Abstract
Background
Visceral leishmaniasis has now emerged as an important opportunistic disease in patients coinfected with human immunodeficiency virus type-1 (HIV-1). Although the effectiveness of HIV-1 protease inhibitors, such as nelfinavir, in antiretroviral therapies is well documented, little is known of the impact of these drugs on Leishmania in coinfected individuals.
Methodology and Principal Findings
Here, we show that nelfinavir generates oxidative stress in the parasite, leading to altered physiological parameters such as an increase in the sub-G1 DNA content, nuclear DNA fragmentation and loss of mitochondrial potential, which are all characteristics of apoptosis. Pretreatment of axenic amastigotes with the caspase inhibitor z-VAD-fmk did not inhibit the increase in sub-G1 DNA content in nelfinavir-treated parasites, suggesting therefore that this antiviral agent does not kill Leishmania amastigotes in a caspase-dependent manner. Furthermore, we observed that the mitochondrial resident protein endonuclease G is involved. We also demonstrate that parasites overexpressing GSH1 (the rate limiting enzyme of glutathione biosynthesis) were more resistant to nelfinavir when compared to untransfected controls.
Conclusions and Significance
These data suggest that nelfinavir induces oxidative stress in Leishmania amastigotes, culminating in caspase-independent apoptosis, in which DNA is degraded by endonuclease G. This study provides a rationale for future, long-term design of new therapeutic strategies to test nelfinavir as a potential antileishmanial agent as well as for possible future use in Leishmania/HIV-1 coinfections.
Author Summary
Visceral leishmaniasis is the most severe form of disease caused by the parasite Leishmania. It is a major concern in South America, Africa, India and the Middle East. Additionally, it has now emerged as an important opportunistic disease in patients coinfected with HIV-1. This is due, in part, to the increasing overlap between urban centers and rural areas endemic for Leishmania. Although more efficient combinatorial antiviral drug regimens for treating HIV-1 infection have been developed, the impact of such therapies on HIV-1/Leishmania coinfection is yet to be explored. In this study, we investigated the effect of nelfinavir, a well-characterized anti-HIV-1 drug, on Leishmania. Treating the parasite with nelfinavir activates events that are hallmarks of programmed cell death (also called apoptosis). Among these are oxidative stress, changes in DNA replication and fragmentation, and release of mitochondrial enzymes. Furthermore, these events occur without the participation of caspases, which are classically linked to apoptosis; however, this atypical apoptosis requires the translocation of endonuclease G from mitochondria to the cytoplasm. These findings provide insights for the design of new anti-parasitic therapies, particularly in the case of Leishmania/HIV-1 coinfections.
Introduction
Leishmania is a family of zoonotic protozoan parasites that cause a variety of human diseases termed leishmaniases, which have a current annual incidence of 2 million new cases in 88 countries. Depending on the species involved, infections in humans may lead to a broad spectrum of clinical manifestations, ranging from ulcerous skin lesions to more serious and potentially fatal visceral diseases. Mobile, flagellate Leishmania promastigotes propagate in the sand fly vector, and eventually differentiate into non dividing metacyclic forms before inoculation into the vertebrate host and phagocytosis by macrophages. The metacyclics subsequently differentiate into amastigotes, which multiply in an intracellular vacuolar compartment, leading to macrophage lysis and serial infection of other surrounding macrophages. Besides being a major tropical disease, leishmaniasis, and particularly visceral leishmaniasis (VL) which is caused primarily by Leishmania infantum (L. infantum) and L. donovani, is now also emerging as an important opportunistic disease found in patients infected with human immunodeficiency virus type-1 (HIV-1) [1]. Indeed, reactivation of latent Leishmania infections have been reported to occur frequently in HIV-1-infected and immunocompromised individuals [2],[3], and, on the other hand, the parasite is able to potentiate and up-regulate virus replication, at least in vitro [4],[5].
The recent development of highly active antiretroviral therapy (HAART) has significantly improved the prognosis of patients infected with HIV-1. This therapeutic strategy consists of at least three anti-retroviral drugs, typically two nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) used in combination with a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (PI). It has been demonstrated that PIs block the active site of aspartyl protease, a viral enzyme known to be essential for the maturation of viral proteins, by mimicking peptides [6]. In patients co-infected with Leishmania and HIV-1, HAART is initiated to partially restore immune functions and it has also been found to prevent VL in individuals infected by Leishmania [7], as reflected by the sharp decrease in the incidence of VL in Europe following the widespread use of HAART [8],[9]. Recently, two HIV-1 PIs (i.e. indinavir/IDV and saquinavir/SQV) were described to exert a dose-dependent antileishmanial activity against L. infantum and L. major promastigotes in vitro [10]. Interestingly, we have recently reported the leishmaniacidal activity of PIs in an experimental cell culture system, in which we show that nelfinavir (NFV) acts as a powerful in vitro inhibitor of the intracellular growth of Leishmania in both monocytoid THP-1 cells and human primary monocyte-derived macrophages [11]. Moreover, subcytotoxic concentrations of NFV and ritonavir (RTV) significantly inhibit the growth of axenic amastigotes in vitro [11]. Although these previous studies clearly show that HIV-1 PIs can inhibit the growth of Leishmania parasites, the exact mode(s) of action of such compounds remains to be established. In mammalian cancer cell lines, NFV is known to induce apoptotic death [12]. Interestingly, it was recently demonstrated that a 24 h treatment with the PI lopinavir (LPV) causes chromatin condensation in L. amazonensis promastigotes, which is suggestive of apoptosis [13]. However, whether the PI NFV can bring about similar effects on Leishmania amastigotes remains unknown.
Previous studies have revealed that apoptosis, which is considered as a tightly regulated form of cell death, plays a dominant role in the interaction between Leishmania and its host. For example, primed macrophages were reported to kill intracellular parasites by inducing apoptosis through a caspase- and cysteine protease-independent mode [14],[15]. Leishmania can undergo apoptosis under in vitro conditions following exposure to various stimuli such as generation of reactive oxygen species (ROS), which will eventually lead to changes in mitochondrial membrane potential, endonuclease activation and DNA laddering. Together these metabolic processes will result in nuclear condensation, cell cycle arrest and surface binding of annexin V to phosphatidylserines. Furthermore, several reports have suggested that Leishmania can undergo apoptosis by caspase activation [16],[17],[18],[19]. However no caspase gene has been identified in the L. major genome sequence database [20]. Recently, several groups have suggested that a mitochondrial nuclease homologous to endonuclease G (EndoG) is involved in a caspase-independent apoptosis in Trypanosoma and Leishmania parasites [21],[22],[23]. Moreover, involvement of EndoG in nucleosomal DNA degradation was also found in sustained endogeneous oxidative stress [21],[22],[24]. It is therefore quite obvious that apoptosis of Leishmania can be induced by distinct pathways [16],[18],[19],[22], although it is unclear if any of these mechanisms are involved in the previously reported NFV-mediated antileishmanial activity [11].
