Abstract
Background
The wide use of gametocytocidal artemisinin-based combination therapy (ACT) lead to a reduction of Plasmodium falciparum transmission in several African endemic settings. An increased impact on malaria burden may be achieved through the development of improved transmission-blocking formulations, including molecules complementing the gametocytocidal effects of artemisinin derivatives and/or acting on Plasmodium stages developing in the vector. Azadirachtin, a limonoid (tetranortriterpenoid) abundant in neem (Azadirachta indica, Meliaceae) seeds, is a promising candidate, inhibiting Plasmodium exflagellation in vitro at low concentrations. This work aimed at assessing the transmission-blocking potential of NeemAzal®, an azadirachtin-enriched extract of neem seeds, using the rodent malaria in vivo model Plasmodium berghei/Anopheles stephensi.
Methods
Anopheles stephensi females were offered a blood-meal on P. berghei infected, gametocytaemic BALB/c mice, treated intraperitoneally with NeemAzal, one hour before feeding. The transmission-blocking activity of the product was evaluated by assessing oocyst prevalence, oocyst density and capacity to infect healthy mice. To characterize the anti-plasmodial effects of NeemAzal® on early midgut stages, i.e. zygotes and ookinetes, Giemsa-stained mosquito midgut smears were examined.
Results
NeemAzal® completely blocked P. berghei development in the vector, at an azadirachtin dose of 50 mg/kg mouse body weight. The totally 138 examined, treated mosquitoes (three experimental replications) did not reveal any oocyst and none of the healthy mice exposed to their bites developed parasitaemia. The examination of midgut content smears revealed a reduced number of zygotes and post-zygotic forms and the absence of mature ookinetes in treated mosquitoes. Post-zygotic forms showed several morphological alterations, compatible with the hypothesis of an azadirachtin interference with the functionality of the microtubule organizing centres and with the assembly of cytoskeletal microtubules, which are both fundamental processes in Plasmodium gametogenesis and ookinete formation.
Conclusions
This work demonstrated in vivo transmission blocking activity of an azadirachtin-enriched neem seed extract at an azadirachtin dose compatible with 'druggability' requisites. These results and evidence of anti-plasmodial activity of neem products accumulated over the last years encourage to convey neem compounds into the drug discovery & development pipeline and to evaluate their potential for the design of novel or improved transmission-blocking remedies.
Background
In the last decade, a renewed commitment to the fight against malaria has arisen from governments of endemic countries and international organizations, with the explicit aim to eliminate the disease in low transmission settings and to reduce its burden in high transmission areas through high coverage application of insecticide treated mosquito nets and artemisinin-based combination therapy (ACT) [1]. An important advantage of ACT is that, when applied on a wide scale, it can impact on transmission intensity of the disease, thanks to the gametocytocidal activity of artemisinin derivatives [2]. Artemether in particular significantly reduces the density of the Plasmodium falciparum sexual stages that persist in the circulation of patients treated with non-gametocytocidal drugs for more than three weeks [3]. The long gametocyte life span of P. falciparum allows the parasite to propagate to blood-seeking mosquitoes for a prolonged period, thus increasing its chances to be transmitted to other human hosts. Moreover, there is evidence that the stress imposed to parasites by drug treatment determines an up-regulation in the production of sexual stages [4]. These observations imply that treating patients with schizonticidal drugs has the unwanted detrimental 'side effect', from a public health perspective, of favouring the transmission of the parasite, thus jeopardising malaria control efforts at community level. Furthermore, this mechanism may enhance the spread of drug resistance, once resistant parasite clones have developed within a plasmodial population [4]. Conversely, it has been demonstrated that the use of ACT can counteract the spread of parasite strains already resistant to one of the partner drugs [5].
A number of clinical studies conducted over the last years to evaluate the curative efficacy of different formulations of ACT have evidenced the transmission-blocking potential of the treatments: artemisinin-, artemether- and artesunate-based combinations, unlike other drugs/drug combinations, such as chloroquine (CQ), quinine and sulphadoxine-pyrimethamine [6-8], were found to decrease the prevalence of gametocytaemia [9,10] and mosquito infection [11,12]. However, membrane feeding assays showed that around 60% and 40% of children treated with artemether-lumefantrine and sulphadoxine-pyrimethamine plus artesunate, respectively, were still infective to mosquitoes on day 14 after the beginning of the treatment [13], indicating that gametocytaemia reduction after ACT treatment is a slow process, requiring 3-4 weeks [14-16]. This represents an obvious limitation for the potential of ACT as a transmission-blocking tool. Also, the first evidences of P. falciparum resistance to artemisinin derivatives [17-19] are casting a shadow over the future of malaria control with ACT.
The search for new transmission-blocking compounds to back up the artemisinins' gametocytocidal action and to reduce the chances for the diffusion of resistant parasites is, therefore, of great importance. Such compounds ideally should be directed against the mature forms of gametocytes, and/or against the stages of the parasite developing in the vector.
Medicinal plants represent a possible source for the discovery of anti-plasmodial molecules. Extracts of the neem tree (Azadirachta indica, Meliaceae) are used traditionally to treat malaria and other illnesses in several endemic countries [20,21]. The efficacy of these extracts is attributed to limonoids, a class of highly oxygenated terpenoids [22], endowed with a range of biological properties including insecticidal, anti-microbial, anti-inflammatory and immuno-modulatory activities [23].
Several studies demonstrated that A. indica leaf, seed and stem bark extracts possess in vitro inhibitory activity on P. falciparum asexual stages [24-29]. In vitro screening of purified limonoids revealed that gedunin and nimbolide are the most active molecules against P. falciparum [24,30-32]. Gedunin was found to be effective against CQ-resistant strains of the parasite, with estimated IC50 of 0.72 and 1.25 in different studies [32,33].
