Abstract
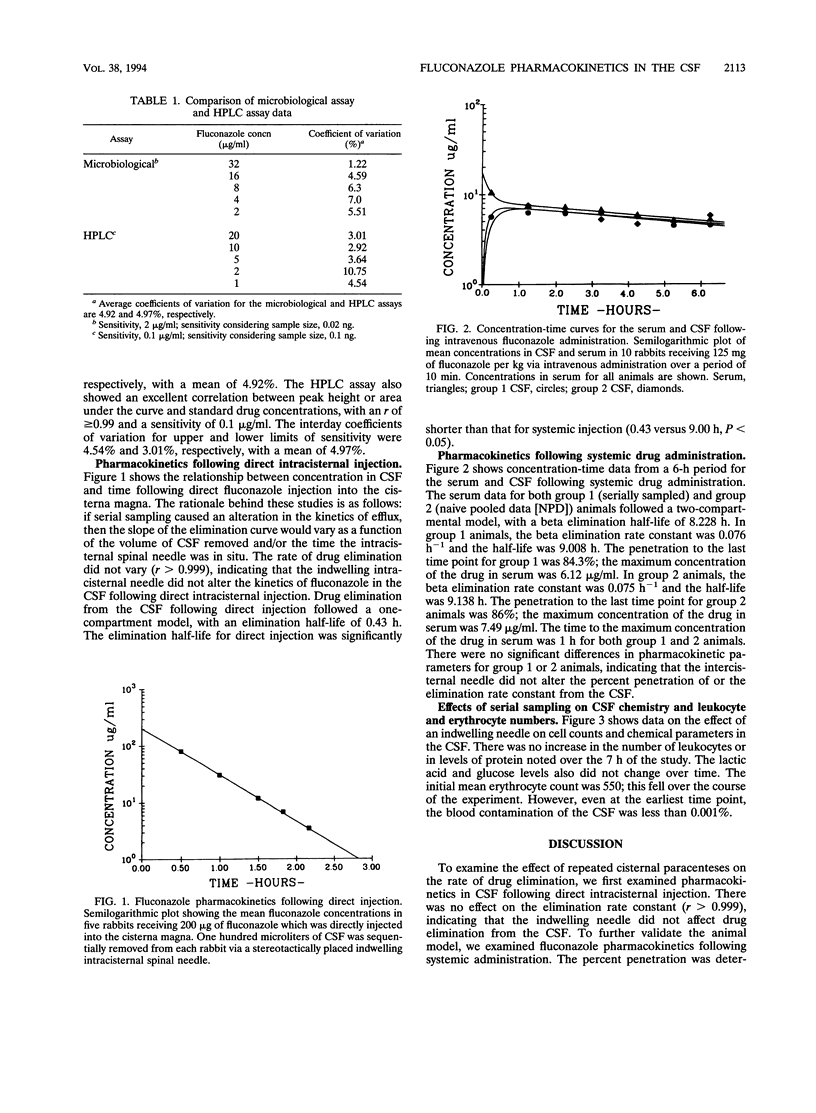
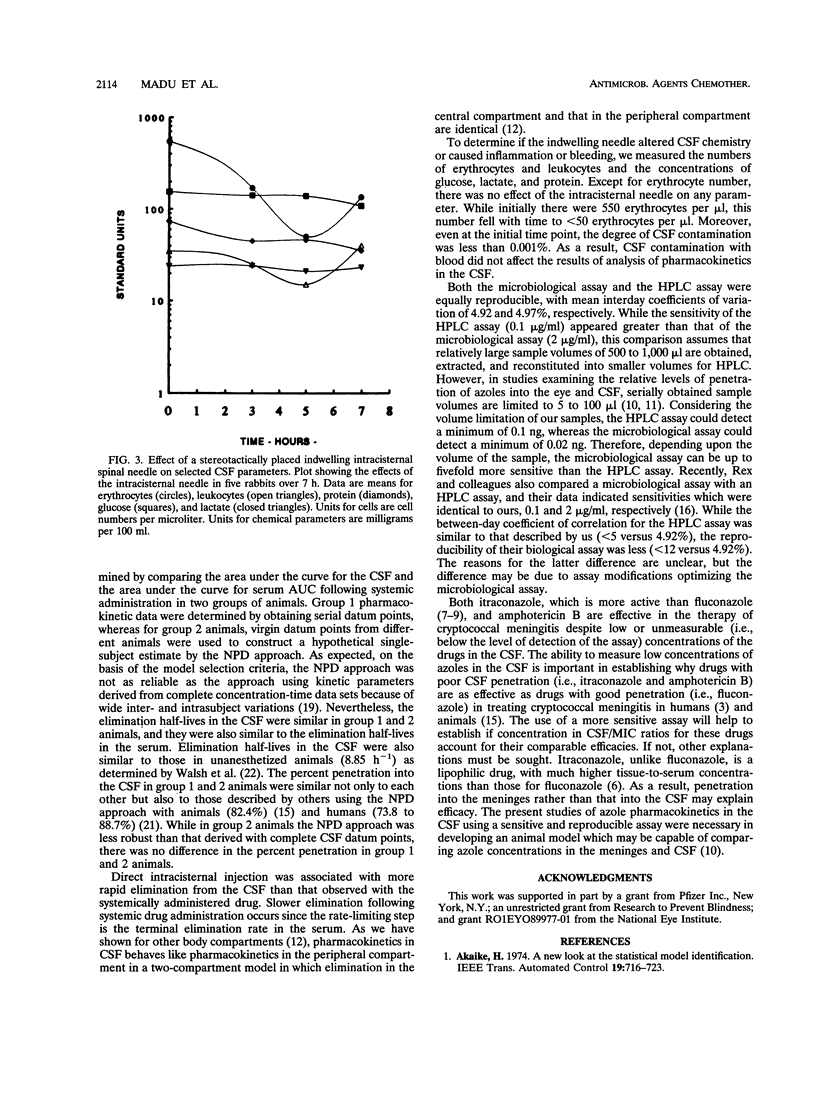
Complete concentration-time data describing the pharmacokinetics of fluconazole in the cerebrospinal fluid (CSF) following a single dose are not available for humans or animals. We studied the pharmacokinetics of fluconazole with an indwelling intracisternal needle as described by R.G. Dacey and M.A. Sande (Antimicrob. Agents Chemother. 6:437-441, 1974). To determine whether the presence of an intracisternal needle alters pharmacokinetics in the CSF, we validated this model with uninfected rabbits by measuring pharmacokinetic constants following direct intracisternal and intravenous administration of fluconazole. Following direct injection, there was no alteration of elimination rates in the CSF with increasing sample number or time. Following intravenous administration, the penetration and kinetic constants were the same in individual animals from which multiple CSF samples were obtained as in a composite subject constructed by pooling virgin samples from different animals. The presence of the intracisternal needle did not alter CSF chemistry or leukocyte counts, and erythrocyte contamination was < 0.001%. While drug concentrations were measured by a microbiological assay, we also compared the sensitivity and reproducibility of a high-performance liquid chromatography (HPLC) assay with those of the microbiological assay. Following a single intravenous dose, the maximum concentration of the drug in serum, the time to maximum concentration of the drug in serum, the terminal elimination half-life in the CSF, and the percent penetration by fluconazole were 6.12 micrograms/ml, 1 h, 9.0 h, and 84.3%, respectively. We conclude that the sampling of CSF via an indwelling needle does not alter fluconazole pharmacokinetics, cause inflammation, or alter chemical parameters; that the microbiological assay is at least equivalent in sensitivity and reproducibility to the HPLC assay; and that robust parameters describing the pharmacokinetics of fluconazole are possible with this model.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacherjee P., Hammond B. R. Inhibition of increased permeability of the blood-aqueous barrier by non-steroidal anti-inflammatory compounds as demonstrated by fluorescein angiography. Exp Eye Res. 1975 Dec;21(6):499–505. doi: 10.1016/0014-4835(75)90031-7. [DOI] [PubMed] [Google Scholar]