In the present study, we report that NFV induces apoptosis in axenically grown Leishmania amastigotes. Moreover, we show that NFV mediates a caspase-independent apoptosis via an oxidative stress, which culminates in mitochondrial membrane depolarization in the protozoan parasite Leishmania. This biochemical event leads to a release of EndoG from the depolarized mitochondria resulting ultimately in DNA fragmentation. Together our results provide the first insights into the cell death mechanisms induced by NFV in the Leishmania parasite.
Materials and Methods
Chemicals
The HIV-1 PI NFV was obtained from the NIH AIDS Repository Reagent Program (Germantown, MD). This compound was resuspended to a concentration of 50 mM in dimethyl sulfoxide (DMSO) for a maximum final concentration in culture of 0.1% DMSO. The benzyloxycarbonyl-Val-Ala-DL-Asp(O-methyl)-fluoromethylketone (z-VAD-fmk) reagent, a caspase inhibitor with broad specificity, was purchased from Sigma-Aldrich (St. Louis, MO), was dissolved in DMSO at 20 mM concentration and stored at −20°C. Aurintricarboxylic acid ammonium salt (ATA, 20 mM, stock solution, Sigma-Aldrich) and N-Acetyl cysteine (NAC, 0.5 M, stock solution, Sigma-Aldrich) were dissolved in water and stored at −20°C. Propidium iodide (Sigma-Aldrich) was also dissolved in water at a final concentration of 1 mg/ml and stored at 4°C. Miltefosine was from Cayman Chemicals (Ann Arbor, MI) and stored at −20°C.
Parasite culture and maintenance
Axenically grown amastigotes of L. donovani field strain 9518 (Ld 9518) were maintained at 37°C with 5% CO2 by weekly subpassages in MAA/20 medium pH (5.6) in 25-cm2 flasks. MAA/20 consists of modified medium 199 (Gibco BRL, Carlsbad, CA) with Hank's salts, supplemented with 0.5% soybean trypto-casein (Pasteur Diagnostics, Marne la Coquette, France), 15 mM D-glucose, 5 mM L-glutamine, 4 mM NaHCO3, 0.023 mM bovine hemin and 25 mM HEPES at a final pH of 6.5 and supplemented with 20% of fetal bovine serum (FBS). These axenic amastigotes show morphological, biochemical and biological characteristics similar to those of amastigotes isolated in vivo. L. infantum (MHOM/MA/67/ITMAP-263) promastigotes transfected with pSPαzeoα and pSPαzeoαGSH1, hereafter called Li-pSP and Li-GSH1, were maintained at pH 7.0 and at a temperature of 25°C and cultured in RPMI-1640 medium (Wisent, St.-Bruno, Quebec) supplemented with 10% FBS, 5 µg/mL hemin, 25 µM HEPES, 2 mM NaHCO3 and 800 µg/ml of the selective agent Zeocin (Invivogen, San Diego, CA) in 25-cm2 flasks. Li-pSP and Li-GSH1 axenic amastigotes were obtained after several days of culture by seeding promastigotes in 25-cm2 flasks containing MAA/20 medium at a final pH of 5.6 as described above except for the addition of 800 µg/ml Zeocin.
Selection of NFV-resistant amastigotes
Axenically grown amastigotes of L. donovani Ld 9518 were subjected to stepwise increasing drug concentrations until resistance to 6.25 and 12.5 µM of NFV was established. A stepwise increase in drug concentration was undertaken only when the drug-exposed cultures showed a growth rate equivalent to that of unexposed cultures. Parasites displaying a resistance phenotype were maintained under a constant drug pressure (i.e. 6.25 and 12.5 µM) throughout our entire experimental procedures. Resistant clones were also stored in −150°C at various times during their establishment.
DNA fragmentation assays
Log-phase axenic amastigotes were seeded at a final concentration of 5×106 parasites/ml and drugs were added to the culture media at the listed concentrations. Total DNA (i.e. 10 µg) was extracted at different intervals by the salting out method [25]. Parasites were then pelleted and resuspended in 300 µl of lysis buffer (i.e. 10 mM Tris-HCl pH 8, 5 mM EDTA, 0.5% SDS, 200 mM NaCl and 100 µg/ml proteinase K) for 45 min at 65°C. Two volumes of ice-cold ethanol (100%) were added and DNA was collected by centrifuging at 13,000 rpm for 15 min in a microfuge. Supernatants were discarded and dried pellets of DNA were resuspended in 50 µl of 10 mM Tris-HCl pH 8 and 0.1 mM EDTA, and treated with RNase A (0.3 µg/ml) for 1 h at 37°C. Qualitative analysis of nuclear DNA fragmentation was performed by 1% agarose gel electrophoresis. In some studies, untreated or NFV-treated axenic amastigotes were washed in PBS. Pelleted cells were permealized in hypotonic lysis buffer (0.1% sodium citrate and 0.1% Triton X-100) and incubated overnight at 4°C containing 50 µg/ml propidium iodide and 100 µg/ml RNase. The fluorescence intensity of propidium iodide was analyzed with a Coulter EPICS XL flow cytometer (Beckman, Miami, FL) and FCS express software (version 3).
Measurement of Δψm
Change in Δψm was measured by Cayman's JC-1 mitochondrial membrane potential assay kit (Cayman Chemical Company, Ann Arbor, MI). This dye accumulates in the mitochondrial matrix under the influence of Δψm and its monomeric form increases in unhealthy or apoptotic cells [26]. Ld 9518 amastigotes were cultured in 24 well plates at a density of 5×105 cells/well in 400 µl of MAA/20 medium in a CO2 incubator overnight at 37°C. Parasites were then treated with 40 µM NFV for different time periods afterwhich 40 µl of JC-1 staining solution (prepared according to the manufacturer's instructions) was added to each well. The cells were then incubated at 37°C in a 5% CO2 incubator for 30 min, and transferred to 96-well black culture plates. Finally, a spectrofluorometer using 485 nm and 535 nm as excitation and emission wavelengths, respectively, was used to measure Δψm.
