Abstract
Context: Steroid 11β-hydroxylase (CYP11B1) deficiency (11OHD) is the second most common form of congenital adrenal hyperplasia (CAH). Cases of nonclassic 11OHD are rare compared with the incidence of nonclassic 21-hydroxylase deficiency.
Objective: The aim of the study was to analyze the functional consequences of seven novel CYP11B1 mutations (p.M88I, p.W116G, p.P159L, p.A165D, p.K254_A259del, p.R366C, p.T401A) found in three patients with classic 11OHD, two patients with nonclassic 11OHD, and three heterozygous carriers for CYP11B1 mutations.
Methods: We conducted functional studies employing a COS7 cell in vitro expression system comparing wild-type (WT) and mutant CYP11B1 activity. Mutants were examined in a computational three-dimensional model of the CYP11B1 protein.
Results: All mutations (p.W116G, p.A165D, p.K254_A259del) found in patients with classic 11OHD have absent or very little 11β-hydroxylase activity relative to WT. The mutations detected in patients with nonclassic 11OHD showed partial functional impairment, with one patient being homozygous (p.P159L; 25% of WT) and the other patient compound heterozygous for a novel mild p.M88I (40% of WT) and the known severe p.R383Q mutation. The two mutations detected in heterozygous carriers (p.R366C, p.T401A) also reduced CYP11B1 activity by 23 to 37%, respectively.
Conclusion: Functional analysis results allow for the classification of novel CYP11B1 mutations as causative for classic and nonclassic 11OHD, respectively. Four partially inactivating mutations are predicted to result in nonclassic 11OHD. These findings double the number of mild CYP11B1 mutations previously described as associated with mild 11OHD. Our data are important to predict phenotypic expression and provide important information for clinical and genetic counseling in 11OHD.
The in vitro and in silico analysis of novel CYP11B1 mutations proves their association with classic and non-classic 11β-hydroxylase deficiency.
Congenital adrenal hyperplasia (CAH) is one of the most common metabolic diseases. It is caused by a severe or partial impairment of adrenal steroidogenesis affecting cortisol biosynthesis. Approximately 5–8% of all cases are due to steroid 11β-hydroxylase deficiency (11OHD; OMIM +202010), which occurs in approximately 1:100,000 to 1:200,000 live births in nonconsanguineous populations (1).
Steroid 11β-hydroxylase (CYP11B1, EC 1.14.15.4) converts 11-deoxycortisol and 11-deoxycorticosterone (DOC) to cortisol and corticosterone, respectively. Inactivating CYP11B1 mutations cause an impairment of these two reactions. Therefore 11OHD results in an accumulation of the precursor steroids, which are shunted into the adrenal androgen synthesis pathway, resulting in virilization of female external genitalia (46,XX disordered sex development). Postnatal androgen excess causes precocious pseudopuberty, rapid somatic growth, and accelerated bone maturation in both sexes. The accumulation of DOC, which binds and activates the mineralocorticoid receptor, leads to hypertension in about two thirds of patients (1).
A milder, nonclassic 11OHD form (2,3), with a phenotype resembling nonclassic 21-hydroxylase deficiency (4), is caused by partial impairment of CYP11B1 function (2). The frequency of this mild form is unknown, but it appears to be rare (2,3). Nonclassic 11OHD can manifest with mild virilization and precocious pseudopuberty in children and with signs and symptoms suggestive of polycystic ovary syndrome in adult women. However, unlike classic 11OHD, arterial hypertension is not commonly found in the nonclassic form (2).
The CYP11B1 gene is localized on chromosome 8q21, approximately 40 kb from the paralog aldosterone synthase gene (CYP11B2) (5). A mutation cluster in exons 2, 6, 7, and 8 has been suggested (6,7). However, a large number of CYP11B1-inactivating mutations are localized to other gene regions (Table 1 and Supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). In addition, chimeric CYP11B2/CYP11B1 genes abolishing 11β-hydroxylase function have been described (8,9,10).
Table 1.
CYP11B1 gene mutations
| g.DNA | Exon/ introna | c.DNA | Proteinb | Localization | 11OH activity (%)c | Ref. |
|---|---|---|---|---|---|---|
| g.55C>T | e1 | c.55C>T | p.Q19X | Pre-protein | ND | 15 |
| g.96delC | e1 | c.96delC | p.R33GfsX18 | N-term | ND | 6 |
| g.124C>T | e1 | c.124C>T | p.P42S | N-term | 15 | 2 |
| g.128G>A | e1 | c.128G>A | p.R43Q | N-term | 30–50 | 16,21 |
| g.635T>G | e2 | c.248T>G | p.L83S | A-B connecting region | 3 | 16 |
| g.651G>A | e2 | c.264G>A | p.M88I | A-B connecting region | 73 | |
| g659_660dup | e2 | c.272_273dup | p.M92X | A-B connecting region | ND | 32 |
| g.668C>T | e2 | c.281C>T | p.P94L | B helix | 0–2 | 13,33 |
| g.702_729del | e2 | c.315_342del | p.S105SfsX19 | B-C loop | ND | 7 |
| g.733T>G | e2 | c.346T>G | p.W116G | B-C loop | 0 | |
| g.734G>A | e2 | c.347G>A | p.W116X | B-C loop | 0 | 12 |