- Chotmongkol V., Jitpimolmard S. Itraconazole in the treatment of cryptococcal meningitis. J Med Assoc Thai. 1992 Feb;75(2):85–88. [PubMed] [Google Scholar]
- Dacey R. G., Sande M. A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974 Oct;6(4):437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds G., Brennan D. R., Wajszczuk C., Catanzaro A., Garg D. C., Knopf W., Rinaldi M., Weidler D. J. Fluconazole penetration into cerebrospinal fluid in humans. J Clin Pharmacol. 1988 Apr;28(4):363–366. doi: 10.1002/j.1552-4604.1988.tb03159.x. [DOI] [PubMed] [Google Scholar]
- Grant S. M., Clissold S. P. Itraconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs. 1989 Mar;37(3):310–344. doi: 10.2165/00003495-198937030-00003. [DOI] [PubMed] [Google Scholar]
- Graybill J. R. New antifungal agents. Eur J Clin Microbiol Infect Dis. 1989 May;8(5):402–412. doi: 10.1007/BF01964056. [DOI] [PubMed] [Google Scholar]
- Harris S. C., Wallace J. E., Foulds G., Rinaldi M. G. Assay of fluconazole by megabore capillary gas-liquid chromatography with nitrogen-selective detection. Antimicrob Agents Chemother. 1989 May;33(5):714–716. doi: 10.1128/aac.33.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Alexander G. A., Graybill J. R., Drutz D. J. Sensitive bioassay for ketoconazole in serum and cerebrospinal fluid. Antimicrob Agents Chemother. 1981 Jul;20(1):59–62. doi: 10.1128/aac.20.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. H., Madu A., Samathanam G., Rush D., Madu C. N., Mathisson K., Mayers M. Fleroxacin pharmacokinetics in aqueous and vitreous humors determined by using complete concentration-time data from individual rabbits. Antimicrob Agents Chemother. 1992 Jan;36(1):32–38. doi: 10.1128/aac.36.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y., Tanaka M. Effects of pilocarpine and paracentesis on occluding junctions between the nonpigmented ciliary epithelial cells. Exp Eye Res. 1981 May;32(5):635–647. doi: 10.1016/s0014-4835(81)80012-7. