1. Introduction
Bladder cancer is the fourth most common cancer in men and the eighth most common cause of cancer death in USA. In 2009 the number of new cases anticipated in the USA is estimated at about 1.5 million, of which 7% will correspond to bladder cancer (American Cancer Society, 2009). In USA more than 93% of bladder tumors are transitional cell carcinomas (Lynch and Cohen, 1995), the other types of bladder cancer which include squamous cell and adenocarcinoma are less common. There are many risk factors for bladder cancer development including cigarette smoking (Silverman et al., 1992, Boffeta, 2008), occupational exposure to aromatic amines (Baan et al., 2008), the use of phenacetin (Silveraman et al., 1992), cyclophosphamide(Khan et al., 1998), and environmental exposure to arsenic (Guo et al., 1997; Smith et al., 1998).
The mechanisms of arsenic-induced carcinogenesis have not been fully developed, however, there is substantial evidence suggesting that inflammation can play a direct role in the development of cancer. In general, it is well known that inflammatory diseases increase the risk of developing many types of cancer including the bladder, cervical, gastric, intestinal, ovarian, prostate and thyroid cancer (Balkwill et al., 2001) and inflammatory cytokines and chemokines are present in tumor microenvironment in all tumors including animals and humans (Balkwill et al., 2003). In support of this observation, the use of drugs which target inflammatory mediators or key transcription factors involved in the inflammatory proteins expression (nuclear factor Кβ [NFКβ] and signal transducers and activator of transcription-3 [STAT3]) decreases the incidence and spreading of cancer (Coimbra et al, 2009).
Cytokines areknow mediators of inflammatory processes. Cytokines like IL-1, IL-2, IL-6, IL-8, IL-10 and IL-12 have been shown to participate in inflammation-associated carcinogenesis (Rose-John and Schooltink, 2007.; Xie, 2001.; Black et al., 2007.; Lin and Karin, 2007), and the associated mechanisms involve the cell cycle genes modulation, apoptosis inhibition, cell survival promotion, increase of the invasiveness, and angiogenesis promotion.
The relationship between human chronic arsenic exposures with high risk for bladder cancer development has been documented. The association between inflammation derived from chronic bladder infections, as schistosomal infections, endemic in some developing countries, and squamous cell carcinoma of the bladder is well established (Lynch et al., 1995). However, different tissue injuries and irritants like the use of catheters, the presence of renal, bladder and urether stones (Chow et al., 1997), sexually transmitted diseases (Mommsen et al., 1983), and as mentioned before chemical induced cystitis or exposure to some toxicants are also associated with bladder cancer development Such irritants can in some way induce the activation of inflammatory cells in bladder. As a consequence, the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) occurs. In a chronic inflammation, these ROS and RNS can produce DNA breaks or can directly modify different proteins leading to cell growth and tumor promotion by activating different signal-transduction pathways (Hussain et al., 2003; Klaunig et al., 2004).
Supporting the notion that chronic inflammation is an important factor for bladder cancer development, in vitro exposure of human aortic endothelial cells to 10 µM sodium arsenite leads to an over-expression of interleukin-8 (IL-8) gene (Simeonova et al, 2003) a well known pro-inflammatory cytokine and an angiogenic chemokine. In mice, exposure to 200 ppb sodium arsenite leads to up-regulation of interleukin-6 (IL-6), tumor necrosis factor-aplha (TNF-α), inducible nitric oxide sintetase (iNOS), and macrophage inflammatory protein 2 (MIP-2) (Wu et al., 2008). Additionally, the sub-chronic or chronic exposure to sodium arsenite lead to the over-expression of cyclooxygenases (COX-1 and 2) and prostaglandins in mice (Bunderson et al., 2004; Trouba and Germolec, 2004; Aguirre-Bañuelos et al. 2008; Lantz and Hays, 2006; Wu, et al., 2008). The gene expression or protein activation of NFκβ and the activator protein 1 (AP-1) which regulate the expression of inflammatory mediators are also increased in human exposed to arsenic (Yamamoto et al., 2008; Shen et al., 2008; Fry et al., 2007; Mathews et al.; 2007, Drobna et al., 2002; Tsai et al., 2002).
