Abstract
Background & Aims
p38α is a mitogen-activated protein kinase that mediates inflammatory responses, but its role in inflammatory bowel disease (IBD) is unclear. The effects of p38α inhibitors have been inconsisten in animal models and clinical studies of IBD, possibly arising from the different functions of p38α in different tissues or cell types. We investigated the effects of p38α inhibition in myeloid vs. the colonic epithelium.
Methods
We studied mice with myeloid cell-specific and intestinal epithelial cell-specific disruption p38α (LtrLysCre-p38αΔ/Δ mice and VillinCre-p38αΔ/Δ mice), as well as p38β, γ, and δ knockout. Colitis was induced using Dextran Sodium Sulfate (DSS) or 2.4.6-trinitrobenzene sulfonic acid (TNBS).
Results
Mice with myeloid cell-specific deletion of p38α had less inflammation and an improved disease condition, compared with wild-type mice, whereas mice with intestinal epithelial cell-specific deletion of p38α had increased progression of colitis that resulted from disrupted intestinal epithelial homeostasis. The distinct effects of p38α disruption in different tissue types might underlie the unsuccessful therapeutic application of p38 inhibitors to colitis. We found that a γ-secretase inhibitor, which functions opposite that of a p38 inhibitor in the regulation of intestinal epithelial homeostasis, can significantly improve the effects of a p38 inhibitor in reducing colitis.
Conclusion
p38α has distinct functions in mouse myeloid cells vs. colonic epithelium; these differences should be taken into consideration in defining the role of p38α in inflammation and developing p38 inhibitors as therapeutics.
Keywords: p38α, colitis, knockout mice
INTRODUCTION
Inflammatory bowel diseases (IBDs), represented mainly by ulcerative colitis (UC) and Crohn’s disease (CD), are disorders characterized by chronic relapsing inflammation of the gastrointestinal tract 1. Although the precise etiology remains unknown, a widely accepted hypothesis is that commensal intestinal bacteria trigger an inappropriate mucosal immune response that mediates intestinal tissue damage in genetically susceptible individuals 2-5. Inhibition of inflammation should therefore be beneficial for IBD patients; however, current therapies, such as glucocorticoids, provide only transient or marginal effects. Clinical trials and animal studies with chemicals that inhibit inflammatory signaling pathways, such as NF-κB and p38, have not yielded promising results 6.
p38 group mitogen activated protein kinase (MAPK) has four members, with p38α being the prototypic member of this group kinase 7. The functional importance of p38α in inflammatory diseases has been well documented 8. p38α is also assumed to be a major mediator of inflammation in IBD, showing the strongest increase in activity among MAPKs within the inflamed intestinal mucosa of IBD patients 9, 10. However, in clinical trials p38 inhibitors have yielded controversial results 11, 12. In patients with moderate to severe Crohn’s disease, the p38 and JNK inhibitor CNI-1493 has demonstrated clinical improvement 11, while the p38 inhibitor BIRB796 did not in a multicenter trial 12. Similarly, p38 inhibitors in mouse experimental colitis models have yielded contradicting results, with some reporting improvement 13, 14 and others reporting improvement in some parameters but worsening in others 15, 16. The role of the p38 pathway in IBD needs to be clarified before determining whether p38α inhibitors have therapeutic potential in IBD.
Because the function of p38α might be different in different cell types or tissues, we hypothesized that the antiinflammatory effect of p38 inhibition in myeloid cells is actually beneficial while inhibition of p38 in colonic epithelial cells is harmful. Using mice with myeloid cell-specific deletion of p38α and mice with intestinal epithelial cell-specific deletion of p38α, we found that p38α deletion in myeloid cells indeed improves the condition of experimental colitis, while p38α deletion in intestinal epithelial cells increases susceptibility to colitis by inhibiting the differentiation and enhancing the proliferation of colonic epithelial cells. p38α has distinct functions in different cell types, which should be carefully taken into consideration to understand the role of p38α in diseases.
MATERIALS AND METHODS
Experimental animals
LtrLysCre-p38αΔ/Δ mice were described previously 17. p38β, γ, and δ knockout mice were generated by protamine-Cre breeding with their floxed alleles, respectively 18.
Experimental procedures
The detailed methods of mice, colitis induction, colonic injury scoring, proteins and total RNA isolation from inflammatory colonic mucosa, primary intestinal epithelial cells isolation, electrophoretic mobility-shift assay, histology, immunohistochemistry, immunofluorescence, in situ intestinal proliferation analysis, hemoglobin and hematocrit analysis, inhibitors, western blotting analysis, real-time PCR, semi-quantitative RT-PCR analysis, and statistical analysis are described in the Online Supplementary Materials.