Cell fractionation and immunoblotting
Ld 9518 axenic amastigotes (5×106 cells/ml) were treated with 40 µM of NFV for different time periods before monitoring the cytoplasmic translocation of EndoG. In some studies, parasites were first treated with NAC (20 mM) prior to exposure to NFV. Cell fractionation was carried out using the ApoAlert Cell fractionation kit (Clontech, Mountain View, CA). After the separation of cytosolic and mitochondrial fractions, 5 µg of protein from each fraction was electrophoresed in different sets of 12% SDS-PAGE and transferred to nitrocellulose membranes. Thereafter, membranes were blocked using 5% skimmed milk and immunoblotted with rabbit polyclonal anti-human EndoG (Santa Cruz Biotech, Santa Cruz, CA), followed by anti-rabbit HRP-conjugated antibodies. For loading controls corresponding to mitochondrial and cytosolic fractions, the same blots were reprobed with anti-COX IV antibodies provided with the ApoAlert Cell fractionation kit and anti-human DHFR antibodies (Santa Cruz Biotech) followed by the appropriate HRP-conjugated secondary antibodies. The anti-EndoG, anti-COX IV and anti-DHFR antibodies show cross-reactivity with the corresponding Leishmania proteins.
Statistical analysis
The statistical significance of the results was defined by performing a 1-way analysis of variance with Bonferroni's multiple comparison tests and unpaired t test. All statistical analyses were performed using Prism software 3.03. P values of less than 0.05 were considered statistically significant.
Results
NFV induces an apoptosis-like death in Leishmania
Our initial studies were performed with the 9551 field strain of L. donovani. However, we were unable to obtain axenic amastigotes from this viscerotropic strain of Leishmania. Therefore, we used the sodium stibogluconate (SbV)-resistant L. donovani field strain 9518 (Ld 9518) in the majority of experiments described in the present work, of which we regularly could obtain axenic amastigotes in our laboratory. It should be noted that this L. donovani field isolate was previously shown to be sensitive to NFV in promastigote-infected human primary monocyte-derived macrophages [11].
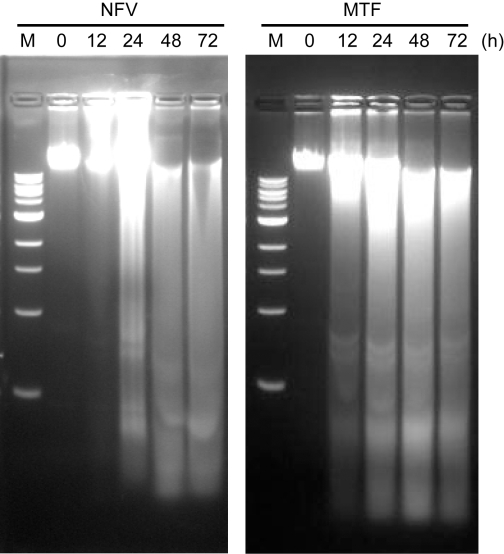
In order to investigate the mechanism(s) by which NFV affects Leishmania growth, we analysed the integrity of nuclear DNA of untreated and NFV-treated Ld 9518 amastigotes by agarose gel electrophoresis. A combination of ladder and degradation patterns of nuclear DNA fragments was observed beginning at 24 h of NFV treatment, although more pronounced degradation of DNA was achieved after longer treatment (Fig. 1). Miltefosine (MTF) was used as a positive control in this study, having been already shown to induce apoptosis in Leishmania [27],[28],[29]. Therefore, as expected, a comparable degradation of nuclear DNA fragments was also observed after a 12 h treatment with MTF, culminating to complete degradation after 24 h of treatment. DNA of untreated parasites, or that of parasites treated with the highest concentration of diluent (i.e. 0.05% DMSO), showed no fragmentation (data not shown).
Figure 1. NFV induces degradation of genomic DNA isolated from L. donovani amastigotes.
Genomic DNA was isolated from Ld 9518 amastigotes which have been treated with NFV or MTF (25 µM in both instances) for the indicated time periods and was resolved on 1% agarose gel. M corresponds to 1 Kb DNA ladder.
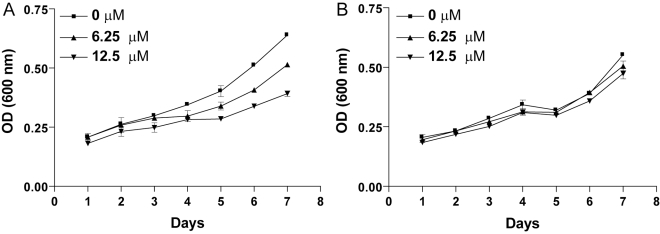
To investigate more deeply the effect of NFV on Leishmania, we generated NFV-resistant mutants by increasing the drug concentration in several steps, as previously described [30]. Parasites were continuously maintained under drug pressure and optical density was measured at every passage. After 7 days of culture, we observed a 21% and 33% decrease in parasite growth when using NFV at 6.25 and 12.5 µM, respectively (P<0.001) (Fig. 2A). After 8 consecutive passages, the growth rates of parasites treated with the two tested concentrations of NFV were similar to the untreated parasites (Fig. 2B), thus suggesting that we are able to derive parasites displaying a resistant phenotype.
Figure 2. Development of in vitro NFV resistance by direct drug pressure on axenic amastigotes.
(A) Growth curve was determined at the initial seeding. (B) Growth curve was determined after 8 passages. The growth of parasites was followed by measuring the optical density (OD) at 600 nm at the listed days.