| g.735G>C | e2 | c.348G>C | p.W116C | B-C loop | 3 | 14 |
| g.745_749dup | e2 | c.358_362dup | p.H122DfsX13 | B-C loop | ND | 34 |
| g.761A>G | e2 | c.374A>G | p.H125R | B-C loop | 30–50 | 16 |
| g.772G>A | e2 | c.385G>A | p.V129M | B-C loop | 0 | 7 |
| g.2593A>C | e3 | c.397A>C | p.N133H | B-C loop | 17 | 2 |
| g.2599C>T | e3 | c.403C>T | p.P135S | C helix | 2–10 | 16 |
| g.2611T>C | e3 | c.415T>C | p.F139L | C helix | 30–50 | 16 |
| g.2617C>T | e3 | c.421C>T | p.R141X | C helix | ND | 35 |
| g.2669T>C | e3 | c.473T>C | p.L158P | D helix | 3 | 16 |
| g.2672C>T | e3 | c.476C>T | p.P159L | D helix | 40 | |
| g.2677_2679del | e3 | c.481_483del | p.V161del | D helix | ND | 35 |
| g.2690C>A | e3 | c.494C>A | p.A165D | D helix | 2 | |
| g.2716A>T | e3 | c.520A>T | p.K174X | D-E loop | ND | 6 |
| g.2782A>G | e3 | c.586A>G | p.T196A | E helix | 30–50 | 16 |
| g.2807G>T | i3 | c.595 + 16G>T | p.? | ND | 9 | |
| g.3073G>A | e4 | c.740G>A | p.W247X | G helix | ND | 7 |
| g.3093_3110del | e4 | c.760_777del | p.K254_A259del | G helix | 0 | |
| g.3126C>T | e4 | c.793C>T | p.Q265X | G helix | ND | 10 |
| g.3132G>A | e4 | c.799G>A | p.? | G helix | ND | 20 |
| g.3132G>C | e4 | c.799G>C | p.G267R | G helix | ND | 19 |
| g.3137G>T | i4 | c.799 + 5G>T | p.? | ND | 36 | |
| g.3419G>A | e5 | c.800G>A | p.G267D | G helix | ND | 19 |
| g.3515T>C | e5 | c.896T>C | p.L299P | H-I loop | 1 | 14,17 |
| g.3536C>T | e5 | c.917C>T | p.A306V | I helix | ND | 24 |
| g.3559G>C | e5 | c.940G>C | p.G314R | I helix | 0 | 10 |
| g.3571A>C | e5 | c.952A>C | p.T318P | I helix | ND | 24 |
| g.3572C>T | e5 | c.953C>T | p.T318M | I helix | 0 | 6 |
| g.3572C>T | e5 | c.953C>G | p.T318R | I helix | ND | 15 |
| g.3573G>C | e5 | c.954G>C | p.? | ND | 37 | |
| g.3573G>A | e5 | c.954G>A | p.? | ND | 38 | |
| g.3574G>A | i5 | c.954 + 1G>A | p.? | ND | 15 | |
| g.3574G>C | i5 | c.954 + 1G>C | p.? | ND | 39 | |
| g.3935G>A | i5 | c.955–1G>A | p.? | ND | 19 | |
| g.3937C>T | e6 | c.956C>T | p.T319M | I helix | 37 | 2 |
| g.3942T>G | e6 | c.961T>G | p.F321V | I helix | ND | 21 |
| g.3973C>T | e6 | c.992C>T | p.A331V | I helix | 0 | 7 |
| g.3993C>T | e6 | c.1012C>T | p.Q338X | J helix | ND | 6 |
| g.4002C>A | e6 | c.1021C>A | p.R341S | J helix | ND | 21 |
| g.4047C>T | e6 | c.1066C>T | p.Q356X | J-K loop | ND | 6 |
| g.4077C>T | e6 | c.1096C>T | p.R366C | K helix | 38 | |
| g.4084C>A | e6 | c.1103C>A | p.A368D | K helix | 1 | 13 |
| g.4093A>G | e6 | c.1112A>G | p.E371G | K helix (ERR triad) | 0 | 7 |
| g.4102G>A | e6 | c.1121G>A | p.R374Q | K helix (ERR triad) | 0 | 6 |
| g.4530C>G | e7 | c.1150C>G | p.R384G | K-L loop | ND | 18 |
| g.4531G>A | e7 | c.1151G>A | p.R384Q | K-L loop | 0 | 6 |
| g.4537C>T | e7 | c.1157C>T | p.A386V | K-L loop | ND | 21 |
| g.4559_4560dup | e7 | c.1179_1180dup | p.N394RfsX37 | K-L loop | ND | 40 |
| g.4561delA | e7 | c.1181delA | p.N394TfsX36 | K-L loop | ND | 41 |
| (Continued) | ||||||
Table 1A.
Continued
| g.DNA | Exon/ introna | c.DNA | Proteinb | Localization | 11OH activity (%)c | Ref. |
|---|---|---|---|---|---|---|
| g.4660A>G | e8 | c.1201A>G | p.T401A | K-L loop | 60 | |
| g.4669dup | e8 | c.1210dup | p.R404Pfs×18 | K-L loop | ND | 20 |
| g.4728T>G | e8 | c.1269T>G | p.Y423X | K-L loop | ND | 2 |
| g.4739G>A | e8 | c.1280G>A | p.R427H | K-L loop (ERR triad) | ND | 19 |
| g.4772_4774del | e8 | c.1313_1315del | p.F438del | K-L loop | 0 | 14 |
| g.4781T>G | e8 | c.1322T>G | p.V441G | K-L loop | 0 | 6 |
| g.4790G>A | e8 | c.1331G>A | p.G444D | Cys pocket | ND | 23 |
| g.4801C>T | e8 | c.1342C>T | p.R448C | Cys pocket | 0 | 7 |
| g.4802G>A | e8 | c.1343G>A | p.R448H | Cys pocket | 0 | 6,11 |
| g.4817G>A | e8 | c.1358G>A | p.R453Q | L helix | 1 | 32 |
| g.4841T>C | e8 | c.1382T>C | p.L461P | L helix | ND | 22 |
| g.4849_4851dup | e8 | c.1390_1392dup | p.L464dup | L helix | 0 | 7 |
| g.4861A>G | i8 | c.1398 + 4A>G | p.? | ND | 37 | |
| g.5395T>C | e9 | c.1466T>C | p.L489S | C-term | ND | 3 |
The location of the first nucleotide used for g.DNA and c.DNA numbering is the A of the ATG translation initiation codon of the reference sequence [GenBank NC_000008 (CYP11B1 g.DNA), GenBank NM_000497 (CYP11B1 c.DNA)]. Mutations characterized in the report are given in bold letters. ND, not determined.
″e″ denotes exon, and ″i″ denotes intron.
p.? denotes mutations that produce an aberrant splicing, and thus, result in an alteration of the normal protein.
11OH, 11β-hydroxylase activity.
Herein, we have characterized seven novel CYP11B1 mutations identified in three patients with classic 11OHD, two patients with a mild form (nonclassic) of the disease, and three heterozygote carriers for CYP11B1 mutations. To prove the disease-causing effect of the novel CYP11B1 variants, the mutations were functionally characterized using an in vitro expression system. The results of the in vitro analysis confirmed the molecular diagnosis of 11OHD in five patients and allowed the 11OHD carrier status to be established in the other three individuals.