In vitro gametocytocidal activity of neem leaf and seed extracts on P. falciparum has been reported [25,28]. In a recent study, in particular, a neem leaf extract was demonstrated to eliminate more than 90% of P. falciparum immature and mature gametocytes in culture, at a concentration of 2.5 μg/ml [29]. Azadirachtin, a limonoid present in neem leaves, and particularly abundant in neem seeds, was observed to inhibit in vitro P. falciparum and P. berghei microgamete exflagellation [34]. Cellular studies revealed that azadirachtin prevents the microgametocyte from completing the three endomitoses required for microgametogenesis, and inhibits cytoskeletal functions [35].
However, the in vivo inhibitory effect of azadirachtin on male gametogenesis, and the effect of azadirachtin and other limonoids on parasite stages developing in the vector have not been explored yet. This work aims at assessing the transmission blocking potential of NeemAzal® (a standardized, azadirachtin enriched extract of neem seeds), using a rodent malaria model.
Methods
Experimental model
The rodent malaria model P. berghei/An. stephensi/BALB/c mouse was used for the experiments. This model has been validated as appropriate for obtaining inferences on the potential of compounds as P. falciparum transmission-blocking agents [36]. The An. stephensi colony was maintained at 30°C, >95% RH and a photoperiod of 12 h. BALB/c mice, used as vertebrate hosts, were reared in the animal facilities of the Department of Experimental Medicine and Public Health of the University of Camerino, Italy. Female mice weighing 16 - 19 g were used in the experiments. Blood meals were carried out on mice anaesthetized with xylazine : acepromazine 6.5 : 3.5, at a dosage of 11 mg/kg of the mixture. Experimental animal rearing and handling were fully compliant with the Italian Directive 116 of 10/27/92 on the "use and protection of laboratory animals", and in adherence with the European regulation (86/609) of 11/24/86 (licence no. 125/94A, issued by the Italian Ministry of Health).
The P. berghei ANKA (CQ-sensitive) strain was maintained by weekly blood transfer from infected to healthy mice. Every second month a cyclic passage was carried out by infecting mosquitoes through a blood meal on gametocytemic mice and, three weeks afterwards, by transferring the parasite to healthy mice through the bites of the sporozoite carrying mosquitoes. Mosquitoes infected with P. berghei were kept at 19 ± 1°C for the whole duration of the sporogonic cycle [37].
Neem products
The following neem products were used: i) NeemAzal® technical grade (NA; Trifolio-M GmbH, Lahnau, Germany), a standardized extract of A. indica seed kernels, containing limonoids at a concentration of 57.6% [38] and azadirachtin A at 34% (table 1); ii) azadirachtin tech grade (AZA; Sigma). Stock solutions of the neem products were prepared in absolute ethanol, at an azadirachtin A concentration of 50 mg/ml, according to manufacturers' information about azadirachtin content in the extracts.
Table 1.
Limonoid content of NeemAzal®
| azadirachtin A | 34% |
| other azadirachtins (azadirachtin B to K) | 16% |
| salannins | 4% |
| nimbins | 2% |
| Total limonoids | 56% |
Experimental mice received intraperitoneal (ip) inoculations of the various test solutions in a volume of 100 μl/mouse, obtained by dilution of the stock solutions with PBS (pH 6.5) with the addition of Tween 80 and DMSO, to final solvent concentrations of 5 - 20% ethanol, 7,5% Tween 80 and 10% DMSO. Control mice were inoculated with solutions containing solvents only, with 20% ethanol, the maximum alcoholic concentration present in the highest test dosage.
Evaluation of the impact on the sporogonic development
Experimental mice were infected by ip inoculation of 107 infected red blood cells and, four days after infection, the presence of mature gametocytes was verified on Giemsa stained blood smears. Three gametocytemic mice were used for each of the five treatments, namely NA 13.2 mg/kg, NA 25 mg/kg, NA 50 mg/kg, AZA 50 mg/kg, and control solutions. Sixty minutes after receiving the treatment, the mice were narcotized and placed, according to the treatment, over cages containing ~250 An. stephensi females, 5 - 7 day old. Nearly all of the mosquitoes appeared successfully fed within 30 - 45 minutes. Unfed mosquitoes were discarded 24 hours after the blood meal. Ten days after the blood meal, samples of about 40 mosquito females per treatment group were dissected and their midguts examined under the light microscope (400×) to assess oocyst presence and count their number. Log transformed oocyst densities were compared using the Student's t test. Oocyst prevalences were compared using the χ2 test. The experiment was replicated three times.
Assessment of mosquito infectivity
As another measure to assess sporogonic cycle blockage by the neem treatments, healthy mice were challenged by 10 - 15 bites each, using the treated mosquitoes, three weeks after the infective blood meal. Two mice were used per treatment group and Giemsa-stained thin smears were observed to ascertain mouse positivity for P. berghei one week after the challenge.
Evaluation of the impact on early sporogonic stages, developing in the midgut lumen
To determine the effect of neem substances on gamete-to-ookinete development mice were treated and exposed to mosquitoes as described above and mosquitoes from each treatment group were dissected to detect and count ookinetes in the midgut lumen, 18 and 20 hours after the blood meal, i.e. at a time when P. berghei gametogenesis, fecundation and ookinete development are completed. Midguts were excised in a drop of calf serum, transferred to Eppendorf tubes in pools of three and homogenized in 4 μl of calf serum. Three microliters of the homogenate were smeared onto a glass slide and stained with Giemsa. Ten to eleven slides (30-33 mosquitoes) were prepared for each treatment group. Zygotes, immature (retort stage to elongated shape with marginal nucleus) and mature ookinetes were counted over 300 microscopic fields (1,000×) on each slide, following a cross-shaped transect. The experiment was repeated twice. Log transformed midgut stages' densities were compared using the Student's t test.