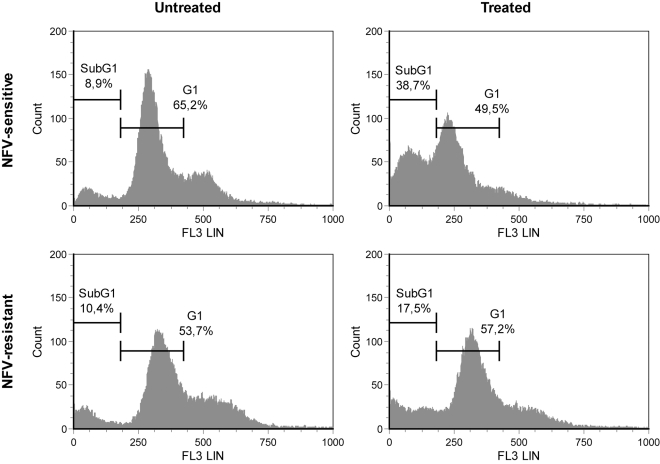
Next, to further confirm and evaluate the NFV-mediated induction of apoptosis, we performed an in vitro apoptosis detection assay by staining the sub-G1 DNA content of both NFV-sensitive (i.e. wild-type/WT) and -resistant Ld 9518 amastigotes (i.e. 12.5 µM) with propidium iodide. Next, the percentages of parasites containing sub-G1 DNA (below the G0/G1 peak) were measured by flow cytometry. After a 24 h treatment with NFV, the percentage of drug-sensitive amastigotes in the sub-G1 phase (i.e. M1 region) obtained was 38.7%, compared to 49.5% in the G1 fraction (i.e. M2 region) (Fig. 3). As expected, a low sub-G1 fraction is detected in untreated parasites (i.e. 8.9%), therefore confirming that NFV induces DNA fragmentation in axenic amastigotes after 24 h of treatment. Importantly, the percentage of parasites with a sub-G1 content are also less important in both untreated (i.e. 10.4%) and drug-treated NFV-resistant axenic amastigotes (i.e. 17.5%). Again, these observations are further suggesting that NFV causes an apoptosis-like death process in Leishmania.
Figure 3. NFV induces an increase in the sub-G1 DNA-containing population.
The DNA content degradation profiles of drug-sensitive and NFV-resistant axenic amastigotes (i.e. usually maintained under a constant drug pressure of 12.5 µM), which were either left untreated or treated with NFV (10 µM), were assessed by flow cytometry after cell permeabilisation and PI staining. DNA fragmentation was quantified by measuring the cell population in the sub-G1 DNA region indicated by M1 and G1 DNA peak indicated by M2. The data shown are representative of three independent experiments.
NFV induces a caspase-independent death process and oxidative stress in Leishmania
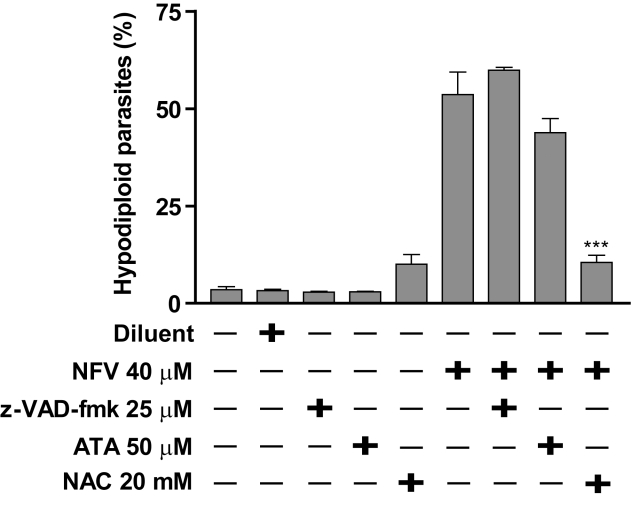
As apoptosis involves endonuclease-mediated DNA fragmentation and caspases activate the endonuclease responsible for cleaving cellular DNA, a pan-caspase inhibitor (i.e. z-VAD-fmk) was used to investigate if these two pathways are involved in NFV-induced apoptosis in Leishmania. Pretreatment of the parasites with z-VAD-fmk (25 µM) did not bring any decrease in the percentage of sub-G1 DNA content in amastigotes with respect to NFV treatment, as the percentage of sub-G1 DNA in NFV-treated parasites, either pre-treated with z-VAD-fmk (57%) or not (54%), were similar (P>0.05) (Fig. 4). Similar observations were obtained when using higher concentrations of z-VAD-fmk (i.e. up to 100 µM) (data not shown). Therefore, NFV induces apoptosis in Leishmania in a caspase-independent manner.
Figure 4. NFV generates an oxidative stress-mediated caspase-independent death process.
Drug-sensitive axenic amastigotes were either left untreated or treated for 2 h with the indicated compounds, i.e. caspase inhibitor z-VAD-fmk (25 µM), Endo-G inhibitor ATA (50 µM) and antioxidant NAC (20 mM). Next, parasites were subjected to a treatment for 24 h with NFV (40 µM) or the diluent (i.e. DMSO). Percentage of apoptotic cells was measured by staining with propidium iodide as described in Materials and Methods. Data are expressed as mean ± SD of three independent experiments.
We next examined the role of endonucleases in NFV-mediated DNA degradation. In this regard, parasites were pretreated with the endonuclease inhibitor aurin tricarboxylic acid (ATA), prior to NFV. Treatment of these parasites with both ATA and NFV exhibited an 18% reduction in sub-G1 DNA content in comparison to amastigotes only treated with NFV (Fig. 4). However, statistical analysis showed that this reduction was not significant (P>0.05).
Finally, we determined whether NFV induces oxidative stress in Leishmania. To this end, parasites were treated with the antioxidant N-acetyl-cysteine (NAC) prior to NFV. Our results show that NAC pre-treatment caused a 80% reduction in the sub-G1 DNA induced by NFV (Fig. 4), a statistically significant decrease when compared to the amount of sub-G1 DNA in NFV-treated parasites (P<0.001). Pre-treatment with NAC would therefore inhibit the production of ROS potentially generated by NFV, protecting cells from oxidative stress.