But is arsenic directly inducing the inflammatory response or the inflammatory response in a secondary response of molecular changes induced for arsenic? Mantovani et al. (2008) have summarized that there are two ways in which the inflammation can be linked with cancer: the intrinsic and the extrinsic pathways. The first one is activated by genetic events that normally cause neoplasia such as the activation of various types of oncogenes by mutation, chromosomal rearrangement or amplification. Cells transformed through this pathway over-produce inflammatory mediators creating and inflammatory microenvironment around the tumor. For example, this is the case of breast cancer and papillary thyroid carcinoma. However, in the extrinsic pathway, a pre-existing inflammatory condition increases the risk for cancer development. In any case, the process results in the activation of NFКβ, STAT3 and hypoxia factor 1-α (HIF1a) in immune cells as well as other tissues; these factors in turn moderate the expression of inflammatory mediators (cytokines, chemokines, COX2), activating immune cells and generating of ROS and RNS. Cytokines, chemokines, prostaglandins, ROS and RNS activate the same transcription factors, keeping a sustained inflammatory state.
Our studies have found that following chronic in vitro exposure of the UROtsa cells (an immortalized, non-tumorigenic urothelial human cell line) to either 1 µM As(III) or 50 nM of monomethylarsenous acid [MMA(III)] for 12 mo leads to the malignant transformation of the cells (Bredfeldt et al., 2006). The transformed cells show the characteristic of neoplasic cells with anchorage independent growth and tumorigenicity in nude mouse. Using this model Eblin et al (2009) has been able to show that MMA(III)-transformed cells show an increase in endogenous ROS production and an over-expression in COX2 and the epidermal growth factor receptor B2 (ErbB2). The increase in COX2 (Eblin et al. 2007) and Ras (small GTPases) (Eblin et al., 2009) were also found in a time-dependent fashion in cells chronically exposed to 50 nM MMA(III). The activation of inflammatory- signal transduction factors including ErbB2, the extracellular signal-regulated kinase 2 (Erk2)and c-Jun/AP-1 was demonstrated after only 15 or 30 min of cell exposure to 50 nM of MMA(III) (Eblin et al., 2007). These results suggest that MMA(III) can be acting through the extrinsic pathway since inflammation-associated pathways are activated very early in the transformation process. However other inflammatory mediators like cytokines or signal transcription or transduction factors associated with the inflammatory response have not been evaluated in acute and chronic studies using this model to better understand the role of inflammation in MMA(III)-induced malignant transformation. This study proposes that inflammatory cytokines play a key role in the transformation of UROtsa cells by chronic low level exposure to MMA (III). This occurs through the activation of signaling pathways associated with cell proliferation and survival. To probe such hypothesis cytokine production and activation of signal pathways associated with the production and response to cytokines (NFКβ, STAT-3, AP-1, Erk1/2 and p38 MAPK) was profiled after acute and chronic exposure of UROtsa cells to 50 nM MMA(III) to evaluate the inflammation-associated mechanisms involved in MMA(II)-induced cell transformation.
1. Materials and Methods
2.1 Cells
UROtsa cells were generously provided by Drs. Mary Ann and Donald Sens (University of North Dakota). Cell culture conditions were the previous described by Bredfelt el al. (2004). Stock cells cultures were grown on 100 mm tissue culture flasks using DMEM enriched with 5% FBS and 1% antibiotic-antimycotic at 37° C in 5% CO2. Cells were allowed to become 85–90% confluent before the experiments were conducted. MMA (III)-transformed cells (MSC52) were obtained in our laboratory according with Bredfeldt et al. (2006) and correspond to UROtsa cells that were chronically exposed to 50 nM of MMA(III) for 12 mo. These cells were used in this study as a positive control or end point.
2.2 Passage Matched Controls
In our laboratory we have developed a simple way to keep all the exposed and unexposed UROtsa cells in the same passage. We start one new exposed culture from the same unexposed control (which is kept growing together with the exposed cells) every month. In this way unexposed and all exposed cells have the same passage number.