RESULTS
Global inhibition of p38α inhibits inflammatory cell infiltration into colitis mucosa but does not improve clinical symptoms
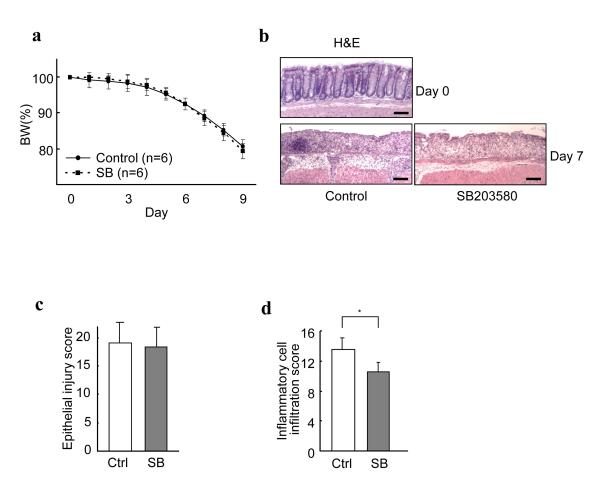
Due to the role of p38α in inflammation, inhibitors of p38α/β have been used to evaluate whether inhibition of p38α could be a useful approach in treating IBD. Unfortunately, the data are controversial 13-16. We also evaluated the effects of a p38α/β inhibitor SB203580 on mouse colitis. Since weightloss is a hallmark of severe intestinal inflammation in mice and is one of the criteria for determining IBD and its severity, we monitored the body weight of mice that were given 3.5% DSS in drinking water for 6 days. SB203580 did not affect body weight loss associated with DSS-induced colitis (Fig. 1a). SB203580 itself has no effect on mouse body weight (Supplementary Fig. 1). Histological examination showed that although the inflammatory cell infiltration into the colon were significantly less in the mice treated with SB203580, the degree of epithelial injury were very similar between control mice and the mice treated with SB203580 (Fig. 1b, c, d). SB203580 indeed inhibits the inflammatory reaction in the colonic mucosa to some extent, but the global inhibition of p38α/β did not reduce susceptibility of colonic mucosa to colitis injury and did not improve clinical results.
Figure 1.
The p38 inhibitor SB203580 inhibits the inflammatory cell infiltration into colonic mucosa during DSS-induced colitis, but does not improve clinical symptoms. (a) No difference in body weight changes during the course of colitis between control and SB203580 (SB)-treated mice. C57Bl/6 mice were treated daily with control vehicle or SB. Mice were given 3.5% DSS in drinking water for 6 days, and body weight was recorded daily. Data are presented as mean ± s.d. (b) More inflammatory cell infiltration is seen in the colonic mucosa of control mice than those treated with SB. Representative photomicrographs of each hematoxylin and eosin (H&E)-stained colons at approximately 30 mm from the anal canal of mice treated with control vehicle or SB203580 at 7 days after the initiation of DSS administration. The section before colitis induction (day 0) is shown as a reference (top panel). Four other mice sets showed similar results. Bars, 100 μm. (c, d) Histological scoring of epithelial injury in colons (c) and inflammatory cell infiltration into colonic tissues of mice (d) treated with control vehicle or SB at 7 days after the initiation of DSS administration. The degree of epithelial injury was similar but the inflammatory cell infiltration was greater in SB-treated mice. The scoring was performed as described in the “Methods” section. Results are presented as mean ± s.d (n = 8 per group from two independent experiments). * p < 0.05.
Because SB203580 not only inhibits p38α but also p38β, and because these two kinases have different functions, we used p38β knockout mice to determine whether inhibition of p38β contributed to the puzzling result observed in SB203580-treated colitis mice (Fig. 1a-d). p38β knockout mice are viable and apparently healthy, and did not show any clinical and pathological differences compared with those in control littermates during colitis (Supplementary Fig. 2a, b). We also examined the effects of p38γ and p38δ on DSS-induced colitis and eliminated their involvement in colitis (Supplementary Fig. 3; the genotyping of these knockout mice are shown in Supplementary Fig. 4).
SB203580 is known as a p38 inhibitor, but it can also inhibit RICK 13, 14. To exclude the contribution of the non-specificity of SB203580 in studying the role of p38 in colitis, we needed to use p38 knockout mice.
Specific deletion of p38α in myeloid cells reduces the disease activity of DSS-induced mouse colitis
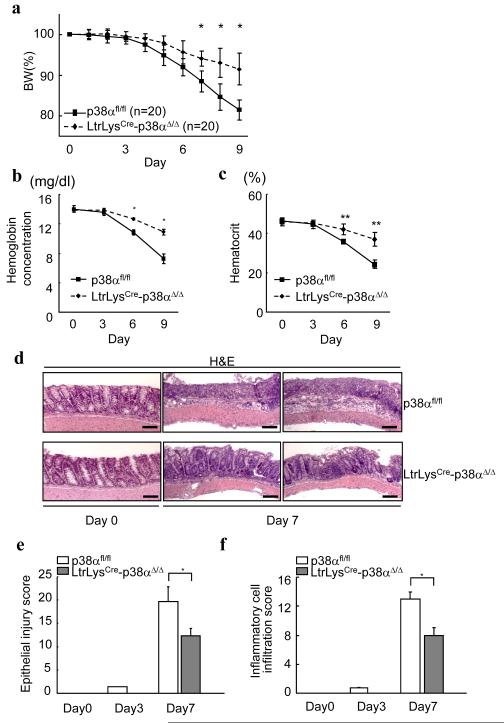
Since p38α has diverse roles in different cells 19, inhibition of p38α in one cell type in the colonic mucosa might be beneficial, while in another it could be harmful. Since p38α in myeloid cells plays a role in inflammatory reactions 17, we hypothesized that the antiinflammatory effects of p38α inhibition in myeloid cells are beneficial in colitis and examined this using mice with myeloid cell (macrophages and neutrophils) specific deletion of p38α (LtrLysCre-p38αΔ/Δ). LtrLysCre-p38αΔ/Δ mice had a significantly smaller body weight reduction in comparison with littermate p38αfl/fl control mice when we feed the mice with DSS (Fig. 2a). Colons after the administration of DSS revealed more severe bleeding in control p38αfl/fl mice relative to LtrLysCre-p38αΔ/Δ mice (Supplementary Fig. 5). Concordant with this, LtrLysCre-p38αΔ/Δ mice showed less anemia at multiple time points, as determined by the measurement of hemoglobin and hematocrit concentration in circulating peripheral blood (Fig. 2b, c). The specific deletion of p38α in myeloid cells reduces the disease activity of DSS-induced colitis.