Patients and Methods
Patients with classic CYP11B1 deficiency
Patient 1. p.[W116G]+[R448H]
The male patient of Eastern German origin first presented 34 yr ago at the age of 2 yr when admitted with vomiting. Signs of precocious pseudopuberty were noted, including pubic hair, penile growth, and accelerated bone age (BA) of 7 yr. He was mildly hypertensive (110/85 mm Hg). The history revealed that the penile growth, growth acceleration, and pubic hair development had started at age 11 months. He was treated with prednisolone until the age of 22 yr, when dexamethasone was introduced. His final height was well within the normal range (182 cm). At present, he is mildly overweight (body mass index, 26.87 kg/m2) and normotensive (100/70 mm Hg). A current clinical assessment revealed normal sexual function, normal sperm count, and androgens in the normal male range.
Patient 2. p.[Q19X]+[A165D]
The male patient of British Caucasian origin was diagnosed 41 yr ago at the age of 21 months. He presented with enlarged penis, pubic hair, and acne. At diagnosis he was hypertensive (130/90 mm Hg; 140/90 mm Hg). Currently he receives glucocorticoid treatment with prednisolone and antihypertensive therapy with a combination of spironolactone, amiloride, and furosemide.
Patient 3. p.[K254_A259del]+[K254_A259del]
The 13-yr-old patient of Kuwaiti origin was born with an apparently male genital phenotype. It is reported that shortly thereafter he was diagnosed as suffering from CAH. The patient was commenced on hydrocortisone and fludrocortisone replacement and was reared as a boy. At age 11 yr, he was hypertensive (140/100 mm Hg), with a height of 141.1 cm [−1.74 sd score (SDS)] and body mass index of 19.39 kg/m2 (+0.28 SDS). He had a BA of 15.5 yr. On examination, he had a 6-cm stretched phallus with penoscrotal hypospadias, fused rugose scrotum with no palpable gonads, and internal female genitalia. Chromosome analysis established the diagnosis of 46,XX disordered sex development. The patient is now on hydrocortisone replacement and has been gonadectomized.
Patients with nonclassic CYP11B1 deficiency
Patient 4. p.[M88I]+[R384Q]
The boy (46,XY karyotype) from Spain presented at age 10.3 yr with precocious puberty. Physical examination revealed pubic hair stage P3, genital stage G3, a testicular volume of 8 ml, and acne. He was normotensive. The parents reported that they had noted penile growth continuously since birth. His height was 159.2 cm (+3.26 SDS) with an advanced BA of 14 yr. Blood pressure was within the normal range. Basal and cosyntropin-stimulated 11-deoxycortisol levels were elevated at 114 and 135 nmol/liter, respectively (normal values, <11.6 and <23.1 nmol/liter).
Patient 5. p.[P159L]+[P159L]
The female patient (46,XX karyotype) of Eastern German origin presented 33 yr ago at the age of 7 yr with premature pubarche, but no further signs of androgen excess such as virilization of the external genitalia or growth acceleration. The diagnosis of non-salt wasting CAH was established, and the patient was treated with prednisolone. Menarche occurred at age 13.5 yr, followed by regular periods. She had four uncomplicated pregnancies with normal outcome (three girls, one boy).
Carriers for CYP11B1-deficient alleles
Patient 6. p.[P159L]+[=]
The girl from Spain presented at age 7.9 yr with premature pubarche and slightly advanced BA of 8.8 yr. Blood pressure was normal (110/70 mm Hg). Biochemical analysis showed a normal basal 11-deoxycortisol of 2.7 nmol/liter (normal, <11.6 nmol/liter), but increased 11-deoxycortisol of 52.1 nmol/liter (normal, <23.1 nmol/liter) 60 min after cosyntropin administration. Basal and stimulated serum concentrations of 17-hydroxyprogesterone (17OHP) [basal, 0.8 nmol/liter (0.2–5.2 nmol/liter); stimulated, 5.3 nmol/liter (<10.0 nmol/liter)] and androstenedione [basal, 0.9 nmol/liter (0.4–1.7 nmol/liter); stimulated, 1.4 nmol/liter (<3.4 nmol/liter)] were in the normal range as was the cortisol response to cosyntropin.
Patient 7. p.[R366C]+[=]
A 24-yr-old woman (46,XX karyotype) of Spanish origin was referred because of hirsutism. She was normotensive (130/70 mm Hg). Baseline 11-deoxycortisol was in the upper normal range [10.4 nmol/liter (normal, <11.6 nmol/liter)], and serum androstenedione was increased [33.9 nmol/liter (normal range, 1.4–11.9 nmol/liter)]. A cosyntropin stimulation test showed a normal response of 17OHP with 3.2 nmol/liter (1.8–7.0 nmol/liter) at baseline and 6.3 nmol/liter (<10.0 nmol/liter) 60 min after ACTH, thereby ruling out 21-hydroxylase deficiency.
Patient 8. p.[T401A]+[=]
The girl from Spain presented at the age of 9.1 yr with accelerated growth. Her height was 148.7 cm (+2.46 SDS), and she had an advanced BA of 11 yr. Her clinical appearance was fully prepubertal (B1, P1); blood pressure was normal (106/60 mm Hg). Basal and stimulated 11-deoxycortisol concentrations were slightly elevated [13.3 nmol/liter (normal, <11.6 nmol/liter); and 29.5 nmol/liter (normal, <23.1 nmol/liter), respectively]. Basal androstenedione was high normal (1.7 nmol/liter; normal range, 0.4–1.7 nmol/liter). The 17OHP response to cosyntropin was also normal [basal, 2.5 nmol/liter (normal range, 0.2–5.2 nmol/liter); stimulated, 7.3 nmol/liter (normal, <10.0 nmol/ liter)]. She had her menarche at 13 yr and achieved a final height of 161.4 cm (3.4 cm above midparental target height).