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Durack D. T. Penetration of imidazoles and triazoles into cerebrospinal fluid of rabbits. J Antimicrob Chemother. 1985 Jul;16(1):81–86. doi: 10.1093/jac/16.1.81. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Savani D. V., Durack D. T. Comparison of itraconazole and fluconazole in treatment of cryptococcal meningitis and candida pyelonephritis in rabbits. Antimicrob Agents Chemother. 1986 Apr;29(4):579–583. doi: 10.1128/aac.29.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex J. H., Hanson L. H., Amantea M. A., Stevens D. A., Bennett J. E. Standardization of a fluconazole bioassay and correlation of results with those obtained by high-pressure liquid chromatography. Antimicrob Agents Chemother. 1991 May;35(5):846–850. doi: 10.1128/aac.35.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Sande S., Cioffe C., Wolpe S., Sherry B., Cerami A., Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990 Feb 1;171(2):439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Loock C. A., Thomas M. L. Pharmacokinetics and bacteriologic efficacy of moxalactam, cefotaxime, cefoperazone, and rocephin in experimental bacterial meningitis. J Infect Dis. 1981 Feb;143(2):156–163. doi: 10.1093/infdis/143.2.156. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B., Rosenberg B., Marathe V. V. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977 Oct;5(5):445–479. doi: 10.1007/BF01061728. [DOI] [PubMed] [Google Scholar]
- Tucker R. M., Williams P. L., Arathoon E. G., Levine B. E., Hartstein A. I., Hanson L. H., Stevens D. A. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum in human coccidioidal meningitis. Antimicrob Agents Chemother. 1988 Mar;32(3):369–373. doi: 10.1128/aac.32.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. J., Foulds G., Pizzo P. A. Pharmacokinetics and tissue penetration of fluconazole in rabbits. Antimicrob Agents Chemother. 1989 Apr;33(4):467–469. doi: 10.1128/aac.33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]