Figure 2.
Less severity of clinical and pathological parameters of LtrLysCre-p38αΔ/Δ mice during DSS-induced colitis. (a) Mice with p38α wildtype and myeloid deletion (LtrLysCre-p38αΔ/Δ) were given 3.5% DSS in their drinking water for 6 days and body weight was recorded. Data are presented as mean ± s.d. *p < 0.05. (b) Hemoglobin concentration and (c) Hematocrit values of peripheral blood taken at indicated time points after the initiation of DSS administration. Data are presented as mean ± s.d (n = 4 per group). * p < 0.01, ** p < 0.05. (d) Representative photomicrographs of hematoxylin and eosin (H&E)-stained colons of p38αfl/fl and LtrLysCre-p38αΔ/Δ mice at 0 and 7 days after the initiation of DSS administration. No significant differences in the colon appearances between p38αfl/fl and LtrLysCre-p38αΔ/Δ mice at day 0, but p38αfl/fl mice exhibited more severe inflammation at day 7. Similar results were obtained from three other mice sets. Bars, 100 μm. (e, f) Histological scoring of epithelial injury in colons (e) and inflammatory cell infiltration into colonic tissues of mice (f) at 0, 3, and 7 days after the initiation of DSS administration. The scoring algorithm is described in the “Methods” section. Results are presented as mean ± s.d (n = 4 per group). * p < 0.01.
Microscopic analysis showed that the tissue damage in the mucosa was less in LtrLysCre-p38αΔ/Δ mice in comparison with p38αfl/fl, and no significant differences in the colon appearances were observed before DSS administration (Fig. 2d). The severity and extent of glandular mucodepletion, epithelial damage, and inflammatory cell infiltration into the mucosa were analyzed by histopathological scoring. Both epithelial injury scoring and inflammatory cell infiltration scoring were lower in LtrLysCre-p38αΔ/Δ mice compared to control p38αfl/fl mice at days 3 and 7 after colitis induction (Fig.2e, f). The specific deletion of p38α in myeloid cells reduces the inflammatory responses and subsequent colon epithelial damage during DSS-induced colitis.
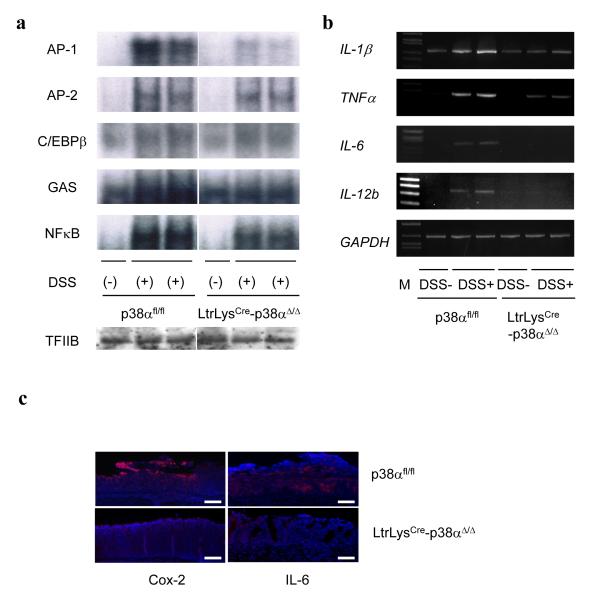
We sought to determine whether there are any differences in the activities of inflammation-related transcriptional factors in the colonic mucosal tissues of LtrLysCre-p38αΔ/Δ mice and control p38αfl/fl mice by comparing the DNA binding activities of several transcriptional factors. DSS activated AP-1, AP-2, C/EBPβ, Interferon-γ activated sequences (GAS), and NF-κB signaling in both LtrLysCre-p38αΔ/Δ mice and control p38αfl/fl mice. The activities of AP-2 and C/EBPβ in colons were similar between LtrLysCre-p38αΔ/Δ mice and control p38αfl/fl mice, while the activation of AP-1, GAS, and NF-κB were less in the colons of LtrLysCre-p38αΔ/Δ mice compared to control p38αfl/fl mice (Fig. 3a). Next, the amounts of mRNAs encoding proinflammatory cytokines (IL-1β, TNFα, IL-6, and IL-12 p40) were examined, and those were found to be less in LtrLysCre-p38αΔ/Δ mice colons (Fig. 3b). Cox-2 and IL-6 protein-expressing cells were much less abundant in the colons of LtrLysCre-p38αΔ/Δ mice compared to control p38αfl/fl mice in the mucosal and submucosal inflammatory lesions (Fig. 3c), suggesting that p38α in myeloid cells directly or indirectly regulates inflammatory responses in colonic mucosa during the course of DSS-induced colitis.
Figure 3.