To verify whether NA treatment altered the zygote and/or ookinete morphology and whether it inhibited the formation of mature ookinetes, 9 out of 21 slides from the two NA 50 mg/kg treatment replicates were extensively screened over a total area of 628 mm2 (15% of total smear surface). Totally, 90 zygotes and ookinetes were found and inspected.
Evaluation of the impact on oocyst maturation
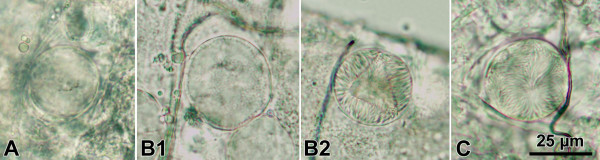
To establish if neem substances interfere with the maturation of oocysts, infected (untreated) mosquitoes were offered a second blood meal on neem-treated mice. About 1,800 An. stephensi females were infected with P. berghei through a blood meal on gametocytaemic mice. On day 7 after the infective blood meal, the mosquitoes were offered a second blood meal, according to the procedure described above, on three groups of three uninfected mice treated with 50 mg/kg NA, 50 mg/kg AZA, or control solutions. On day 10 after the first, infective blood meal (day 3 after the second blood meal), samples of mosquitoes from the three experimental groups were dissected, and oocyst numbers and their stage of development were recorded. The oocyst developmental status was coded into three categories, namely: s1, immature oocyst, before the formation of sporoblasts (Figure 1A); s2, immature oocyst, with visible sporoblasts and budding sporozoites (Figure 1B1 and 1B2); s3, mature oocyst containing fully developed sporozoites (Figure 1C). Log transformed oocyst densities were compared using the Student's t test.
Figure 1.
Light microscope images (400×) of Plasmodium berghei oocyst development stages (day 10 after mosquito infection). Immature oocyst, with uniform content, A; immature oocysts, with sporoblasts and budding sporozoites, B1 and B2; mature oocyst, with fully developed sporozoites visible, C.
Results
Block of the sporogonic development
The development of P. berghei in An. stephensi mosquitoes was blocked when the sexual stages of the parasite were ingested by females taking a blood meal on NA-treated, gametocytaemic mice. Within the NA 50 mg/kg treatment group, none of the 138 mosquitoes examined in three consecutive experiments revealed the presence of oocysts (Table 2).
Table 2.
Effect of NeemAzal® on P. berghei oocyst prevalence and density on mosquito midguts.
| Treatment* | Experiment† | % Prevalence (CI95) | Oocysts/mosquito (CI95)# | Examined mosquitoes |
|---|---|---|---|---|
| control | 1 | 90 (81 - 99) | 17.5 (11.1 - 27.2) | 40 |
| 2 | 83 (68 - 99) | 29.9 (15.8 - 55.8) | 24 | |
| 3 | 100 | 111.2 (83.8 - 148.9) | 40 | |
| 4 | 86 (72 - 100) | 35.2 (21.6 - 57.6) | 22 | |
| 5 | 88 (78 - 98) | 11.3 (7.2 - 17.4) | 42 | |
| 6 | 89 (80 - 97) | 26.1 (15.8 - 42.4) | 53 | |
| tot‡ | 90 (86 - 94) | 27.7 (22.3 - 34.2) | 221 | |
| NA 13.2 mg/kg | 4 | 82 (64 - 100) | 49.9 (24.5 - 100.5) | 17 |
| 5 | 79 (66 - 91) | 12.7 (8.6 - 18.7) | 42 | |
| 6 | 100 | 39.4 (30.5 - 50.9) | 58 | |
| tot | 90 (84 - 95) | 28.9 (23.0 - 36.3) | 117 | |
| NA 25 mg/kg | 3 | 90 (81 - 99) | 42.8 (26.7 - 69.1) | 40 |
| 5 | 69 (55 - 84) | 8.9 (5.4 - 14.2) | 39 | |
| 6 | 96 (91 - 100) | 30.5 (22.3 - 41.1) | 54 | |
| tot | 86 (81 - 92) | 25.6 (19.9 - 32.8) | 133 | |
| NA 50 mg/kg | 1 | 0 | 0 | 55 |
| 2 | 0 | 0 | 43 | |
| 3 | 0 | 0 | 40 | |
| tot | 0 | 0 | 138 | |
| AZA 50 mg/kg | 3 | 95 (88 - 100) | 23.3 (15.0 - 36.0) | 41 |
| 5 | 88 (78 - 98) | 24.3 (17.2 - 34.5) | 42 | |
| 6 | 83 (68 - 99) | 3.2 (2.0 - 5.0) | 24 | |
| tot | 90 (73 - 100) | 16.1 (12.2 - 21.2) | 107 | |
Oocyst prevalence and intensity was assessed on day 10 after the infective blood meal carried out on mice treated with the indicated products and doses. * NA = NeemAzal® technical grade (Trifolio-M GmbH); AZA = azadirachtin tech grade (Sigma). † each number corresponds to one experimentation involving different treatment groups. # geometric means, only positive mosquitoes included. ‡ values refer to pooled data.
This blockage of the sporogonic development was confirmed by mouse challenge: all the healthy mice (n = 6) exposed to 10 - 15 bites of mosquitoes fed on the NA 50 mg/kg treated gametocytaemic mice failed to develop Plasmodium infections, whereas all the mice (n = 6) exposed to control mosquitoes had positive blood slides on day 7 after the challenge.
No impact on the sporogonic development could be detected in mosquitoes of the other neem treatment groups, namely NA 25 mg/kg, NA 13.2 mg/kg and AZA 50 mg/kg (Table 2). In these groups, the percentage of oocyst-positive mosquitoes was high in all occasions, most often above 80%, and very similar in treatment and control mosquitoes. Also, NA at these dosages and AZA did not appear to be able to reduce the number of oocysts and all the mice challenged with mosquitoes from these treatment groups developed parasitaemia.
