Oh HS, Cha JK. Org Lett. 2007;9:2693–2696. doi: 10.1021/ol070985q. [DOI] [PubMed] [Google Scholar]; (b) Lysenko IL, Oh HS, Cha JK. J Org Chem. 2007;72:7903–7908. doi: 10.1021/jo071272o. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Oh HS, Lee HI, Cha JK. Org Lett. 2002;4:3707–3709. doi: 10.1021/ol026666a. [DOI] [PubMed] [Google Scholar]
- 9.Girard C, Amice P, Barnier JP, Conia JM. Tetrahedron Lett. 1974;37:3329–3332. [Google Scholar]
- 10.For a recent review of the intramolecular Schmidt reaction: Grecian S, Aubé J. In: Organic Azides: Syntheses and Applications. Bräse S, Banert K, editors. John Wiley and Sons; Chichester, UK: 2009. pp. 191–237. For selected examples of domino reactions involving alkyl azides and Lewis acids, see: Reddy DS, Judd WR, Aube J. Org Lett. 2003;5:3899–3902. doi: 10.1021/ol0355130.Zeng Y, Reddy DS, Hirt E, Aube J. Org Lett. 2004;6:4993–4995. doi: 10.1021/ol047809r.Golden JE, Aube J. Angew Chem Int Ed. 2002;41:4316–4318. doi: 10.1002/1521-3773(20021115)41:22<4316::AID-ANIE4316>3.0.CO;2-U.Reddy PG, Varghese B, Baskaran S. Org Lett. 2003;5:583–585. doi: 10.1021/ol027563v.Reddy PG, Baskaran S. J Org Chem. 2004;69:3093–3101. doi: 10.1021/jo035258x.Song D, Rostami A, West FG. J Am Chem Soc. 2007;129:12019–12022. doi: 10.1021/ja071041z.Frankowski KJ, Golden JE, Zeng Y, Lei Y, Aube J. J Am Chem Soc. 2008;130:6018–6024. doi: 10.1021/ja800574m.Huh CW, Somal GK, Katz CE, Pei H, Zeng Y, Douglas JT, Aubé J. J Org Chem. 2009;74:7618–7626. doi: 10.1021/jo901843w.
- 11.A related but stepwise spiroannulation/Schmidt sequence was reported previously: Aubé J, Milligan GL. J Am Chem Soc. 1991;113:8965–8966. For a mechanistically related semipinacol arrangement coupled with the intramolecular Schmidt reaction: Gu P, Zhao YM, Tu YQ, Ma Y, Zhang F. Org Lett. 2006;8:5271–5273. doi: 10.1021/ol062116r.
- 12.Initial attempts to remove the methyl group on compounds like 8a led to complex mixtures that included products arising from hydride shifts and elimination.
- 13.Barton DHR, McCombie SWJ. Chem Soc, Perkin Trans. 1975;1:1574–1585. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.