Molecular genetic analysis of the CYP11B1 gene
The molecular genetic analysis of the CYP11B1 gene was carried out after taking informed consent. DNA was extracted from peripheral blood leukocytes following a standard procedure. The coding sequence of the CYP11B1 gene including exon-intron boundaries was amplified in three nonoverlapping fragments as previously described (11,12). Direct sequencing was carried out using an automated ABI3730XL Sequencer (Applied Biosystems Inc., Foster City, CA). Sequences were analyzed using the Staden Package v.4.1 software (http://staden.sourceforge.net). Southern blotting was adapted (9), digesting 5 μg of DNA with BamHI restriction enzyme. Sequence variants were designated according to Human Genome Variation Society recommendations (www.hgvs.org/rec.html) using the reference sequences GenBank NC_000008 (g.DNA), GenBank NM_000497 (c.DNA), and GenBank NP_000488.3 (protein).
Site-directed mutagenesis, transient transfection, enzymatic activity assays, and enzyme kinetics
A pcDNA3.1 expression vector construct with the CYP11B1 cDNA as insert (pcDNA3.1-CYP11B1 construct) was used as previously described (13). Site-directed mutagenesis was performed using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene, Amsterdam, The Netherlands). Insertion of the mutations and the integrity of the insert were checked by direct sequencing. Approximately 3.5 × 105 COS7 cells were plated 24 h before transfection. Cells were transiently transfected with 1 μg of each pcDNA3.1-CYP11B1 construct, 0.5 μg of adrenodoxin (pECE-Adx), and 0.5 μg of Adx reductase (pECE-ADR) expression vectors (kindly provided by Professor W. L. Miller, Department of Pediatrics, University of California, San Francisco, CA), using Fugene-HD transfection reagent according to the manufacturer’s protocol (Roche Applied Sciences, Indianapolis, IN). COS7 cells were incubated with the transfection reagents for 24 h at 37 C in DMEM supplemented with 200 mm glutamine and 10% fetal calf serum, followed by a further 24-h incubation in fresh full DMEM media.
The kinetic constants of CYP11B1 in intact COS7 cells were determined 48 h after transfection. The cells were incubated for 90 min at 37 C with 1000 μl DMEM containing 5 μmol/liter 18β-glycyrrhetinic acid (an 11β-hydroxysteroid dehydrogenase inhibitor to inhibit the 11β-hydroxysteroid dehydrogenase type 2 activity endogenous to COS7 cells, which otherwise facilitates the further conversion of the reaction product cortisol to cortisone), 0.2 μCi 3H-labeled 11-deoxycortisol, and 2, 5, 10, or 15 μmol/liter unlabeled 11-deoxycortisol. Steroids were extracted with 5 ml dichloromethane, concentrated by evaporation at 55 C, and separated by thin-layer chromatography on PE SIL G/UV silica gel plates (Whatman, Maidstone, Kent, UK) using a 300:20:1 dichloromethane:methanol:water solvent system. Substrates and conversion products were identified by comparison with comigration of unlabeled reference steroids and quantified using a Bioscan 2000 image analyzer (Lablogic, Sheffield, UK). All assays were performed in at least three independent triplicate experiments, and data are presented as means ± sem. Kinetic parameters were established by nonlinear regression, using the Michaelis-Menten equation to determine the Michaelis-Menten constants Km and Vmax. Catalytic efficiency was defined as the ratio Vmax/Km expressed as percentage of wild-type. Mutations with undetectable activity under the above conditions were incubated for 24 h at 37 C with 1 ml full DMEM containing 0.2 μCi 3H-labeled 11-deoxycortisol and 250 nmol/liter unlabeled 11-deoxycortisol as previously described (13). Cells were scraped from the plates, resuspended in cold 1xPBS, and sonicated for cell lysation. A Bio-Rad assay (Bio-Rad Laboratories, Hercules, CA) was used for total protein assessment. The 11β-hydroxylase activity of the mutants was expressed as a percentage of substrate conversion in nanomoles per milligram of total protein per minute, defining CYP11B1 wild-type activity as 100%. Enzyme kinetic parameters and enzymatic activity were calculated using the GraphPad Prism software version 4.0 (GraphPad, Inc., San Diego, CA).
To ensure comparable levels of expression and translation of CYP11B1 wild-type and mutant proteins, Western blot analysis was performed using an antihuman-CYP11B rabbit antiserum (kindly provided by Dr. H. Takemori, Department of Molecular Physiological Chemistry, Osaka University Medical School, Osaka, Japan) as previously described (13).
Molecular modeling
The detailed generation of the human CYP11B1 three-dimensional structure model using the x-ray structure of the mammalian cytochrome CYP2C5 (Pdb code 1DT6) as a template has been described previously (13,14). The structural representations were generated using the programs Deep View/Swiss-Pdb Viewer (http://www.expasy.org/spdbv/) and Molsoft ICM Browser Pro (Molsoft L.L.C, La Jolla, CA).
Results
Molecular genetic analysis of the CYP11B1 gene
Seven novel mutations in eight nonrelated individuals were detected (Table 1 and Supplemental Fig. 1). Patient 1 was a heterozygous for the novel T to G transversion at position 733 (g.733T>G, p.W116G) and the known p.R448H mutation (11) on the other allele. Patient 2 was a compound heterozyogous for a novel C to A transversion at position 2690 (g.2690C>A; p.A165D) and a described nonsense mutation (p.Q19X) (15). Patient 3 carried a novel homozygous 18-bp deletion resulting in a six-amino acid in-frame deletion (g.3093_3110del, p.K254_A259del).
Patient 4 was a compound heterozygous for the novel G to A substitution at position 651 (g.651G>T; p.M88I) on the maternal allele and for the severe mutation p.R384Q (6) on the paternal allele. A novel heterozygous C to T transition at nucleotide 2672 was found in the unrelated patients 5 and 6 (g.2672C>T; p.P159L). Patient 5 was homozygous for the p.P159L mutation, whereas patient 6 was a heterozygous carrier for the p.P159L allele. Patient 7 was confirmed to be a heterozygous carrier for a novel C to T transition at position 4077 (g.4077C>T, p.R366C). Patient 8 was also a heterozygous carrier having a novel A to G transition at nucleotide 4660 (g.4660A>G, p.T401A). Partial CYP11B1 gene deletions in the carriers were ruled out by Southern blot analysis.
Functional 11β-hydroxylase in vitro assays
The seven novel CYP11B1 mutations were functionally analyzed using transiently transfected COS7 cells measuring the conversion of 11-deoxycortisol to cortisol. The three mutations detected in patients with classic 11OHD (p.W116G; p.A165D; p.K254_A259del) had absent enzymatic activity.