Impact on early sporogonic stages, developing in the midgut lumen
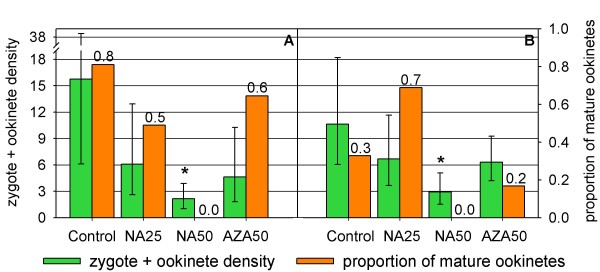
Mean early midgut stage (zygotes + ookinetes) densities were severely affected by NA action, as evidenced by mosquito midgut smears prepared 18 and 20 hours after the infective blood meal (Figure 2). Mean zygotes + ookinetes densities, estimated through counts on 300 microscopic fields (mf), amounted in the control mosquitoes 15.7 (CI95 6.1 - 38.4) and 10.6 (CI95 6.2 - 18.2) in the 1st and 2nd experiment, respectively, compared to only 2.1 (CI95 1.0 - 3.9) and 2.9 CI95 1.5 - 5.1) in mosquitoes fed on 50 mg/kg NA treated mice. These results show that NA at 50 mg/kg significantly reduced the number of parasites able to reach the zygote stage (p = 0.001 and p = 0.002 for the 1st and 2nd experiment, respectively).
Figure 2.
Effects of the neem products on P. berghei midgut stages (zygotes to ookinetes). Parasites were counted on 300 microscopic fields (1000× magnification) of midgut content smears of A. stephensi females that had fed 18 (A), and 20 (B) hours before on mice treated with the indicated neem products and doses. NA25, NeemAzal® 25 mg/kg; NA50, NeemAzal® 50 mg/kg; AZA50, azadirachtin 50 mg/kg. Geometric means of zygote + ookinete densities, evaluated over ten smears (30 mosquitoes), with 95% confidence intervals (green bars; left axis). Mature ookinetes/total midgut forms ratio (orange bars; right axis). * means differ significantly from control (Student's t test; p ≤ 0.002)
The mean zygotes + ookinetes densities of mosquitoes fed on NA 25 mg/kg and AZA 50 mg/kg treated mice were lower than those of the controls, ranging from 4.6 to 6.7 (NA 25 mg/kg 1st exp.: 6.1, CI95 2.6 - 12.9; 2nd exp.: 6.7, CI95 3.7 - 11.7; AZA 50 mg/kg 1st exp.: 4.6, CI95 1.8 - 10.3; 2nd exp.: 6.3, CI95 4.2 - 9.2), but the difference was not statistically significant.
In both experimental groups, examined 18 or 20 hours after the infective blood meal, no mature ookinetes were detected on the midgut slides of NA 50 mg/kg treated mosquitoes (Figure 2), suggesting that the treatment interferes with the process of ookinete development. Accordingly, the additional extensive screening of 9 slides from this group, covering 15% of the total smear area, revealed the presence of 90 zygotes and post-zygotic forms, with no mature ookinete. For comparison, in control mosquitoes, 1/3 to 5/6 of the midgut stages present 18 or 20 hours after the infective blood meal were mature ookinetes (Figure 2).
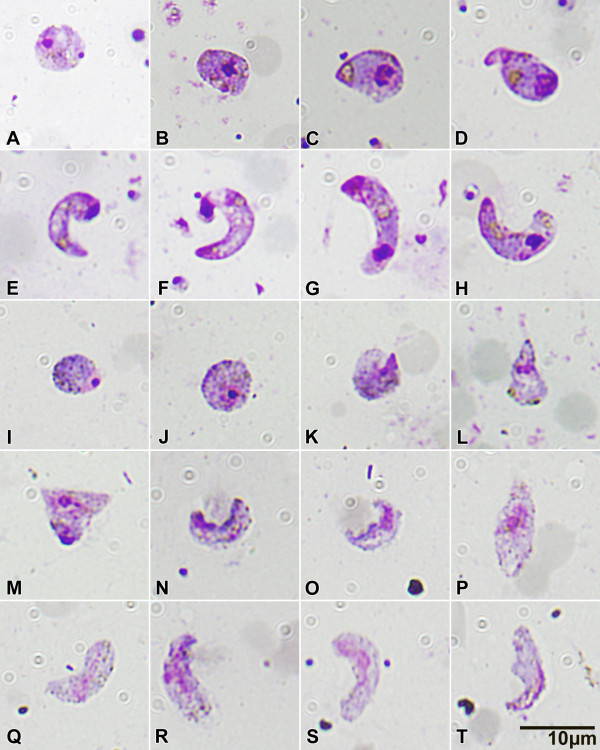
The morphological comparison of the zygotes and post-zygotic forms traced on NA 50 mg/kg slides with controls allowed alterations of the post-zygotic stages to be detected (Figure 3), whereas zygotes did not appear to differ. Giemsa-stained zygotes from both groups were similar in size, displaying rounded, condensed nuclei, intense cytoplasmic coloration and comparable haemozoin granules and crystalloid precursors (Figure 3A, 3I to 3J). In the control group, the ookinete development from retort stages (Figure 3B to 3F) to mature ookinetes (Figure 3G, 3H) was characterized by the completion of the elongation process, with the disappearance of the residual bulbous end containing the condensed nucleus, an intense staining of the apical region and the appearance of well defined haemozoin-surrounded crystalloid organelles. Several morphological alterations of the post-zygotic forms were found in the NA 50 mg/kg group (Figure 3K to 3T):
Figure 3.