(a) Induction of inflammation-related transcriptional factor activities in the colons of DSS-treated mice. Nuclear extracts of colonic mucosa prepared 0 and 9 days after initiation of DSS administration were analyzed for AP-1, AP-2, C/EBPβ, GAS (IFN-γ-activated sequences), and NF-κB DNA-binding activities (Electrophoretic mobility-shift assay; EMSA). Equal protein recovery in nuclear extracts was verified by immunoblotting with antibodies against TFIIB. Results using two independent p38αfl/fl and LtrLysCre-p38αΔ/Δ mice each are shown. (b) Induction of inflammation-associated genes in the colons of DSS-treated mice. Colonic RNA isolated 9 days after the initiation of DSS treatment was analyzed by semi-quantitative RT-PCR for IL-1β, TNFα, IL-6, IL-12b (IL-12p40), and GAPDH. Results from two separate p38αfl/fl and LtrLysCre-p38αΔ/Δ mice after DSS treatment are shown. (c) Representative photographs of immunofluorescent detection of Cox-2 and IL-6 in the inflammatory lesions. Colon sections prepared at 7 days after the initiation of DSS administration were analyzed by indirect immunofluorescent staining for Cox-2 and IL-6 (red), and counterstaining of DNA with DAPI (blue). Bars, 100 μm.
Specific deletion of p38α in intestinal epithelial cells affects cell proliferation and goblet cell differentiation and thus promotes the course of colitis
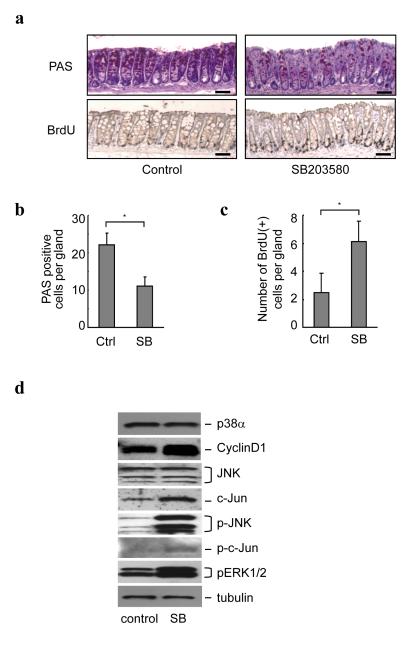
Our results show that the specific inhibition of p38α in myeloid cells reduces the severity of DSS-induced mouse colitis, confirming that inhibition of inflammation is beneficial for the disease. The lack of any beneficial effect using SB203580 in treating colitis could be due to the fact that inhibition of p38α in non-myeloid cells was harmful. Intestinal epithelial cells are major components of the mucosa of the colon and are targets during colitis. We found that mice treated with SB203580 in vivo had a decreased differentiated mucus-producing goblet cell population in colonic mucosa (Fig. 4a, b). Because intestinal homeostasis is maintained based on the balance of differentiation and proliferation of the epithelial cells, we next analyzed the proliferative state of colonic crypts in mice treated with SB203580. Proliferating cells were labeled by intraperitoneal injection of BrdU 2 hrs before harvesting colons. Analysis of BrdU-positive cells revealed that SB203580 significantly increased the number of proliferating cells in the colon (Fig. 4a, c). Much evidence indicates that the increase in cell proliferation and the impaired homeostasis, especially the decreased number of goblet cells, which normally have a protective role in the mucosa, result in greater susceptibility to injuries 20, 21, and therefore the changes in differentiation and proliferation of the epithelial cells in SB203580-treated mice could lead to an increased susceptibility to DSS-induced colitis injuries. Indeed, as shown above, the mice treated with SB203580 show severe DSS-induced colon epithelial damage, although the number of inflammatory cells in the mucosal lesions was less than that of the control mice (Fig.1b, c, d). The SB203580-induced increase of proliferating cells in the colon mucosa is associated with increases in cyclin D1 and c-Jun expression, as well as JNK and ERK1/2 phosphorylation, consistent with the p38 function reported in a variety of cell types (Fig. 4d) 18, 19, 22-24. Our data suggest that inhibition of p38α in colonic epithelial cells affects intestinal homeostasis.
Figure 4.
p38 inhibitor inhibits intestinal goblet cell differentiation and promotes intestinal epithelial cell proliferation. (a) PAS-positive goblet cells were less, but BrdU-positive proliferating cells were more in intestinal epithelial cells of SB203580-treated mice. Representative photomicrographs of PAS- and BrdU-stained colons at approximately 30 mm proximal from the anal canal of C57Bl/6 mice with and without SB203580 treatment for 3 days are shown. Mice were injected with 1mg/ml BrdU and sacrificed 2 hrs later. Adjacent sections were used for these PAS and BrdU staining. Four mice were used in one group and showed similar results. Bars, 100 μm. (b, c) Average numbers of PAS-positive cells (b) and BrdU-positive cells (c) per gland are shown. Cells were counted in 80 randomly chosen glands from four mice in each group. Error bars represent ± s.d. * p < 0.01. (d) Total extracts from the colon mucosa of C57Bl/6 control mice (control) and mice treated with SB203580 for 48 hrs (SB) were analyzed by immunoblotting using the indicated antibodies. The result shown is a representative of three independent experiments using three mouse sets.