Only the p.A165D mutation showed some conversion for 11-deoxycortisol to cortisol after incubating these three mutations with a lower 11-deoxycortisol concentration (250 nmol/liter) for 24 h. The conversion rate of p.A165D was not expressed in percentage CYP11B1 wild-type activity because it would overestimate the residual activity.
The other four mutations (p.M88I, p.Pro159Leu, p.R366C, and p.T401A) detected in patients with mild 11OHD and heterozygous carriers resulted in partial 11β-hydroxylase impairment. The p.M88I mutation reduced activity to 39.8 ± 6.4% of wild-type. p.P159L and p.R366C showed a similar impairment with 25.8 ± 3.3% and 23.0 ± 3.1% of wild-type activity, respectively. p.T401A had 37.5 ± 3.8% of the normal 11β-hydroxylase activity (Fig. 1A).
Figure 1.
Comparison of residual 11β-hydroxylase activity of the CYP11B1 variants. A, The activities of the mild mutants are expressed as percentage of wild-type activity (cortisol synthesis rate, 4.7 ± 0.7 nmol/mg protein/min), which is defined as 100%. Values are depicted for the conversion of 11-deoxycortisol to cortisol at a substrate concentration of 10 μmol/liter of unlabeled steroid. Error bars represent the mean ± sem (%). B, Lineweaver-Burk plots of 11β-hydroxylase activity converting 11-deoxycortisol (S) to cortisol assessed by incubation of transiently transfected COS7 cells coexpressing human wild-type (WT) or mutant CYP11B1, and human Adx reductase and Adx with 2–15 μm 11-deoxycortisol and [3H]-11-deoxycortisol. Error bars represent the mean ± sem (%). The p.P159L mutation is not shown because this mutation did not reach substrate saturation under the employed reaction conditions.
Determination of kinetic constants showed similar Km values for p.M88I, p.R366C, and p.T401A with significantly impaired Vmax compared with wild-type (Fig. 1B). The p.P159L mutation did not reach substrate saturation under the established reaction conditions.
Western blot analysis demonstrated that all mutations apart from the p.K254_A259del mutation had translation efficiency similar to wild-type. The p.K254_A259del mutation resulted in a smaller band of decreased intensity (data not shown).
Discussion
Herein, we have characterized three severe and four mild CYP11B1 mutations found in three patients with classic 11OHD, in two patients with nonclassic 11OHD, and in three heterozygous carriers. Apart from the p.P159L mutation, all mutations were detected in different individuals. However, there was no obvious connection between the patient homozygous for the p.P159L from Eastern Germany and the heterozygous carrier from Spain. The in vitro activity of the less severely affected allele was consistent with the clinical phenotype in all affected patients. Diagnostic criteria such as an 11-deoxycortisol response after 1-24ACTH stimulation to differentiate between heterozygous carriers and patients with mild 11OHD are not well established, such as differentiation by 17OHP response to 1-24ACTH in 21-hydroxylase deficiency. The two genetically proven carriers studied had normal or mildly elevated basal 11-deoxycortisol levels and showed a variable increase of 11-deoxycortisol to 1-24ACTH stimulation. This variability has previously been reported in patients with mild or nonclassic 11OHD (2).
Functional effects of CYP11B1 mutations
In vitro 11β-hydroxylase activity of less than 5% can be considered severe and is most likely associated with classic 11OHD (6,7,10,13,14,16,17). However, a significant number of detected CYP11B1 missense mutations have not been functionally analyzed yet (15,18,19,20,21,22,23,24) (Table 1), which does not allow for exact phenotype prediction.
We demonstrated a partially impaired CYP11B1 activity in four of the novel mutations, which are associated with nonclassic 11OHD. Until now, only three mild CYP11B1 mutations detected in three patients with nonclassic 11OHD have been functionally analyzed (2). The residual 11β-hydroxylase enzyme activity of these previously described mutations (p.P42S, p.N133H, and p.T319M) ranged from 15 to 40% of wild-type activity. This is a similar magnitude as the in vitro activity of our p.M88I, p.P159L, p.R366C, and p.T401A mutations. Four other CYP11B1 variants (p.R43Q, p.P135S, p.F139L, and p.T196A) resulted in approximately 30–50% residual CYP11B1 activity (16). These variants were found in a cohort study analyzing the effect of CYP11B1 variants on the etiology of hypertension, and no data on the sex hormone status are available. All previously described in vitro studies on mild CYP11B1 mutations employed significantly longer incubation times for the analysis of the p.P42S, p.N133H, and p.T319M mutations (15–24 h) (2) and for the analysis of the p.R43Q, p.P135S, p.F139L, and p.T196A variants (24 h) (16). This potentially overestimates the in vitro CYP11B1 activity of mutations. Using similar incubation times for our mild CYP11B1 mutations resulted in a significant overestimation with an increase to 55–63% of wild-type activity (data not shown). The residual activity of mild mutations described in this paper (p.M88I, p.P159L, p.R366C, and p.T401A) are within the well-established range of 21-hydroxylase mutations causing nonclassic CAH with a residual 21-hydroxylase activity of about 20–50% (25,26).
Putative effects of severe CYP11B1 mutations
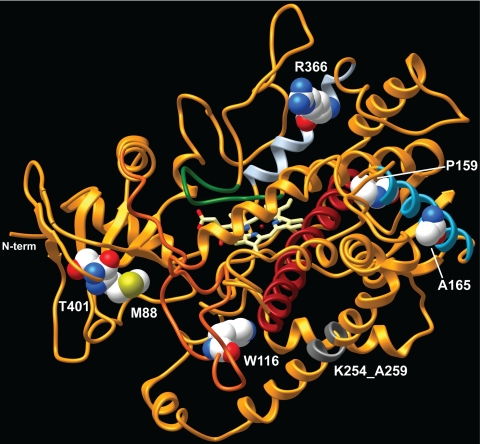
The tryptophan 116 residue is highly conserved in the CYP11 family across species (Fig. 2), and two severe mutations at codon 116 have already been described (12,14). Trp116 lies in the B-C loop (Fig. 3), which contains the substrate recognition site 1, one of six postulated substrate recognition sites (27). The side chains of Trp116 and Leu113 interact with Phe223 and Trp247. Thus, the change from the aromatic tryptophan to the small aliphatic glycine might alter the conformation of the substrate access channel in a similar fashion as the p.W116C mutation (14). This alteration might impact on substrate access and product release, thereby abolishing CYP11B1 function.