Light microscope images (1000×) Giemsa stained P. berghei zygotes and ookinetes from A. stephensi midgut content smears, 18 - 20 hours after the infective blood meal. Control mosquitoes, A to H; NeemAzal® 50 mg/kg treated mosquitoes, I to T. See text for details.
i) irregular cell shape (Figure 3L, 3M) and jagged cell borders (Figure 3K, 3N, 3O, 3R to 3T);
ii) lack of intense nuclear staining, with the nuclear material appearing dispersed within the cytoplasm (Figure 3O to 3T);
iii) weak staining, if any, of the apical region (Figure 3L, 3O to 3Q, 3S, 3T);
iv) lack of crystalloid assembly, the organelle granules remaining dispersed as in the zygote stage (Figure 3K, 3M to 3S), or partial crystalloid assembly (Figure 3L, 3T).
Evaluation of impact on oocyst maturation
The exposure of early P. berghei oocysts to NA and AZA through a second treated blood meal on day 7 after mosquito infection did not affect the oocysts' subsequent development. In both neem treated groups oocysts underwent regular sporoblast formation and sporozoite maturation, as in untreated mosquitoes. Also, the proportion of mature oocysts containing fully developed sporozoites (stage s3) was similar in treated and control groups. Salivary glands were successfully invaded by sporozoites in both groups, indicating that neem treatment was not able to interfere with the sporogonic cycle (Table 3).
Table 3.
P. berghei oocyst number/mosquito and development stage proportions after a secondary, treated blood meal.
| Treatment | Replication | Stage of development* | Total (CI95%) | Examined mosquitoes | ||
|---|---|---|---|---|---|---|
| s1 (%) | s2 (%) | s3 (%) | ||||
| control | 1 | 25 (38) | 23 (37) | 12 (25) | 70 (50 - 98) | 27 |
| 2 | 15 (33) | 12 (31) | 13 (36) | 54 (42 - 70) | 92 | |
| NA 50 mg/kg | 1 | 10 (36) | 10 (36) | 8 (28) | 31 (19 - 49) | 23 |
| 2 | 30 (42) | 21 (33) | 11 (25) | 82 (68 - 100) | 112 | |
| AZA 50 mg/kg | 1 | 19 (34) | 20 (35) | 18 (31) | 61 (40 - 93) | 27 |
Secondary blood meals were carried out on mice treated with the indicated products and doses on day 7 after mosquito infection. Oocysts were evaluated on day 3 after the secondary blood meal (day 10 post infection). *s1: immature oocysts, before the formation of sporoblasts (fig. 1A); s2: immature oocysts, with visible sporoblasts and budding sporozoites (fig. 1 B1 - B2); s3: mature oocysts containing fully developed sporozoites (fig. 1C). Geometric means, only positive mosquitoes included.
Discussion
NeemAzal® (NA), a commercial A. indica seed extract, containing the limonoid azadirachtin as the main component, completely blocked the development of P. berghei in An. stephensi mosquitoes fed on gametocytaemic mice treated with the extract at 50 mg/kg body weight. The treated mosquitoes did not produce oocysts and were thus unable to infect healthy mice. The examination of slides prepared from the midgut content of experimental mosquitoes showed that NA activity was directed to the early sporogonic stages: the total number of zygotes and post-zygotic forms was reduced in the NA-treated group compared to controls, indicating that NA components interfere with parasite development already before zygote formation. In addition, post-zygotic forms from NA treated mosquitoes displayed evident morphological alterations and no mature ookinetes could be detected. These results indicate that NA may act on gametogenesis and ookinete formation.
In vitro studies conducted by Jones and colleagues [34] showed that azadirachtin interferes with the exflagellation process of P. falciparum and P. berghei microgametocytes, inducing an interruption of the endomitotic divisions and the formation of rigid extensions on axonemes, preventing their motility [34]. In a subsequent study it was demonstrated by Billker et al [35] that azadirachtin disrupts cytoplasmic and axonemal microtubule organization, possibly by compromising the functionality of the microtubule organizing centres (MTOC), including spindle plaques. The results presented in this report suggest that azadirachtin inhibits microgametogenesis in vivo.
The morphological alterations observed on NA-treated post-zygotic forms are compatible with the hypothesis that azadirachtin interferes with ookinete formation via a possibly similar, microtubule-targeted mechanism of action. The irregular cell shape, the jagged cell membrane, the inability to form the typically strongly stained apical complex and to perform the elongation process are compatible with an interference with the microtubule organizing processes involved in cytoskeletal and organelle rearrangements. Electron microscopy studies have shown that ookinete development is depending on the differentiation of a MTOC at the apical pole, from which subpellicular, longitudinal microtubules radiate and the triple membrane (pellicle) is formed [39,40]. Further evidence for azadirachtin action on microtubule organizing processes is available from studies of Salehzadeh and colleagues [41], who showed that the antimitotic activity of azadirachtin observed on cultured insect cells was related to its interference with tubulin polymerization, through a mode of action resembling that of colchicine. Colchicine and other compounds of natural origin, added to zygote cultures of Plasmodium gallinaceum, have been demonstrated to inhibit ookinete formation [42].
The fact that a transmission blocking action was observed with NA, but not with AZA tested at the same dose, might suggest that other bioactive limonoids present in the former (which is an azadirachtin-enriched extract, not a pure azadirachtin molecule) could explain the difference in activity. However, a HPLC analysis conducted according to the protocol of Kaushik [43] did not evidence additional compounds present in significant amounts in NA, but revealed that the azadirachtin A content of AZA was about half the amount declared by the manufacturer, which could well explain the difference in transmission blocking effect observed with the two products.