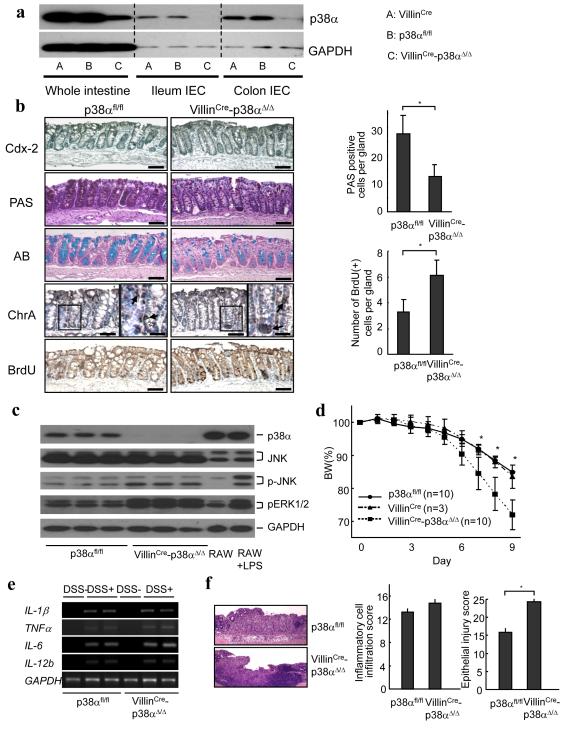
To evaluate this notion, we generated mice lacking p38α in intestinal epithelial cells (VillinCre-p38αΔ/Δ) by crossing p38αfl/fl mice and VillinCre mice, which express Cre recombinase under the control of the promoter of the intestinal epithelial cell-specific gene encoding villin 25. Although live VillinCre-p38αΔ/Δ mice were obtained at about one third of the expected ratio from over 100 pups analyzed, due to still unknown reasons (Supplementary Table 1 and Supplementary Results), VillinCre-p38αΔ/Δ mice appear to be healthy without any apparent clinical symptoms. Specific deletion of p38α in intestinal epithelial cells in VillinCre-p38αΔ/Δ mice was confirmed by comparing the p38α protein expression in the whole intestinal tissues and in the isolated intestinal epithelial cells (Fig. 5a). The trace amounts of p38α may be due to small amounts of non-epithelial cells unavoidably contaminating the isolated epithelial cells, or due to the fact the slight mosaicism of cre expression in the colon of Villin-Cre mice.
Figure 5.
p38α activity in intestinal epithelial cells is required for intestinal epithelial homeostasis and protection from DSS-induced colitis. (a) Specific deletion of p38α in the intestinal epithelial cells in VillinCre-p38αΔ/Δ mice. p38α expression in the whole colon tissue, isolated ileum epithelial cells, and isolated colon epithelial cells of VillinCre, p38αfl/fl, and VillinCre-p38αΔ/Δ mice were analyzed by immunoblotting. The result is representative of three independent experiments using different mice. (b) (Left panel) Histological and immunohistochemical analyses of the p38αfl/fl and VillinCre-p38αΔ/Δ mice colon. No gross differences were detected in the number of Cdx-2 positive enterocytes (Cdx-2), and the number of Chromogranin A positive enteroendocrine cells (ChrA), but PAS staining (PAS) and Alcian Blue staining (AB) revealed a reduction in goblet cell population in the colon epithelium of VillinCre-p38αΔ/Δ mice. BrdU-positive proliferating cells were greater in VillinCre-p38αΔ/Δ mice colons. The sections are 30 mm proximal from the anal canal. Adjacent sections were used for all the staining. Bars are 100 μm, except enlarged photos in chromogranin A staining. Close examination of the colon denoted by the boxes in the left panels of chromogranin A staining are shown at a higher magnification in the right panels (bars, 25 μm) and the positive cells are indicated by arrows. Similar results were obtained from three independent mice sets. (Right panel) Average numbers of PAS-positive cells (upper) and BrdU-positive cells (lower) per gland in the p38αfl/fl and VillinCre-p38αΔ/Δ mice colon are shown. Cells were counted in 80 randomly chosen glands from three mice in each group. Error bars represent ± s.d. * p < 0.01. (c) Protein extracts from isolated colon epithelial cells of p38αfl/fl and VillinCre-p38αΔ/Δ mice were analyzed by immunoblotting using the indicated antibodies. The results from three separate mice in each group are shown. As controls, protein extracts from RAW264.7 cells with and without LPS stimulation for one hour were used. (d) More severe body weight loss in VillinCre-p38αΔ/Δ mice during the DSS-induced colitis. p38αfl/fl control and VillinCre-p38αΔ/Δ mice were given 3.5% DSS in their drinking water for 6 days and body weight was recorded daily. Mice expressing villin-cre only with no p38 floxed alleles (VillinCre) were also included to rule out the possibility of the influence of cre. Data are presented as mean ± s.d. *p < 0.05. (e) Expression of inflammation-associated genes in the colons of p38αfl/fl and VillinCre-p38αΔ/Δ mice after DSS treatment. Colonic RNA was isolated 7 days after the initiation of DSS treatment and analyzed by semi-quantitative RT-PCR. Results from two separate p38αfl/fl and VillinCre-p38αΔ/Δ mice are shown. (f) (Left panel) Representative photomicrographs of hematoxylin and eosin-stained colons of p38αfl/fl and VillinCre-p38αΔ/Δ mice at 7 days after the initiation of DSS administration. Mice in both groups exhibited severe intestinal inflammation, but VillinCre-p38αΔ/Δ mice showed more severe and extensive epithelial injury. Similar results were obtained from the other three mice sets. Bars, 100 μm. (Right panel) Histological scoring of inflammatory cell infiltration and epithelial injury in colonic tissues of mice at 7 days after the initiation of DSS administration. Results are presented as mean ± s.d (n = 4 per group). * p < 0.05.