2.3 Chemicals
Diiodomethylarsine (MMA(III) iodide CH3AsI2) was prepared by the Synthetic Chemistry Facility Core (Southwest Environmental Health Science Center, Tucson, AZ, USA) according with the method of Millar et al. (1960). Fresh stock solutions of 25 mM MMA(III) were made in distilled, deionized and ultrapure water. A 5 µM MMA(III) working solution was used to dose the cells every other day to assure constant exposure to MMA(III). To have passage matched cells a new dosed culture was started every month from normal unexposed UROtsa cells.
2.4 Protein extraction
The cells were rinsed with cold phosphate-buffered saline, removed from plates by scraping in radioimmunoprecipitation lysis buffer containing 50 mM Tris-HCl (pH 8.6), 1% NP-40, 0.25% C24H39NaO4, 150 mM NaCl, 1 mM PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 mM NaF, 1 mM Na3VO4, 1 mM EDTA, and 10 µg/ml protease inhibitor cocktail. The lysates were sonicated and centrifuged at 14,000 rpm for 5 min at 4° C to eliminate the cell debris. Supernatant protein concentrations were determined by the Bradford method using Protein Assay (BIO-RAD).
2.5 Cytokine production
For studies in chronically exposed cells, the UROtsa cells were exposed to 50 nM MMA(III) for 1, 3, 5 and 12 mo. At 90% of confluence the cells were harvested and cultured in 12 well plates in a concentration of 1 × 105 cells/well in absence of MMA(III) and in complete DMEM. To evaluate a sustained cytokine production, the cells were allowed to settle down for 24 h, the media was then replaced for serum free media [without MMA(III)] and the supernatants were recovered 24 h later for cytokines assay. For acute studies, unexposed UROtsa cells were plated in 12 well plates in the same quantity and allowed to settle down for 24 h. Afterwards, MMA(III) was added to a final concentration of 50 nM, the supernatants were recovered at 12 and 36 h. The cytokine production profile for acute and chronic studies was evaluated using the Q-Plex™ Human Cytokine Screen Plus (16-plex) commercial system (Quansys Biosciences) following the provider directions. The image was acquired using Chemi-DocXRS (BIORAD) and the Quantity-one software (BIORAD). The image was analyzed with Q-view software (Quansys) and the data were analyzed with the Quansys Analysis Template Human cytokines 3.2 v.
All the assays were done in triplicate; the results were adjusted to picograms per 100,000 cells (pg/1 × 105 cells) in accordance with the doubling times corresponding to the untreated and treated cells. The adjusted quantities were presented as an average of the data ± standard deviation (SD).
2.6 Macrophage Inhibitory Factor (MIF) p-P38 MAPK, p-P44/42 and p-cJun expression
Thirty µg of protein from each sample was loaded onto 4–20% or 10%sodium dodecyl sulfate (SDS)/polyacrylamide gels. Samples were separated via SDS–polyacrylamide gel electrophoresis (PAGE) with Mini-Protean II (BioRad, Hercules, CA) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA) and blocked 1 h at room temperature with protein Free T20 (PBS) blocking buffer (Thermo Scientific). Blots were incubated overnight at 4° C with primary antibodies for MIF, p-P38 MAPK, (Santa Cruz Biotechnology), p-cJun, p-P44/42 [ERK1/2], and α- tubulin (Cell Signaling Biotechnologies) at manufacturer's recommended dilution. The appropriate secondary antibody linked to horseradish peroxidase was used for detection of primary antibody. Chemiluminescent detection was performed with enhanced chemiluminescence Western blotting substrate (Pierce Biotechnology, Inc., Rockford, IL or GE Healthcare, Piscataway, NJ). Images were scanned with a Scanjet 5370C (Hewlett Packard, Palo, Alto, CA) at maximum resolution and prepared in Adobe Photoshop 3.0 (San Jose, CA). The corresponding images were analyzed using imageJ, the results are expressed as relative intensity to α-tubulin.