NFV leads to a loss of mitochondrial potential and translocation of EndoG
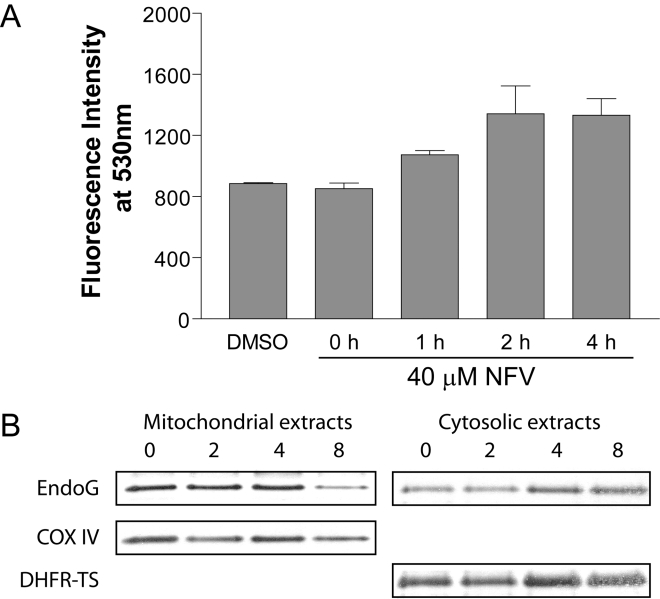
A characteristic feature of apoptosis is the loss of mitochondrial potential (ψm), which induces ROS formation and oxidative stress inside the parasite [22]. Using a dye (JC-1) sensitive to the loss in Δψm, we monitored the mitochondrial membrane potential by spectrofluorometry. As shown in Fig. 5A, fluorescence increased with time upon NFV treatment. We observed a plateau in fluorescence intensity, corresponding to a complete loss of Δψm, after 2 h following initial contact with NFV.
Figure 5. Loss of mitochondrial potential and translocation of EndoG in NFV-treated L. donovani amastigotes.
(A) L. donovani axenic amastigotes were exposed to the diluent or NFV (40 µM) for the indicated time periods. Mitochondrial membrane potential (Δψm) was assessed through the use of the JC-1 Mitochondrial Membrane Potential Detection Kit. Data are expressed as mean ± SD of three independent experiments. (B) Western blot analysis of the mitochondrial and cytosolic fractions obtained from NFV-treated amastigotes at different intervals. Anti-EndoG immunoblots of cytosolic and mitochondrial fractions are shown, along with the loading controls for mitochondria (i.e. COX IV) and cytosol (i.e. DHFR-TS), respectively.
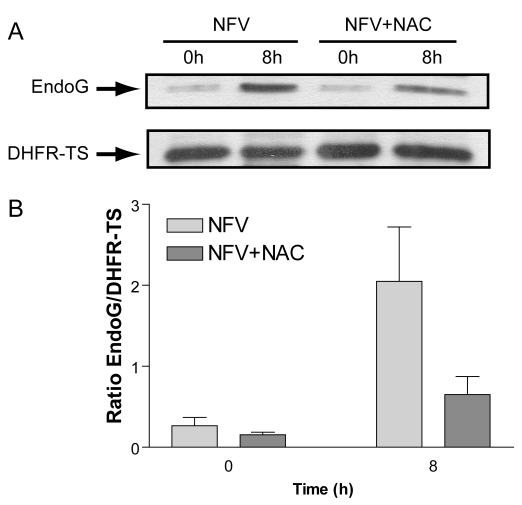
EndoG is involved in caspase-independent apoptosis and is a mitochondrial resident protein which cleaves the nuclear DNA [21],[31],[32], after its release from depolarized mitochondria through transition pores. Since our previous results suggested that NFV induces a loss of mitochondrial potential, we set out to determine if NFV also caused mitochondrial release of EndoG. Mitochondrial and cytosolic extracts prepared from both untreated and NFV-treated parasites were separated on SDS-PAGE, transferred to membranes and immunoblotted with anti-EndoG antibodies. The gradual translocation of EndoG to the cytoplasm was observed with time after NFV treatment (Fig. 5B). No variation in band intensities corresponding to COX IV (for the mitochondrial extracts) and DHFR-TS (for the cytosolic extracts) were observed, and were used as loading controls. Furthermore, this cytoplasmic translocation of EndoG was inhibited by the addition of NAC (Fig. 6).
Figure 6. Cytosolic translocation of EndoG in NFV-treated L. donovani amastigotes is inhibited by addition of antioxidant.
(A) L. donovani axenic amastigotes were exposed to NFV (40 µM) for 0 or 8 h with or without pre-treatment with NAC (20 mM). Western blot analysis of the cytosolic fractions obtained from the amastigotes was then performed using anti-EndoG (upper panel) and DHFR-TS (lower panel, as loading control). Densitometric analysis of blots from three of these experiments are shown in (B).
Expression of GSH1 protects amastigotes against NFV-induced cell death
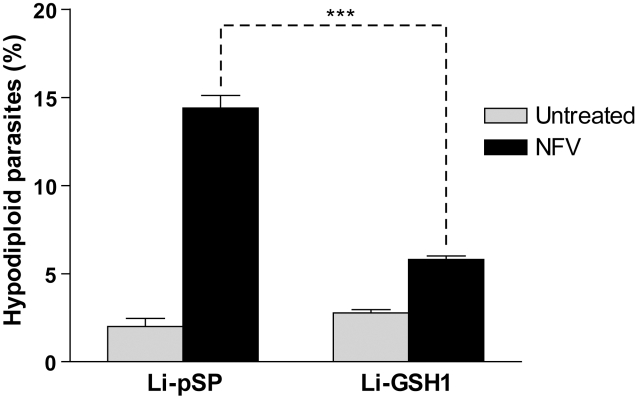
Glutathione (GSH) has been shown to protect cells from oxidative stress, as well as from the effect of several drugs. In order to further our understanding of the possible effect of NFV-induced ROS, we tested L. infantum axenic amastigotes transfected with the empty control vector pSPαzeoα (called Li-pSP) or a pSPαzeoα vector containing the gsh1 gene (called Li-GSH1). The gsh1 gene codes for the heavy subunit of γ-glutamylcysteine synthase (γ-GCS), the rate limiting enzyme of GSH biosynthesis in Leishmania [33],[34]. When Li-GSH1 parasites were subjected to a 12 h treatment with NFV, we observed a significant decrease (57%) in sub-G1 DNA content, when compared to NFV-treated control Li-pSP parasites (Fig. 7). This confirmed our previous observations that parasites generate ROS after exposure to NFV, and overexpression of GSH1 inhibits the effects of ROS in these cells.
Figure 7. GSH1 overexpression suppresses NFV-induced cell death in parasites.