Numerous studies demonstrated that azadirachtin and other limonoids present in neem extracts are active on insects [44], including malaria vectors [45-47]. Exploring the insecticidal properties of NA in An. stephensi, it was found that mosquitoes fed with artificial blood meals or sugar solutions containing a NA concentration corresponding to 100 μg/ml azadirachtin displayed reduced blood feeding capability and produced fewer eggs. This reduction in oviposition was mirrored by a degenerative tissue damage provoked by NA on ovarian follicles [48].
These multiple and diverse effects on Plasmodium midgut stages and female mosquitoes are of remarkable interest in view of the development of transmission blocking molecular tools, directed against both the malaria parasite and the malaria vector, based on neem extracts. To challenge their potential, the identification of the bioactive molecules and the characterization with respect to target stage and mode of action represent important research questions to be addressed. Also, the 'druggability' of limonoids may represent a major challenge: the few studies that explored limonoid pharmacokinetics indicate a relatively short half-life and low bioavailability of the compounds. Manners et al [49] report that limonin and related metabolites were not detectable in plasma samples of human volunteers 24 hours after the administration of 30 mg/kg of pure limonin glucoside, which reached its peak plasma concentration 6 hours post-administration. Lack of in vivo curative activity of gedunin against P. berghei has been ascribed to its poor absorption in the digestive tract [20].
Developing transmission blocking drug candidates from neem compounds is a long and challenging way to go. Still, evidences accumulated over the years on the anti-plasmodial and mosquito-toxic activity of plants encourage medicinal chemistry studies aiming to develop limonoid leads with improved bioavailability characteristics. Once limonoid leads are obtained, drug interaction studies with ACT would be required and possible interactions with the immune system should be assessed, before considering them for further development as transmission-blocking components of combination therapies.
As an equally challenging alternative, the development of neem-based transmission blocking remedies by improving traditional herbal treatments could be explored. Extract fractions displaying a high activity against gametocytes and/or against the Plasmodium stages developing in the vector can be identified applying bio-guided fractionation methods. Combining the most active fractions should result in remedies with an increased overall transmission blocking efficacy, thanks to potentially additive or synergistic effects of azadirachtin, other limonoids and other components present in extracts [50]. In addition the presence of various bioactive molecules in the extracts might reduce the likelihood of selecting resistant strains in the target organisms [45]. On the other hand, toxicity and variability of preparations must be taken into account when considering plant extracts for therapeutic use, as the useful metabolites' concentration may vary widely according to environmental conditions, geographic origin, mode of plant collection and storage [51]. In the case of neem preparations, not only toxicity risks, but possible immuno-modulatory effects also need to be explored before considering a product for human use [52].
Conclusion
The neem tree, A. indica, produces and stores in its organs and tissues secondary metabolites that have been demonstrated to be active against different malaria parasite stages in the vertebrate host and to possess insecticidal and insect growth regulatory properties on malaria vectors as well. The transmission blocking activity of a standardized, azadirachtin rich neem extract described in this work represents an additional element contributing to the potential of neem as a resource for malaria control. The available evidence strongly encourages to convey neem compounds in the drug discovery and development pipeline and also to evaluate the potential of plant extracts for the development of improved traditional remedies.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LL participated in study design, carried out the in vivo experiments, performed the statistical analysis, and drafted the manuscript; RSY participated in study design and in the execution of the experiments; GL carried out the HPLC analysis and helped with study design; LP participated in the execution of the in vivo experiments; FE participated in study design and helped with manuscript revision; AH coordinated the work, participated in study design and critically revised the manuscript. All the authors read and approved the final manuscript.
Contributor Information
Leonardo Lucantoni, Email: leonardo.lucantoni@unicam.it.
Rakiswendé S Yerbanga, Email: yrserge@yahoo.fr.
Giulio Lupidi, Email: giulio.lupidi@unicam.it.
Luciano Pasqualini, Email: luciano.pasqualini@unicam.it.
Fulvio Esposito, Email: fulvio.esposito@unicam.it.
Annette Habluetzel, Email: annette.habluetzel@unicam.it.
Acknowledgements
We wish to thank Dr. S. A. van der Esch (ENEA, Italy) for kindly providing NeemAzal®. The work was financially supported by the University of Camerino (UNICAM), the Italian Malaria Network, the Seventh European Framework Programme project 'TransMalariaBloc' n. 223736 and by the UNICAM PhD Programme on Malaria and Human Development (supported by WHO Global Malaria Programme).