Figure 2.
Multiple CYP11B1 clustalW alignments. The M88, W116, P159, A165, R366, and T401 residues of CYP11B1 and corresponding amino acids of the aligned CYPs are shaded and marked by a triangle. A, Alignment of human CYP11B1 with human CYP11B2 and CYP11A1, the mouse and rat orthologs. B, Alignment of different human steroidogenic CYP enzymes. C, Alignment of mammalian CYP11B1 with different cytochrome P450 type 2 enzymes.
Figure 3.
Total view on the three-dimensional molecular model of CYP11B1. N-term, Amino terminus.; The B-C loop is colored in dark orange, the D helix in blue, the I helix in red, and the K helix in light blue. Amino acid residues affected by missense mutations are shown in ball representation; the region affected by the six-amino acid deletion in the G helix is depicted in gray.
Alanine 165 is localized in the D helix of CYP11B1 (Fig. 3) and conserved in the CYP11 family, including members of hepatic CYP2C monooxygenases (Fig. 2C), with the exception of human CYP11A1 (Fig. 2A). No acidic amino acids such as aspartic acid are present at the corresponding residue in aligned cytochrome P450 enzymes (CYP) (Fig. 2). The change to Asp165 does not lead to obvious steric problems in silico with neighboring residues, but it changes the polarity and structure of the protein surface. This change will most likely not affect redox-partner binding because it takes place on the opposite side of the CYP11B1 protein. Further conclusions on the effect of the p.A165D mutation on CYP11B1 from the comparison with other steroidogenic CYP enzymes are difficult. No mutations in the D helix of CYP17A1 have been reported, and only two mutations without functional data available in CYP21A2 (p.V139W; p.C147R) have been described to result in salt-wasting or simple-virilizing and nonclassic 21-hydroxylase deficiency, respectively (28).
The novel in-frame deletion of the six amino acid residues 254 to 259 affects the G helix (Fig. 3). In general, the F and G helices and F-G-loop together with the B-C loop control access to the active site of mammalian CYP enzymes, with he F and G helices and F/G-loop forming the roof of the substrate-binding pocket (29). Therefore, a deletion within this structure can be predicted to result in an enzyme with absent CYP11B1 function, consistent with our patient’s clinical phenotype and absent in vitro CYP11B1 activity.
Putative effects of mild CYP11B1 mutations
The methionine 88 residue localized in the N-terminal region is not highly conserved and an isoleucine is present at the corresponding position of murine 11β-hydroxylase (Fig. 2). M88 is found in a region connecting the A and B helices (Fig. 3). It lies in a hidden cove on the surface of CYP11B1 and forms an H-bond with E383, which is localized in a β sheet of the K-L loop. The substitution for isoleucine does not lead to any major structural changes. There is only a minor polarity change on the surface of CYP11B1. Therefore, it is unsurprising that the p.M88I mutation only leads to mild reduction of in vitro 11β-hydroxylase activity.
Proline 159 is conserved within the CYP11 family and is found in CYP21A2, CYP3A4, and CYP3A7 (Fig. 2). However, various types of amino acids are present in other CYP enzymes ranging from hydrophobic, over polar to acidic and basic amino acids in other CYP enzymes. P159 is localized in the D helix opposite the L helix. A change to leucine would not affect the protein surface, neither predicted H-bond formation nor interference with residues in the L helix. However, the presence of a leucine residue at this position (L159) removes a small kink from the D helix. This might lead to a minor change in orientation of the E helix but without obvious direct effect on the active center. Overall, these in silico changes are consistent with the partially preserved in vitro activity of p.P159L.
The arginine 366 residue is localized in the K helix (Fig. 3) and conserved within the CYP11B family. A lysine residue is present at the corresponding position of CYP11A1 enzymes (Fig. 2). This lysine residue (K377 in human CYP11A1) appears to be involved in binding the electron providing factor Adx (30). The side chain of R366 faces toward the protein surface, and a change to cysteine (p.R366C) eliminates a positive surface charge. This mutation leaves a cove on the surface, which most likely impacts on Adx binding. Other residues are involved in the CYP-Adx interaction (30,31), including amino acid residues R453 and R454, which remain intact. A mutation of residue R453 (p.R453Q) resulted in almost absent CYP11B1 activity (32) emphasizing the importance of these two arginine residues (R453, R454). The change to cysteine at position 366 might also lead to an interaction with T359. Threonine 359 is localized in the J-K loop, but this proposed new interaction has no obvious effect on the active center. Therefore, p.R366C might affect the interaction with the electron providing factor Adx and thereby partially impair CYP11B1 function.
Although the threonine 401 can be found in a huge number of CYP enzymes, valine residues are present at the corresponding residue in CYP3A4 and CYP3A7 (Fig. 2). The effect of the p.T401A mutation appears less dramatic as expected from the clustalW alignment. Threonine 401 is localized in between the K and L helices. The side chain faces toward the surface providing a surface hydroxyl group. The change to alanine 401 leads to loss of this polar group and leaves a small cove on the surface, but it does not lead to major steric changes. The only minor change is the possible loss of a weak interaction with arginine 80. Therefore, the in silico findings are consistent with mild impairment of the CYP11B1 in vitro activity of the p.T401A mutation.
In conclusion, we have confirmed the inactivating nature of the seven novel CYP11B1 mutations. Furthermore, four CYP11B1 mutations with significant residual activity associated with nonclassic 11OHD were characterized. This doubles the number of reported mutations responsible for mild 11OHD. Our findings emphasize the importance of considering mild 11OHD in patients presenting with signs and symptoms similar to nonclassic 21-hydroxylase deficiency. Therefore, our data are essential for the clinical and genetic counseling, helping to estimate the severity of clinical disease expression in 11OHD.
Supplementary Material
Footnotes
This work was supported by the European Society for Pediatric Endocrinology (Short-term Research Fellowship to S.P.), the European Community’s Seventh Framework Program (Marie Curie Intra-European Fellowship for Career Development under grant agreement PIEF-GA-2008-221058, to N.R.; Collaborative Research Project EuroDSD), the Medical Research Council UK (Program Grant 0900567, to W.A.), and the Wellcome Trust (Clinician Scientist Fellowship GR079865MA, to N.K.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 20, 2010
Abbreviations: Adx, Adrenodoxin; BA, bone age; CAH, congenital adrenal hyperplasia; DOC, 11-deoxycorticosterone; 11OHD, 11β-hydroxylase deficiency; 17OHP, 17-hydroxyprogesterone; SDS, sd score.