Stably transfected L. infantum strains carrying either the empty control vector Pspαzeoα (i.e. Li-pSP) or the GSH1-encoding vector PspαzeoαGSH1 were exposed to NFV (40 µM) for 24 h. Cell death was measured in terms of DNA degradation. Light gray bars refer to untreated parasites, whereas black bars relate to NFV-treated parasites (n = 3) (***, P<0.001).
Discussion
In recent years, the number of worldwide reported cases of Leishmania/HIV-1 coinfections has been increasing steadily given the growing spread of the AIDS pandemic to smaller urban centers and more outlying urban and rural areas where Leishmania infections are more prevalent [1],[35]. Besides this expanding geographical overlap, these two diseases are changing their clinical, epidemiological and therapeutic aspects as they both impact on each other's development. For instance, in Africa and India, where L. donovani/HIV-1 coinfections are emerging, there are high rates of VL relapse and mortality [1],[36],[37]. Furthermore, the use of contaminated syringes by intravenous drug users has now also been identified as a way of transmission of Leishmania, as well as for HIV-1, particularly in Southern Europe and the Mediterranean basin [38]. In addition to these factors, Leishmania and HIV-1 act in a concerted manner in the coinfected individual, both benefiting from the other's presence. Indeed, Leishmania accelerates the progression toward AIDS, whereas HIV-1 can reinitiate or worsen Leishmania-related symptoms [2],[4],[5].
Improper diagnosis and the misuse of leishmaniacidal compounds have also complicated the treatment of Leishmania/HIV-1 coinfected individuals, and have contributed to the rise in the number of coinfected cases. For example, our group has previously reported that SbV, a widely used drug in the treatment against leishmaniasis, can accelerate HIV-1 replication under in vitro conditions [39]. These findings, combined with the fact that little is known on the effect of drugs designed specifically against HIV-1 or Leishmania in coinfected individuals, warrant further investigation into the impact of these compounds and their action on the opposite microbe.
In a recent paper, our group reported that drugs designed against the protease of HIV-1 markedly reduced the intracellular survival of L. infantum and L. donovani in both the macrophage cell line THP-1 and more physiologically relevant human monocyte-derived macrophages [11]. We also observed that, although we did not find any toxic effect of these drugs on promastigotes, the HIV-1 PIs NFV and RTV were effective against Leishmania axenic amastigotes at subcytotoxic levels. Given the long term potential of such findings for the treatment of Leishmania/HIV-1 coinfections, we further investigated by which mechanism HIV-1 PIs, specifically NFV, exert their effect on Leishmania amastigotes in culture. Besides being in the developmental stage of the parasite which is most affected by these drugs, it was also relevant to pursue such a study since amastigotes are the form of Leishmania usually found in humans. However, it should be pointed out that although the NFV concentrations used in this study were much stronger than those found in plasma of treated patients [40], it could be argued that, due to the accumulation of drug in macrophages [41], these higher concentrations may be relevant when used directly in media cultured parasites in order to compensate for this effect.
We first observed that nuclear DNA from NFV-treated axenic L. donovani amastigotes formed a long smear when resolved using agarose gel electrophoresis. Although not a classical ladder pattern associated with apoptosis, this degraded form of DNA is not uncommon in apoptotic L. donovani, as has been previously reported [18]. Similar patterns of DNA degradation have been observed in Leishmania parasites treated, among others, with MTF, a relatively recently developed antileishmanial drug which we have used in control experiments. Indeed, several other better established drugs are known to induce this effect on Leishmania, including pentavalant antimony, pentamidine, and amphotericin B [42]. However, our observations are of potential long term interest in the case of HIV-1/Leishmania coinfections, since NFV acts on both HIV-1 and the amastigote parasites in vitro.
The mechanisms involved in Leishmania apoptosis are not as well understood as in mammalian cells, but have been the object of several investigations in the recent past years [18],[21],[22],[43]. Indeed, some similarities can be made between both organisms. First, in both cases, mitochondria have been identified as major regulators of the apoptotic process, and permeabilization of the mitochondrial membrane by oxidative stress or other stresses leads to the release of proapoptotic enzymes in the cytosol. Relocation of cytochrome C and activation of cellular proteases act as irreversible commitments to the apoptotic death pathway. However, given that the equivalents of mammalian caspases have yet to be identified in Leishmania, at least part of the apoptotic machinery used by the parasite is caspase-independent. In this regard, we observed no effect of the caspase inhibitor z-VAD-fmk in NFV-induced apoptosis in Leishmania, suggesting that the mechanism(s) involved in this case is caspase-independent. Interestingly, Verma and colleagues reported that MTF activates caspase 3-like proteases in arsenite-resistant L. donovani promastigotes, and that their action was sensitive to the CED3/CPP32-specific inhibitor DEVD-fmk [44]. The recent identification of metacaspases in Trypanosoma brucei and L. major may further shed light on the specific pathways involved in Leishmania apoptotic cell death in response to drug treatments [43]. However, although metacaspases and caspases may be structurally similar, the catalytic activity of metacaspases in Leishmania is quite different from that of caspases, suggesting that different apoptotic pathways are involved.
Recently, several papers have proposed a role for EndoG, a mitochondrial protease, in chromatin degradation of trypanosomatids such as Leishmania and Trypanosoma. Gannavaram and co-workers showed that, when transfected with L. major EndoG, over-expressing L. donovani promastigotes exhibited increased sensitivity to oxidative stresses and engaged in translocation of EndoG to the cytosol [21]. Furthermore, Rico and colleagues demonstrated that L. infantum EndoG reacted similarly when these parasites are faced with apoptotic stimuli [23]. Finally, BoseDasgupta et al. further investigated the mechanisms of apoptosis in L. donovani promastigotes induced by the flavone baicalein, a topoisomerase-DNA complex stabilizer [22]. The authors found that, once released, EndoG formed a complex with flap endonuclease-1 (FEN-1), an enzyme involved in DNA repair, and, independently, with a Tat-D-like nuclease, in order to degrade DNA and possibly promote apoptosis. It is therefore possible that this is also the case in NFV-treated amastigotes, given that we also observed ATA-sensitive apoptosis and EndoG translocation to the cytosol. The same authors have also reported that the EndoG release from mitochondria is calcium-dependent and sensitive to the antioxidant NAC or the calcium chelator BAPTA-AM [22]. In this regard, we found that L. donovani axenic amastigotes responded in a similar, NAC-sensitive manner to NFV. Our finding that γ-GCS and GSH overexpression in L. infantum amastigotes, protecting the parasites from oxidative stress, counteracts the pro-apoptotic effects of NFV also suggests that EndoG is released in response to oxidative stress. It is therefore possible that very similar apoptotic pathways are activated in Leishmania promastigotes treated with baicalein, and amastigotes treated with NFV. However, additional experiments are warranted to clearly determine if such is the case.