In the colon, stem cells are located at the base of the crypts, with proliferation and migration occurring upwards while differentiating into mostly enterocytes and goblet cells, with only a few interspersed enteroendocrine cells. We examined the patterns of differentiation and proliferation of intestinal epithelial cells in VillinCre-p38αΔ/Δ mice. No gross morphological differences in the colon were detected between p38αfl/fl and VillinCre-p38αΔ/Δ mice. Immunohistochemistry for Cdx-2, a marker of enterocytes, and for chromogranin A (ChrA), a marker of enteroendocrine cells, revealed no major differences in those cellular populations (Fig. 5b). In contrast, Alcian Blue (AB) staining, which detects acidic mucins, and PAS staining, which detects both neutral and acidic mucins, showed that the goblet cell population was strongly decreased in VillinCre-p38αΔ/Δ mice colon (Fig. 5b). The decreased number of goblet cells was also detected in the small intestine (Supplementary Fig. 6). On the other hand, the number of BrdU-positive cells present in the colons increased in VillinCre-p38αΔ/Δ mice, suggesting that the intestinal cell proliferation was enhanced in VillinCre-p38αΔ/Δ mice similar to the mice treated with the p38 inhibitor (Fig. 5b). Concordant with this, the increase in proliferating colon epithelial cells in VillinCre-p38αΔ/Δ mice was associated with greater activation of JNK and ERK1/2 (Fig. 5c), consistent with the mice treated with the p38 inhibitor.
Because undifferentiated and proliferating cells are generally more susceptible to injury 20, 21, and because less goblet cells result in less mucins and other factors that protect the intestinal mucosa from injury and facilitate tissue repair 26, VillinCre-p38αΔ/Δ mice may be more prone to the induced colitis. This was confirmed by the increased and quicker body weight loss of VillinCre-p38αΔ/Δ mice during DSS-induced colitis (Fig. 5d). The intestinal Cre expression alone showed no effects during the clinical course of colitis (Fig. 5d). Proinflammatory cytokine mRNA levels in the colonic mucosa were almost comparable or slightly higher in VillinCre-p38αΔ/Δ mice than in p38αfl/fl control mice (Fig. 5e). However, histological analyses revealed that, although both p38αfl/fl control mice and VillinCre-p38αΔ/Δ mice exhibited severe intestinal inflammation, VillinCre-p38αΔ/Δ mice had more severe and extensive epithelial injury with larger areas of ulceration (Fig. 5f). Histopathological scoring of colons also showed that the inflammatory cell infiltration was not significantly increased while the epithelial injury was more severe and extensive in VillinCre-p38αΔ/Δ mice after colitis induction (Fig 5f). Similar clinical results on body weight loss during colitis were obtained in another murine IBD model, 2.4.6-trinitrobenzene sulfonic acid (TNBS)-induced colitis (Supplementary Fig. 7). Although TNBS is believed to drive T cell-mediated responses, myeloid cells, including macrophages, are also major cells of its inflammatory reaction 27 and LtrLysCre-p38αΔ/Δ mice still showed clinically less body weight loss compared with control p38αfl/fl mice, which suggests that p38α in myeloid cells also plays a role in the pathogenesis of TNBS colitis. On the other hand, VillinCre-p38αΔ/Δ mice showed increased and sustained body weight loss during the TNBS-induced colitis compared to p38αfl/fl control mice, just as in the DSS-induced colitis model (Supplementary Fig. 7). These results suggest that p38α in intestinal epithelial cells regulates the intestinal epithelial cell differentiation and proliferation, and defective p38α in the intestinal epithelial cells results in greater susceptibility to colitis, which is in contrast to p38α deletion in myeloid cells.
A combination of a p38 inhibitor and a γ-secretase inhibitor confers beneficial effects on colitis
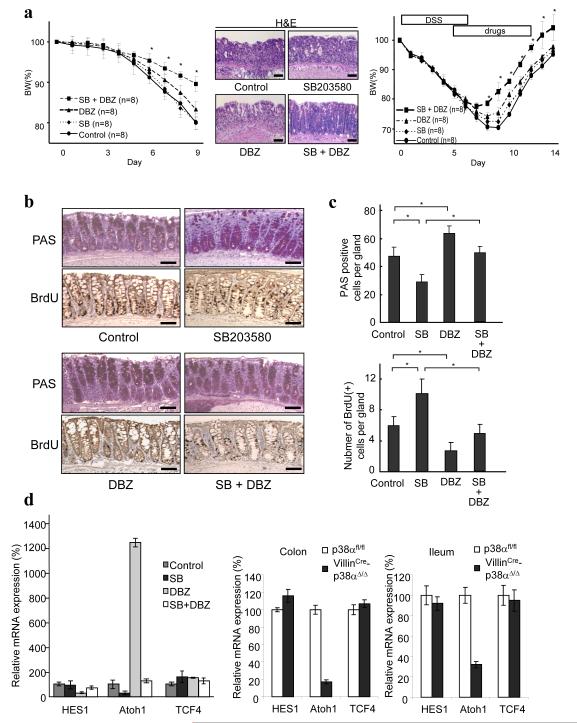
Since distinct functions of p38α in myeloid and intestinal epithelial cells might be the reason for the unsuccessful application of p38 inhibitors in treating colitis, we used a γ-secretase inhibitor dibenzazepine (DBZ), which was reported to affect epithelial cell differentiation and proliferation in a direction opposite that of what we observed with SB203580 28, 29, to compensate for the harmful effect of p38 inhibition on intestinal epithelial cells. DBZ itself had no influence on body weight change without colitis (Supplementary Fig. 8a), and mild protective effects on the course of colitis based on the body weight loss (Supplementary Fig. 8b). We observed a significant protective effect on DSS-induced colitis when the combination of DBZ and SB203580 was used (Fig. 6a left panel left panel and Supplementary Fig. 8b). Similar improvement on body weight loss by the combination of DBZ and SB203580 was obtained in TNBS-induced colitis as well (Supplementary Fig. 9). The histological examination showed that the extent and degree of inflammatory lesions were less in the SB203580 plus DBZ-treated mice compared to those in control, SB203580 only, or DBZ only treated mice (Fig. 6a middle panel and Supplementary Fig. 10). The improvement was observed even when the drug was administered after the establishment of colitis (Fig. 6a right panel). To further analyze the protective effects by this combination, the differentiation and proliferation status of the colon epithelial cells was examined. As described above, SB203580 decreased the number of PAS-positive goblet cells (Fig. 6b, c), DBZ induced significantly more goblet cells (Fig. 6b, c), and the combination eliminated the inhibitory effects of goblet cell differentiation by SB203580 in the colon and small intestine (Fig. 6b, c, and Supplementary Fig. 11). The SB203580-mediated increase of proliferating colonic epithelial cells was also suppressed by DBZ (Fig. 6b, c). The γ-secretase inhibitor masked the effects of the p38 inhibitor on intestinal epithelial cells.