References
- White PC, Curnow KM, Pascoe L 1994 Disorders of steroid 11 β-hydroxylase isozymes. Endocr Rev 15:421–438 [DOI] [PubMed] [Google Scholar]
- Joehrer K, Geley S, Strasser-Wozak EM, Azziz R, Wollmann HA, Schmitt K, Kofler R, White PC 1997 CYP11B1 mutations causing non-classic adrenal hyperplasia due to 11 β-hydroxylase deficiency. Hum Mol Genet 6:1829–1834 [DOI] [PubMed] [Google Scholar]
- Peters CJ, Nugent T, Perry LA, Davies K, Morel Y, Drake WM, Savage MO, Johnston LB 2007 Cosegregation of a novel homozygous CYP11B1 mutation with the phenotype of non-classical congenital adrenal hyperplasia in a consanguineous family. Horm Res 67:189–193 [DOI] [PubMed] [Google Scholar]
- Speiser PW, White PC 2003 Congenital adrenal hyperplasia. N Engl J Med 349:776–788 [DOI] [PubMed] [Google Scholar]
- Mornet E, Dupont J, Vitek A, White PC 1989 Characterization of two genes encoding human steroid 11 β-hydroxylase (P-450(11) β). J Biol Chem 264:20961–20967 [PubMed] [Google Scholar]
- Curnow KM, Slutsker L, Vitek J, Cole T, Speiser PW, New MI, White PC, Pascoe L 1993 Mutations in the CYP11B1 gene causing congenital adrenal hyperplasia and hypertension cluster in exons 6, 7, and 8. Proc Natl Acad Sci USA 90:4552–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley S, Kapelari K, Jöhrer K, Peter M, Glatzl J, Vierhapper H, Schwarz S, Helmberg A, Sippell WG, White PC, Kofler R 1996 CYP11B1 mutations causing congenital adrenal hyperplasia due to 11 β-hydroxylase deficiency. J Clin Endocrinol Metab 81: 2896–2901 [DOI] [PubMed] [Google Scholar]
- Portrat S, Mulatero P, Curnow KM, Chaussain JL, Morel Y, Pascoe L 2001 Deletion hybrid genes, due to unequal crossing over between CYP11B1 (11β-hydroxylase) and CYP11B2 (aldosterone synthase) cause steroid 11β-hydroxylase deficiency and congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:3197–3201 [DOI] [PubMed] [Google Scholar]
- Hampf M, Dao NT, Hoan NT, Bernhardt R 2001 Unequal crossing-over between aldosterone synthase and 11β-hydroxylase genes causes congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:4445–4452 [DOI] [PubMed] [Google Scholar]
- Kuribayashi I, Nomoto S, Massa G, Oostdijk W, Wit JM, Wolffenbuttel BH, Shizuta Y, Honke K 2005 Steroid 11-β-hydroxylase deficiency caused by compound heterozygosity for a novel mutation, p.G314R, in one CYP11B1 allele, and a chimeric CYP11B2/CYP11B1 in the other allele. Horm Res 63:284–293 [DOI] [PubMed] [Google Scholar]
- White PC, Dupont J, New MI, Leiberman E, Hochberg Z, Rösler A 1991 A mutation in CYP11B1 (Arg-448–His) associated with steroid 11 β-hydroxylase deficiency in Jews of Moroccan origin. J Clin Invest 87:1664–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki Y, Kawamoto T, Mitsuuchi Y, Miyahara K, Toda K, Orii T, Imura H, Shizuta Y 1993 A nonsense mutation (TGG [Trp116]–>TAG [Stop]) in CYP11B1 causes steroid 11 β-hydroxylase deficiency. J Clin Endocrinol Metab 77:1677–1682 [DOI] [PubMed] [Google Scholar]
- Krone N, Grischuk Y, Müller M, Volk RE, Grötzinger J, Holterhus PM, Sippell WG, Riepe FG 2006 Analyzing the functional and structural consequences of two point mutations (P94L and A368D) in the CYP11B1 gene causing congenital adrenal hyperplasia resulting from 11-hydroxylase deficiency. J Clin Endocrinol Metab 91:2682–2688 [DOI] [PubMed] [Google Scholar]
- Krone N, Riepe FG, Götze D, Korsch E, Rister M, Commentz J, Partsch CJ, Grötzinger J, Peter M, Sippell WG 2005 Congenital adrenal hyperplasia due to 11-hydroxylase deficiency: functional characterization of two novel point mutations and a three-base pair deletion in the CYP11B1 gene. J Clin Endocrinol Metab 90:3724–3730 [DOI] [PubMed] [Google Scholar]
- Merke DP, Tajima T, Chhabra A, Barnes K, Mancilla E, Baron J, Cutler Jr GB 1998 Novel CYP11B1 mutations in congenital adrenal hyperplasia due to steroid 11 β-hydroxylase deficiency. J Clin Endocrinol Metab 83:270–273 [DOI] [PubMed] [Google Scholar]
- Barr M, MacKenzie SM, Wilkinson DM, Holloway CD, Friel EC, Miller S, MacDonald T, Fraser R, Connell JM, Davies E 2006 Functional effects of genetic variants in the 11β-hydroxylase (CYP11B1) gene. Clin Endocrinol (Oxf) 65:816–825 [DOI] [PubMed] [Google Scholar]
- Riedl S, Nguyen HH, Clausmeyer S, Schulze E, Waldhauser F, Bernhardt R 2008 A homozygous L299P mutation in the CYP11B1 gene leads to complete virilization in 46,XX individuals with 11-β-hydroxylase deficiency. Horm Res 70:145–149 [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Yamada M, Ogawa H, Igarashi Y 1995 Missense mutation in CYP11B1 (CGA[Arg-384]–>GGA[Gly]) causes steroid 11 β-hydroxylase deficiency. Eur J Endocrinol 132:286–289 [DOI] [PubMed] [Google Scholar]
- Skinner CA, Rumsby G, Honour JW 1996 Single strand conformation polymorphism (SSCP) analysis for the detection of mutations in the CYP11B1 gene. J Clin Endocrinol Metab 81:2389–2393 [DOI] [PubMed] [Google Scholar]