References
- World Health Organization. Global malaria control and elimination: report of a technical review. Geneva: World Health Organization; 2008. [Google Scholar]
- Chotivanich K, Sattabongkot J, Udomsangpetch R, Looareesuwan S, Day NP, Coleman RE, White NJ. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob Agents Chemother. 2006;50:1927–1930. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley C, Sutherland C, Bousema JT, Sauerwein RW, Targett GA. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 2006;22:424–430. doi: 10.1016/j.pt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sutherland CJ, Alloueche A, Curtis J, Drakeley CJ, Ord R, Duraisingh M, Greenwood BM, Pinder M, Warhurst D, Targett GA. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg. 2002;67:578–585. doi: 10.4269/ajtmh.2002.67.578. [DOI] [PubMed] [Google Scholar]
- Hallett RL, Sutherland CJ, Alexander N, Ord R, Jawara M, Drakeley CJ, Pinder M, Walraven G, Targett GA, Alloueche A. Combination therapy counteracts the enhanced transmission of drug-resistant malaria parasites to mosquitoes. Antimicrob Agents Chemother. 2004;48:3940–3943. doi: 10.1128/AAC.48.10.3940-3943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines JD, Wilkes TJ, Lyimo EO. Human malaria infectiousness measured by age-specific sporozoite rates in Anopheles gambiae in Tanzania. Parasitology. 1991;102(Pt 2):167–177. doi: 10.1017/S0031182000062454. [DOI] [PubMed] [Google Scholar]
- Klein TA, Tada MS, Lima JB. Infection of Anopheles darlingi fed on patients with Plasmodium falciparum before and after treatment with quinine or quinine plus tetracycline. Am J Trop Med Hyg. 1991;44:604–608. doi: 10.4269/ajtmh.1991.44.604. [DOI] [PubMed] [Google Scholar]
- Sowunmi A, Fateye BA. Plasmodium falciparum gametocytaemia in Nigerian children: before, during and after treatment with antimalarial drugs. Trop Med Int Health. 2003;8:783–792. doi: 10.1046/j.1365-3156.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/S0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- Broek I van den, Kitz C, Al Attas S, Libama F, Balasegaram M, Guthmann JP. Efficacy of three artemisinin combination therapies for the treatment of uncomplicated Plasmodium falciparum malaria in the Republic of Congo. Malar J. 2006;5:113. doi: 10.1186/1475-2875-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley CJ, Jawara M, Targett GA, Walraven G, Obisike U, Coleman R, Pinder M, Sutherland CJ. Addition of artesunate to chloroquine for treatment of Plasmodium falciparum malaria in Gambian children causes a significant but short-lived reduction in infectiousness for mosquitoes. Trop Med Int Health. 2004;9:53–61. doi: 10.1046/j.1365-3156.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GA. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema JT, Schneider P, Gouagna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis. 2006;193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- Mens PF, Sawa P, van Amsterdam SM, Versteeg I, Omar SA, Schallig HD, Kager PA. A randomized trial to monitor the efficacy and effectiveness by QT-NASBA of artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment and transmission control of uncomplicated Plasmodium falciparum malaria in western Kenya. Malar J. 2008;7:237. doi: 10.1186/1475-2875-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangpukdee N, Krudsood S, Srivilairit S, Phophak N, Chonsawat P, Yanpanich W, Kano S, Wilairatana P. Gametocyte clearance in uncomplicated and severe Plasmodium falciparum malaria after artesunate-mefloquine treatment in Thailand. Korean J Parasitol. 2008;46:65–70. doi: 10.3347/kjp.2008.46.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Bousema T, Omar S, Gouagna L, Sawa P, Schallig H, Sauerwein R. (Sub)microscopic Plasmodium falciparum gametocytaemia in Kenyan children after treatment with sulphadoxine-pyrimethamine monotherapy or in combination with artesunate. Int J Parasitol. 2006;36:403–408. doi: 10.1016/j.ijpara.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J. 2009;8:10. doi: 10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar S, Zhang J, MacKinnon S, Leaman D, Durst T, Philogene BJ, Arnason JT, Sanchez-Vindas PE, Poveda L, Tamez PA, Pezzuto JM. Traditionally-used antimalarials from the Meliaceae. Curr Top Med Chem. 2003;3:133–139. doi: 10.2174/1568026033392499. [DOI] [PubMed] [Google Scholar]
- Soh PN, Benoit-Vical F. Are West African plants a source of future antimalarial drugs? J Ethnopharmacol. 2007;114:130–140. doi: 10.1016/j.jep.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Roy A, Saraf S. Limonoids: overview of significant bioactive triterpenes distributed in plants kingdom. Biol Pharm Bull. 2006;29:191–201. doi: 10.1248/bpb.29.191. [DOI] [PubMed] [Google Scholar]
- Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;82:1336–1345. [Google Scholar]
- MacKinnon S, Durst T, Arnason JT, Angerhofer C, Pezzuto J, Sanchez-Vindas PE, Poveda LJ, Gbeassor M. Antimalarial activity of tropical Meliaceae extracts and gedunin derivatives. J Nat Prod. 1997;60:336–341. doi: 10.1021/np9605394. [DOI] [PubMed] [Google Scholar]
- Dhar R, Zhang K, Talwar GP, Garg S, Kumar N. Inhibition of the growth and development of asexual and sexual stages of drug-sensitive and resistant strains of the human malaria parasite Plasmodium falciparum by Neem (Azadirachta indica) fractions. J Ethnopharmacol. 1998;61:31–39. doi: 10.1016/S0378-8741(98)00012-9. [DOI] [PubMed] [Google Scholar]
- Badam L, Deolankar RP, Kulkarni MM, Nagsampgi BA, Wagh UV. In vitro antimalarial activity of neem (Azadirachta indica A. Juss) leaf and seed extracts. Indian J Malariol. 1987;24:111–117. [PubMed] [Google Scholar]
- El Tahir A, Satti GM, Khalid SA. Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Maytenus senegalensis (Lam.) Exell. J Ethnopharmacol. 1999;64:227–233. doi: 10.1016/S0378-8741(98)00129-9. [DOI] [PubMed] [Google Scholar]