Figure 6.
Colitis activities are attenuated by a combination therapy of a p38 inhibitor and a γ-secretase inhibitor. (a) (Left panel) C57Bl/6 mice were treated daily with control vehicle, SB203580 (SB), γ-secretase inhibitor (DBZ), or a combination of SB and DBZ. Mice were given 3.5% DSS in their drinking water for 6 days and body weight was recorded daily. Data are presented as mean ± s.d. *p < 0.05 compared with vehicle group. (Middle panels) Hematoxylin and eosin (H&E)-stained colons at approximately 30 mm from the anal canal of mice treated with vehicle, SB, DBZ, and SB plus DBZ, 7 days after the initiation of DSS administration. Similar results were obtained from three mice in each group. Bars, 100 μm. (Right panel) C57Bl/6 mice were given 3.5% DSS in their drinking water for 6 days and body weight was recorded daily. SB, DBZ, SB+DBZ and control (10% DMSO in normal saline) were injected into mice on Day6 and thereafter as indicated. The combined therapy of SB203580 and DBZ showed statistically significant improvement after the establishment of DSS-induced colitis. Data are presented as mean ± s.d. *p < 0.05 compared with the vehicle-treated control mice. (b) Photomicrographs of PAS-stained and BrdU-stained colons of mice treated with vehicle, SB, DBZ, and SB plus DBZ for 3 days. Mice were injected with 1mg/ml BrdU and sacrificed 4 hrs later. Sections at approximately 30 mm proximal from the anal canal are shown. Adjacent sections were used for both PAS and BrdU staining. Three independent groups showed similar results. Bars, 100 μm. (c) Average numbers of PAS-positive and BrdU-positive cells per gland (45 randomly chosen glands were counted from three mice) are shown. Error bars represent ± s.d. * p < 0.05. (d) (Left panel) The results of real-time PCR are shown using isolated colon epithelial cells from mice treated with vehicle, SB, DBZ, and SB plus DBZ for 3 days. The expression levels in vehicle-treated mice were set at 100. Results are presented as mean ± s.d from three independent experiments (n = 6 in total). (Right panels) The results of real-time RT-PCR using isolated colon and ileum epithelial cells from p38αfl/fl and VillinCre-p38αΔ/Δ mice. The expression levels in p38αfl/fl mice were set at 100. Results are presented as mean ± s.d from three independent experiments (n = 6 in total).
The induction of the goblet cell lineage by the γ-secretase inhibitor was reported through its inhibitory effects on the Notch pathway 28. Hes1, which is transcriptionally controlled by Notch signaling, encodes a bHLH transcriptional repressor and suppresses the expression of Atoh1 30, a key transcriptional factor required for goblet cell lineage differentiation in the intestine 31. As reported previously, we found that the γ-secretase inhibitor inhibited the expression of Hes1 and subsequently enhanced the expression of Atoh1 in the intestinal epithelial cells (Fig. 6d). By contrast, the p38 inhibitor had no effect on the expression of Hes1 but suppressed the expression of Atoh1. Expression of the unrelated molecule Tcf4 was not changed by either γ-secretase inhibitor or p38 inhibitor treatment (Fig. 6d). Hes1 expression was as low in the combination treatment as in the γ-secretase inhibitor treatment, but the expression levels of Atoh1 were almost similar to those in the control intestinal cells, albeit less than in the γ-secretase inhibitor-treated intestinal cells (Fig. 6d). These results suggested that p38α may work downstream of Hes1 or independent from Hes1 to influence the expression of the transcriptional factors that are required for secretory lineage cell differentiation. These were confirmed by examining the expression of the transcriptional factors in VillinCre-p38αΔ/Δ mice intestinal epithelial cells. In the isolated intestinal epithelial cells from VillinCre-p38αΔ/Δ mice, Hes1 expression was not changed, Atoh1 expression was significantly decreased, and the expression of the unrelated molecule Tcf4 was unchanged (Fig. 6d). In addition, DBZ treatment decreased Hes1 expression and restored Atoh1 expression in VillinCre-p38αΔ/Δ mice (Supplementary Fig. 12). These results suggest that p38α works as a positive regulator on secretory cell differentiation in the intestinal epithelia downstream of or independent from Hes1, and that the impairment of this cell differentiation by p38 inhibitors can be compensated by inhibition of the Notch pathway.