With the expanding overlap between HIV-1 infection and leishmaniases in the world, it is now critical to better understand the multifaceted effects of antiviral and antiparasitic drugs in the case of HIV-1/Leishmania coinfected individuals. In this report, we determined how L. donovani amastigotes react to NFV, an HIV-1 PI, and that these processes lead to apoptotic death through a caspase-independent mechanism. Another group has recently described that HIV-1 PIs also affect L. amazonensis proliferation and infection of murine macrophages, and observed chromatin condensation in parasites suggesting apoptosis [13]. Further investigation will determine if such is a general reaction of trypanoplasmids to HIV-1 PIs, hopefully leading to a better understanding of these agents and possibly future therapeutic use in coinfected individuals. However, one must note that HIV-1 PIs may also exert toxicity in humans by inducing a mitochondrial loss of potential and apoptosis (for example, of T cells) [45],[46]. Therefore, careful evaluation of the benefits and pitfalls of such therapies must be evaluated when designing future chemotherapeutic strategies to test NFV as a potential possible antileishmanial agent as well as possibly in Leishmania/HIV-1 coinfections.
Footnotes
The authors have declared that no competing interests exist.
This work was financially supported by an operating grant to Michel J. Tremblay from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program (MOP-84555), a CIHR Group Grant to Michel J. Tremblay and Marc Ouellette (MGC-14500), and a strategic new initiative team grant to Michel J. Tremblay and Marc Ouellette from the FQRNT Centre for Host-Parasite Interactions (RS-87902). Michel J. Tremblay is the recipient of the senior Canada Research Chair in Human Immuno-Retrovirology and Marc Ouellette holds the senior Canada Research chair in Antimicrobial resistance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dedet JP, Pratlong F. Leishmania, Trypanosoma and monoxenous trypanosomatids as emerging opportunistic agents. J Eukaryot Microbiol. 2000;47:37–39. doi: 10.1111/j.1550-7408.2000.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 3.Cruz I, Nieto J, Moreno J, Canavate C, Desjeux P, et al. Leishmania/HIV co-infections in the second decade. Indian J Med Res. 2006;123:357–388. [PubMed] [Google Scholar]
- 4.Zhao C, Papadopoulou B, Tremblay MJ. Leishmania infantum promotes replication of HIV type 1 in human lymphoid tissue cultured ex vivo by inducing secretion of the proinflammatory cytokines TNF-alpha and IL-1 alpha. J Immunol. 2004;172:3086–3093. doi: 10.4049/jimmunol.172.5.3086. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Thibault S, Messier N, Ouellette M, Papadopoulou B, et al. In primary human monocyte-derived macrophages exposed to Human immunodeficiency virus type 1, does the increased intracellular growth of Leishmania infantum rely on its enhanced uptake? J Gen Virol. 2006;87:1295–1302. doi: 10.1099/vir.0.81647-0. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 7.de la Rosa R, Pineda JA, Delgado J, Macias J, Morillas F, et al. Influence of highly active antiretroviral therapy on the outcome of subclinical visceral leishmaniasis in human immunodeficiency virus-infected patients. Clin Infect Dis. 2001;32:633–635. doi: 10.1086/318708. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Velez R. The impact of highly active antiretroviral therapy (HAART) on visceral leishmaniasis in Spanish patients who are co-infected with HIV. Ann Trop Med Parasitol. 2003;97(Suppl 1):143–147. doi: 10.1179/000349803225002615. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal E, Tempesta S, Del Giudice P, Marty P, Desjeux P, et al. Declining incidence of visceral leishmaniasis in HIV-infected individuals in the era of highly active antiretroviral therapy. AIDS. 2001;15:1184–1185. doi: 10.1097/00002030-200106150-00017. [DOI] [PubMed] [Google Scholar]
- 10.Savoia D, Allice T, Tovo PA. Antileishmanial activity of HIV protease inhibitors. Int J Antimicrob Agents. 2005;26:92–94. doi: 10.1016/j.ijantimicag.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Trudel N, Garg R, Messier N, Sundar S, Ouellette M, et al. Intracellular survival of Leishmania species that cause visceral leishmaniasis is significantly reduced by HIV-1 protease inhibitors. J Infect Dis. 2008;198:1292–1299. doi: 10.1086/592280. [DOI] [PubMed] [Google Scholar]
- 12.Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13:5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 13.Santos LO, Marinho FA, Altoe EF, Vitorio BS, Alves CR, et al. HIV aspartyl peptidase inhibitors interfere with cellular proliferation, ultrastructure and macrophage infection of Leishmania amazonensis. PLoS ONE. 2009;4:e4918. doi: 10.1371/journal.pone.0004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzmuller P, Sereno D, Cavaleyra M, Mangot I, Daulouede S, et al. Nitric oxide-mediated proteasome-dependent oligonucleosomal DNA fragmentation in Leishmania amazonensis amastigotes. Infect Immun. 2002;70:3727–3735. doi: 10.1128/IAI.70.7.3727-3735.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zangger H, Mottram JC, Fasel N. Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ. 2002;9:1126–1139. doi: 10.1038/sj.cdd.4401071. [DOI] [PubMed] [Google Scholar]
- 16.Lee N, Bertholet S, Debrabant A, Muller J, Duncan R, et al. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 2002;9:53–64. doi: 10.1038/sj.cdd.4400952. [DOI] [PubMed] [Google Scholar]
- 17.Arnoult D, Akarid K, Grodet A, Petit PX, Estaquier J, et al. On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ. 2002;9:65–81. doi: 10.1038/sj.cdd.4400951. [DOI] [PubMed] [Google Scholar]
- 18.Das M, Mukherjee SB, Shaha C. Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J Cell Sci. 2001;114:2461–2469. doi: 10.1242/jcs.114.13.2461. [DOI] [PubMed] [Google Scholar]