- Soardi FC, Penachioni JY, Justo GZ, Bachega TA, Inácio M, Mendonça BB, de Castro M, de Mello MP 2009 Novel mutations in CYP11B1 gene leading to 11 beta-hydroxylase deficiency in Brazilian patients. J Clin Endocrinol Metab 94:3481–3485 [DOI] [PubMed] [Google Scholar]
- Amin HK, Hoeppner W, Shaarawy M, Barakat M 2002 Disease: congenital adrenal hyperplasia. Hum Genet 110:295 [Google Scholar]
- Chang SH, Lee HH, Wang PJ, Chen JH, Chu SY 2004 Congenital adrenal hyperplasia with 11 β-hydroxylase deficiency. J Formos Med Assoc 103:860–864 [PubMed] [Google Scholar]
- Motaghedi R, Betensky BP, Slowinska B, Cerame B, Cabrer M, New MI, Wilson RC 2005 Update on the prenatal diagnosis and treatment of congenital adrenal hyperplasia due to 11β-hydroxylase deficiency. J Pediatr Endocrinol Metab 18:133–142 [DOI] [PubMed] [Google Scholar]
- Lee HH, Won GS, Chao HT, Lee YJ, Chung BC 2005 Novel missense mutations, GCC [Ala306]–>GTC [Val] and ACG [Thr318]–>CCG [Pro], in the CYP11B1 gene cause steroid 11β-hydroxylase deficiency in the Chinese. Clin Endocrinol (Oxf) 62:418–422 [DOI] [PubMed] [Google Scholar]
- White PC, Speiser PW 2000 Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 21:245–291 [DOI] [PubMed] [Google Scholar]
- Krone N, Dhir V, Ivison HE, Arlt W 2007 Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol (Oxf) 66:162–172 [DOI] [PubMed] [Google Scholar]
- Gotoh O 1992 Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem 267:83–90 [PubMed] [Google Scholar]
- Robins T, Carlsson J, Sunnerhagen M, Wedell A, Persson B 2006 Molecular model of human CYP21 based on mammalian CYP2C5: structural features correlate with clinical severity of mutations causing congenital adrenal hyperplasia. Mol Endocrinol 20:2946–2964 [DOI] [PubMed] [Google Scholar]
- Otyepka M, Skopalík J, Anzenbacherová E, Anzenbacher P 2007 What common structural features and variations of mammalian P450s are known to date? Biochim Biophys Acta 1770:376–389 [DOI] [PubMed] [Google Scholar]
- Grinberg AV, Hannemann F, Schiffler B, Müller J, Heinemann U, Bernhardt R 2000 Adrenodoxin: structure, stability, and electron transfer properties. Proteins 40:590–612 [DOI] [PubMed] [Google Scholar]
- Usanov SA, Graham SE, Lepesheva GI, Azeva TN, Strushkevich NV, Gilep AA, Estabrook RW, Peterson JA 2002 Probing the interaction of bovine cytochrome P450scc (CYP11A1) with adrenodoxin: evaluating site-directed mutations by molecular modeling. Biochemistry 41:8310–8320 [DOI] [PubMed] [Google Scholar]
- Krone N, Grötzinger J, Holterhus PM, Sippell WG, Schwarz HP, Riepe FG 2009 Congenital adrenal hyperplasia due to 11-hydroxylase deficiency—insights from two novel CYP11B1 mutations (p.M92X, p.R453Q). Horm Res 72:281–286 [DOI] [PubMed] [Google Scholar]
- Grigorescu Sido A, Weber MM, Grigorescu Sido P, Clausmeyer S, Heinrich U, Schulze E 2005 21-Hydroxylase and 11β-hydroxylase mutations in Romanian patients with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab 90:5769–5773 [DOI] [PubMed] [Google Scholar]
- Skinner CA, Rumsby G 1994 Steroid 11 β-hydroxylase deficiency caused by a five base pair duplication in the CYP11B1 gene. Hum Mol Genet 3:377–378 [DOI] [PubMed] [Google Scholar]
- Sólyom J, RK, Péter F, Homoki J, Sippell WG, Peter M 2001 Clinical, hormonal and molecular genetic characterization of Hungarian patients with 11β-hydroxylase deficiency. J Pediatr Endocrinol 2:37–44 [Google Scholar]
- Andrew M, Barr M, Davies E, Wallace AM, Connell JM, Ahmed SF 2007 Congenital adrenal hyperplasia in a Nigerian child with a novel compound heterozygote mutation in CYP11B1. Clin Endocrinol (Oxf) 66:602–603 [DOI] [PubMed] [Google Scholar]
- Chabre O, Portrat-Doyen S, Vivier J, Morel Y, Defaye G 2000 Two novel mutations in splice donor sites of CYP11B1 in congenital adrenal hyperplasia due to 11β-hydroxylase deficiency. Endocr Res 26:797–801 [DOI] [PubMed] [Google Scholar]
- Penachioni JY, Bachega TASS, Mendonça BB, Castro M, Moreira AC, de Mello MP 2000 Descrição de uma nova mutação no éxon 8 do gene CYP11B1 em uma paciente com deficiência de 11-β-hidroxilase. Gen Mol Biol 23:591 [Google Scholar]
- Bhangoo A, Wilson R, New MI, Ten S 2006 Donor splice mutation in the 11β-hydroxylase (CypllB1) gene resulting in sex reversal: a case report and review of the literature. J Pediatr Endocrinol Metab 19:1267–1282 [DOI] [PubMed] [Google Scholar]
- Helmberg A, Ausserer B, Kofler R 1992 Frame shift by insertion of 2 basepairs in codon 394 of CYP11B1 causes congenital adrenal hyperplasia due to steroid 11 β-hydroxylase deficiency. J Clin Endocrinol Metab 75:1278–1281 [DOI] [PubMed] [Google Scholar]
- Cerame BI, Newfield RS, Pascoe L, Curnow KM, Nimkarn S, Roe TF, New MI, Wilson RC 1999 Prenatal diagnosis and treatment of 11β-hydroxylase deficiency congenital adrenal hyperplasia resulting in normal female genitalia. J Clin Endocrinol Metab 84:3129–3134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.