- Udeinya IJ, Brown N, Shu EN, Udeinya FI, Quakeyie I. Fractions of an antimalarial neem-leaf extract have activities superior to chloroquine, and are gametocytocidal. Ann Trop Med Parasitol. 2006;100:17–22. doi: 10.1179/136485906X78508. [DOI] [PubMed] [Google Scholar]
- Udeinya JI, Shu EN, Quakyi I, Ajayi FO. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Ther. 2008;15:108–110. doi: 10.1097/MJT.0b013e31804c6d1d. [DOI] [PubMed] [Google Scholar]
- Khalid SA, Farouk A, Geary TG, Jensen JB. Potential antimalarial candidates from African plants: and in vitro approach using Plasmodium falciparum. J Ethnopharmacol. 1986;15:201–209. doi: 10.1016/0378-8741(86)90156-X. [DOI] [PubMed] [Google Scholar]
- Rochanakij S, Thebtaranonth Y, Yenjai C, Yuthavong Y. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66–72. [PubMed] [Google Scholar]
- Bray DH, Warhurst DC, Connolly JD, O'Neill MJ, Phillipson JD. Plants as sources of antimalarial drugs. Part 7. Activity of some species of Meliaceae plants and their constituent limonoids. Phytother Res. 1990;4:29–35. doi: 10.1002/ptr.2650040108. [DOI] [Google Scholar]
- Bickii J, Njifutie N, Foyere JA, Basco LK, Ringwald P. In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae) J Ethnopharmacol. 2000;69:27–33. doi: 10.1016/S0378-8741(99)00117-8. [DOI] [PubMed] [Google Scholar]
- Jones IW, Denholm AA, Ley SV, Lovell H, Wood A, Sinden RE. Sexual development of malaria parasites is inhibited in vitro by the neem extract azadirachtin, and its semi-synthetic analogues. FEMS Microbiol Lett. 1994;120:267–273. doi: 10.1111/j.1574-6968.1994.tb07044.x. [DOI] [PubMed] [Google Scholar]
- Billker O, Shaw MK, Jones IW, Ley SV, Mordue AJ, Sinden RE. Azadirachtin disrupts formation of organised microtubule arrays during microgametogenesis of Plasmodium berghei. J Eukaryot Microbiol. 2002;49:489–497. doi: 10.1111/j.1550-7408.2002.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Nath AK, Schneider I, Song GH, Klein TA, Milhous WK. Prevention of sporogony of Plasmodium falciparum and P. berghei in Anopheles stephensi mosquitoes by transmission-blocking antimalarials. Am J Trop Med Hyg. 1994;50:646–653. doi: 10.4269/ajtmh.1994.50.646. [DOI] [PubMed] [Google Scholar]
- Rastogi M, Pal NL, Sen AB. Effect of variation in temperature on development of Plasmodium berghei (NK 65 strain) in Anopheles stephensi. Folia Parasitol (Praha) 1987;34:289–297. [PubMed] [Google Scholar]
- NeemAzal® - a natural Biocide. http://www.neemazal.de/eng/NeemAzal.htm
- Sinden RE, Hartley RH, Winger L. The development of Plasmodium ookinetes in vitro: an ultrastructural study including a description of meiotic division. Parasitology. 1985;91(Pt 2):227–244. doi: 10.1017/S0031182000057334. [DOI] [PubMed] [Google Scholar]
- Morrissette NS, Sibley LD. Cytoskeleton of apicomplexan parasites. Microbiol Mol Biol Rev. 2002;66:21–38. doi: 10.1128/MMBR.66.1.21-38.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehzadeh A, Akhkha A, Cushley W, Adams RL, Kusel JR, Strang RH. The antimitotic effect of the neem terpenoid azadirachtin on cultured insect cells. Insect Biochem Mol Biol. 2003;33:681–689. doi: 10.1016/S0965-1748(03)00057-2. [DOI] [PubMed] [Google Scholar]
- Kumar N, Aikawa M, Grotendorst C. Plasmodium gallinaceum : critical role for microtubules in the transformation of zygotes into ookinetes. Exp Parasitol. 1985;59:239–247. doi: 10.1016/0014-4894(85)90078-5. [DOI] [PubMed] [Google Scholar]
- Kaushik N. Determination of azadirachtin and fatty acid methyl esters of Azadirachta indica seeds by HPLC and GLC. Anal Bioanal Chem. 2002;374:1199–1204. doi: 10.1007/s00216-002-1638-7. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). Board on Science and Technology for International Development. Neem: a tree for solving global problems: report of an ad hoc panel of the Board on Science and Technology for International Development, National Research Council. Washington, D.C.: National Academy Press; 1992. [PubMed] [Google Scholar]
- Mulla MS, Su T. Activity and biological effects of neem products against arthropods of medical and veterinary importance. J Am Mosq Control Assoc. 1999;15:133–152. [PubMed] [Google Scholar]
- Dhar R, Dawar H, Garg S, Basir SF, Talwar GP. Effect of volatiles from neem and other natural products on gonotrophic cycle and oviposition of Anopheles stephensi and An. culicifacies (Diptera: Culicidae) J Med Entomol. 1996;33:195–201. doi: 10.1093/jmedent/33.2.195. [DOI] [PubMed] [Google Scholar]
- Nathan SS, Kalaivani K, Murugan K. Effects of neem limonoids on the malaria vector Anopheles stephensi Liston (Diptera: Culicidae) Acta Trop. 2005;96:47–55. doi: 10.1016/j.actatropica.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Lucantoni L, Giusti F, Cristofaro M, Pasqualini L, Esposito F, Lupetti P, Habluetzel A. Effects of a neem extract on blood feeding, oviposition and oocyte ultrastructure in Anopheles stephensi Liston (Diptera: Culicidae) Tissue Cell. 2006;38:361–371. doi: 10.1016/j.tice.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Manners GD, Jacob RA, Breksa AP III, Schoch TK, Hasegawa S. Bioavailability of citrus limonoids in humans. J Agric Food Chem. 2003;51:4156–4161. doi: 10.1021/jf0300691. [DOI] [PubMed] [Google Scholar]
- Koul O, Multani JS, Singh G, Daniewski WM, Berlozecki S. 6beta-hydroxygedunin from Azadirachta indica. Its potentiation effects with some non-azadirachtin limonoids in neem against lepidopteran larvae. J Agric Food Chem. 2003;51:2937–2942. doi: 10.1021/jf021049m. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Aqil F, Owais M. Modern phytomedicine: turning medical plants into drugs. Weinheim: Wiley-VCH; 2006. [Google Scholar]
- Goswami S, Bose A, Sarkar K, Roy S, Chakraborty T, Sanyal U, Baral R. Neem leaf glycoprotein matures myeloid derived dendritic cells and optimizes anti-tumor T cell functions. Vaccine. 2010;28:1241–1252. doi: 10.1016/j.vaccine.2009.11.018. [DOI] [PubMed] [Google Scholar]