- 19.Sen N, Banerjee B, Das BB, Ganguly A, Sen T, et al. Apoptosis is induced in leishmanial cells by a novel protein kinase inhibitor withaferin A and is facilitated by apoptotic topoisomerase I-DNA complex. Cell Death Differ. 2007;14:358–367. doi: 10.1038/sj.cdd.4402002. [DOI] [PubMed] [Google Scholar]
- 20.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.BoseDasgupta S, Das BB, Sengupta S, Ganguly A, Roy A, et al. The caspase-independent algorithm of programmed cell death in Leishmania induced by baicalein: the role of LdEndoG, LdFEN-1 and LdTatD as a DNA ‘degradesome’. Cell Death Differ. 2008;15:1629–1640. doi: 10.1038/cdd.2008.85. [DOI] [PubMed] [Google Scholar]
- 23.Rico E, Alzate JF, Arias AA, Moreno D, Clos J, et al. Leishmania infantum expresses a mitochondrial nuclease homologous to EndoG that migrates to the nucleus in response to an apoptotic stimulus. Mol Biochem Parasitol. 2009;163:28–38. doi: 10.1016/j.molbiopara.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara Y, Shimamoto N. Involvement of endonuclease G in nucleosomal DNA fragmentation under sustained endogenous oxidative stress. J Biol Chem. 2006;281:6726–6733. doi: 10.1074/jbc.M510382200. [DOI] [PubMed] [Google Scholar]
- 25.Rotureau B, Gego A, Carme B. Trypanosomatid protozoa: a simplified DNA isolation procedure. Exp Parasitol. 2005;111:207–209. doi: 10.1016/j.exppara.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 27.Seifert K, Matu S, Javier Perez-Victoria F, Castanys S, Gamarro F, et al. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int J Antimicrob Agents. 2003;22:380–387. doi: 10.1016/s0924-8579(03)00125-0. [DOI] [PubMed] [Google Scholar]
- 28.Verma NK, Dey CS. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob Agents Chemother. 2004;48:3010–3015. doi: 10.1128/AAC.48.8.3010-3015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vergnes B, Gourbal B, Girard I, Sundar S, Drummelsmith J, et al. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol Cell Proteomics. 2007;6:88–101. doi: 10.1074/mcp.M600319-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Sereno D, Cavaleyra M, Zemzoumi K, Maquaire S, Ouaissi A, et al. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob Agents Chemother. 1998;42:3097–3102. doi: 10.1128/aac.42.12.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 32.van Loo G, Schotte P, van Gurp M, Demol H, Hoorelbeke B, et al. Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ. 2001;8:1136–1142. doi: 10.1038/sj.cdd.4400944. [DOI] [PubMed] [Google Scholar]
- 33.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 34.Grondin K, Haimeur A, Mukhopadhyay R, Rosen BP, Ouellette M. Co-amplification of the gamma-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J. 1997;16:3057–3065. doi: 10.1093/emboj/16.11.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desjeux P, Alvar J. Leishmania/HIV co-infections: epidemiology in Europe. Ann Trop Med Parasitol. 2003;97(Suppl 1):3–15. doi: 10.1179/000349803225002499. [DOI] [PubMed] [Google Scholar]
- 36.ter Horst R, Collin SM, Ritmeijer K, Bogale A, Davidson RN. Concordant HIV infection and visceral leishmaniasis in Ethiopia: the influence of antiretroviral treatment and other factors on outcome. Clin Infect Dis. 2008;46:1702–1709. doi: 10.1086/587899. [DOI] [PubMed] [Google Scholar]
- 37.den Boer ML, Alvar J, Davidson RN, Ritmeijer K, Balasegaram M. Developments in the treatment of visceral leishmaniasis. Expert Opin Emerg Drugs. 2009;14:395–410. doi: 10.1517/14728210903153862. [DOI] [PubMed] [Google Scholar]
- 38.Molina R, Gradoni L, Alvar J. HIV and the transmission of Leishmania. Ann Trop Med Parasitol. 2003;97(Suppl 1):29–45. doi: 10.1179/000349803225002516. [DOI] [PubMed] [Google Scholar]
- 39.Barat C, Zhao C, Ouellette M, Tremblay MJ. HIV-1 replication is stimulated by sodium stibogluconate, the therapeutic mainstay in the treatment of leishmaniasis. J Infect Dis. 2007;195:236–245. doi: 10.1086/510398. [DOI] [PubMed] [Google Scholar]
- 40.Goujard C, Legrand M, Panhard X, Diquet B, Duval X, et al. High variability of indinavir and nelfinavir pharmacokinetics in HIV-infected patients with a sustained virological response on highly active antiretroviral therapy. Clin Pharmacokinet. 2005;44:1267–1278. doi: 10.2165/00003088-200544120-00005. [DOI] [PubMed] [Google Scholar]
- 41.Jones K, Hoggard PG, Sales SD, Khoo S, Davey R, et al. Differences in the intracellular accumulation of HIV protease inhibitors in vitro and the effect of active transport. AIDS. 2001;15:675–681. doi: 10.1097/00002030-200104130-00002. [DOI] [PubMed] [Google Scholar]
- 42.Shaha C. Apoptosis in Leishmania species & its relevance to disease pathogenesis. Indian J Med Res. 2006;123:233–244. [PubMed] [Google Scholar]
- 43.Lee N, Gannavaram S, Selvapandiyan A, Debrabant A. Characterization of metacaspases with trypsin-like activity and their putative role in programmed cell death in the protozoan parasite Leishmania. Eukaryot Cell. 2007;6:1745–1757. doi: 10.1128/EC.00123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma NK, Singh G, Dey CS. Miltefosine induces apoptosis in arsenite-resistant Leishmania donovani promastigotes through mitochondrial dysfunction. Exp Parasitol. 2007;116:1–13. doi: 10.1016/j.exppara.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Estaquier J, Lelievre JD, Petit F, Brunner T, Moutouh-De Parseval L, et al. Effects of antiretroviral drugs on human immunodeficiency virus type 1-induced CD4(+) T-cell death. J Virol. 2002;76:5966–5973. doi: 10.1128/JVI.76.12.5966-5973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petit F, Fromenty B, Owen A, Estaquier J. Mitochondria are sensors for HIV drugs. Trends Pharmacol Sci. 2005;26:258–264. doi: 10.1016/j.tips.2005.03.006. [DOI] [PubMed] [Google Scholar]