DISCUSSION
Here we show that genetic deletion of p38α in myeloid and intestinal epithelial cells leads to opposite effects on the development of colitis in vivo.
The role of p38α in myeloid cells is known to be primarily in inflammatory responses 17, and here we show that p38α in intestinal epithelial cells is important for regulating goblet cell differentiation and epithelial cell proliferation. Secretory lineage cell differentiation in the intestine is regulated by regulating transcriptional factors Atoh1 and Hes1. Our results suggested that p38α may work downstream of or independent from Hes1 to influence the expression of Atoh1. Atoh1 expression can also be regulated by the transcriptional factor Pax6, and p38 is necessary for Pax6 to achieve its transactivation activity 32, the decreased expression of Atoh1 in the intestine could be due to the defect in p38-mediated activation of Pax6 activity.
Using a γ-secretase inhibitor we confirmed that p38 inhibition caused reduction of intestinal cell differentiation into goblet cells and that increasing intestinal cell proliferation is harmful in colitis. Since γ-secretase inhibitors were originally developed for Alzheimer’s disease, with some currently in Phase III clinical trials, and p38 inhibitors are also in clinic trials (http://clinicaltrials.gov/), a combination therapy might be a viable therapeutic option for colitis in the near future.
In conclusion, we show that p38α exerts different functions in myeloid cells and intestinal epithelial cells in the course of colitis in mice. The unclear and undesired outcomes by global inhibition of p38α may arise from the different effects of p38α inhibition in different tissues or cell-types. Based on this notion and the recent attention on tissue-specific functions of various signaling molecules 33-35, tissue-specific p38α inhibition or simultaneous rescue of the undesired effects induced by global p38 inhibition, as shown here, should be taken into consideration for more efficacious intervention in this pathway.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Prof. M. German (University of California San Francisco) for the Cdx-2 antibody and Ryan Cook for critical reading of the manuscript.
Grant Support: This work was supported by grants from National Institutes of Health grants AI41637,AI68896, 973 program 2009CB522200, and grant 30830092 from NSF of China.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
REFERENCES
- 1.Loftus CG, Loftus EV, Jr., Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 3.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartor RB. Targeting enteric bacteria in treatment of inflammatory bowel diseases: why, how, and when. Curr Opin Gastroenterol. 2003;19:358–365. doi: 10.1097/00001574-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5:549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- 7.Han J, Lee J-D, Bibbs L, et al. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 9.Waetzig GH, Seegert D, Rosenstiel P, et al. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–5351. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- 10.Waetzig GH, Schreiber S. Review article: mitogen-activated protein kinases in chronic intestinal inflammation - targeting ancient pathways to treat modern diseases. Aliment Pharmacol Ther. 2003;18:17–32. doi: 10.1046/j.1365-2036.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 11.Hommes D, van den BB, Plasse T, et al. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s disease. Gastroenterology. 2002;122:7–14. doi: 10.1053/gast.2002.30770. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber S, Feagan B, D’Haens G, et al. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:325–334. doi: 10.1016/j.cgh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbach E, Vieth M, Roessner A, et al. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem. 2005;280:14981–14988. doi: 10.1074/jbc.M500966200. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbach E, Neumann M, Vieth M, et al. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004;18:1550–1552. doi: 10.1096/fj.04-1642fje. [DOI] [PubMed] [Google Scholar]
- 15.ten HT, van den BB, Pronk I, et al. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50:507–512. doi: 10.1136/gut.50.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malamut G, Cabane C, Dubuquoy L, et al. No evidence for an involvement of the p38 and JNK mitogen-activated protein in inflammatory bowel diseases. Dig Dis Sci. 2006;51:1443–1453. doi: 10.1007/s10620-006-9116-2. [DOI] [PubMed] [Google Scholar]
- 17.Kang YJ, Chen J, Otsuka M, et al. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- 18.Perdiguero E, Ruiz-Bonilla V, Gresh L, et al. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J. 2007;26:1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim C, Sano Y, Todorova K, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipkin M. Cell proliferation in gastrointestinal disease. Natl Cancer Inst Monogr. 1969;30:199–207. [PubMed] [Google Scholar]
- 21.Bell B, Deschner E, Almy TP, et al. Patterns of cell proliferation in gastrointestinal disease. Dis Colon Rectum. 1967;10:107–111. doi: 10.1007/BF02617356. [DOI] [PubMed] [Google Scholar]
- 22.Engel FB, Schebesta M, Duong MT, et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui L, Bakiri L, Mairhorfer A, et al. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- 24.Ventura JJ, Tenbaum S, Perdiguero E, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 25.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 26.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol. 1991;260:C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
- 27.Neurath MF, Pettersson S, zum Buschenfelde KH Meyer, et al. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 28.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 29.Fre S, Huyghe M, Mourikis P, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 30.Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 31.Yang Q, Bermingham NA, Finegold MJ, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 32.Mikkola I, Bruun JA, Bjorkoy G, et al. Phosphorylation of the transactivation domain of Pax6 by extracellular signal-regulated kinase and p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:15115–15126. doi: 10.1074/jbc.274.21.15115. [DOI] [PubMed] [Google Scholar]
- 33.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Eckmann L, Nebelsiek T, Fingerle AA, et al. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thornton TM, Pedraza-Alva G, Deng B, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.