2.7 NFКβ and c-Jun translocation into the nucleus
UROtsa cells chronically exposed to MMA(III) were plated in a 96 well plate in a cell density of 5 × 103 cells/well. The cells were then incubated 24 h at 37° C in 5% CO2. For c-Jun stimulation, the cells were serum-starved for 24 h and then some wells were re-fed with fresh medium containing 1% FCS for 2 h. For acute exposure 50 nM MMA(III) was added to corresponding wells for 30 min. The cells were then fixed with 2% of formaldehyde for 15 min at room temperature. The cells were then permeabilized and incubated with the corresponding primary and secondary antibodies following the provider directions (Thermo Scientific). For NFКβ detection, the secondary antibody labeled with Dylight 549 leads to the cells to fluoresce in red, while the secondary antibody for p c-Jun detection labeled to Dylight 488 leads to the cells fluoresce in green-yellow using the corresponding filters. The cells were analyzed in a Delta Vision Deconvolution fluorescence microscope.
2.8 ELISA for NFKb activation
Chronically exposed UROtsa cells were grown in 100-mm plates to 85–90% confluence. In acute studies, cells were dosed with 50nM MMA(III) for 30 min. Nuclear fractionation protocol was adapted from Kosugi et al. (2001). Briefly, treated cells were removed from plates by scraping into hypotonic buffer containing 20 mM HEPES, pH 7.4, 5 mM KCl, 2 mM MgCl2, 0.1% NP-40, 40 mM Na2H2P2O7, 0.5% ß-mercaptoethanol, 1 mM PMSF,40 mM ß-glycerophosphate, 1 mM Na3VO4, and phosphatase inhibitors (Panomics). Nuclei and other debris were pelleted via centrifugation at 14,000 rpm for 3 min. Pellet was resuspended in 150 µl of hypotonic buffer solution containing protease and phosphatase inhibitors (Panomics), incubated for 1 h and centrifugated at 14,000 rpm for 5 min. The supernatants were collected and stored at −80° C until use. The proteins were determined using RC DC Protein Assay (BIO-RAD). The activation of NFκβ in nuclear extracts was assessed using a TF ELISA kit (Panomics) following the provider’s instructions. In the plate the reaction provided a colorimetric readout which was quantified using a Versa Max microplate reader (Molecular Devices, Sunnyvale, CA) at 450 nm.
2.9 Total phospho-AKT, phospho P44/42 MAPK, phospho P-38 MAPK and phospho-STAT3
The cytoplasmic proteins from UROtsa cells chronically exposed to MMA(III) were isolated as described previously. The protein concentration was adjusted to 3 mg/ml. 50 µl of each sample was added to a Q-Plex™ inflammation 4-Plex array (Quansys Biosystems) plate which has attached the corresponding antibodies against total AKT, phospho-P44/42 MAPK, phospho-P38 MAPK and phospho-STAT3. The procedure to the detection of these proteins was done following the provider’s instructions. The chemiluminecent image was acquired using Chemi-DocXRS (BIORAD) and the Quantity-one software (BIORAD). The image was analyzed using the imageJ software and the results were expressed as integrated density.
2.10 Statistical methods
Data analysis was carried out using GraphPad Prism 5.0 Software (GraphPad Software, Inc., San Diego, CA). One or two-way ANOVA was used to found differences between groups followed by a Dunnett's Multiple Comparison or Bontferroni post-test analysis. Corresponding graphs were generated in GraphPad Prism 5.0 or in Microsoft Excel (Microsoft Corp., Redmon, WA). Data are expressed as the average of three independent experiments and are represented as the mean ±SD.
3. Results
Several common tumor cells are rich in inflammatory cytokines, growth factors, and chemokines (Ariztia et al., 2006). Cytokines such as TNF-a, IL-1 and the chemokine IL-8, which are present in tumors, seem to be responsible of tumor progression and spread. In the present study the inflammatory cytokines produced for the UROtsa cells exposed acute and chronically to 50 nM MMA(III) were profiled to know the role of inflammation in cell transformation.
3.1 MMA(III) induce the over- production of inflammatory cytokines in UROtsa cells
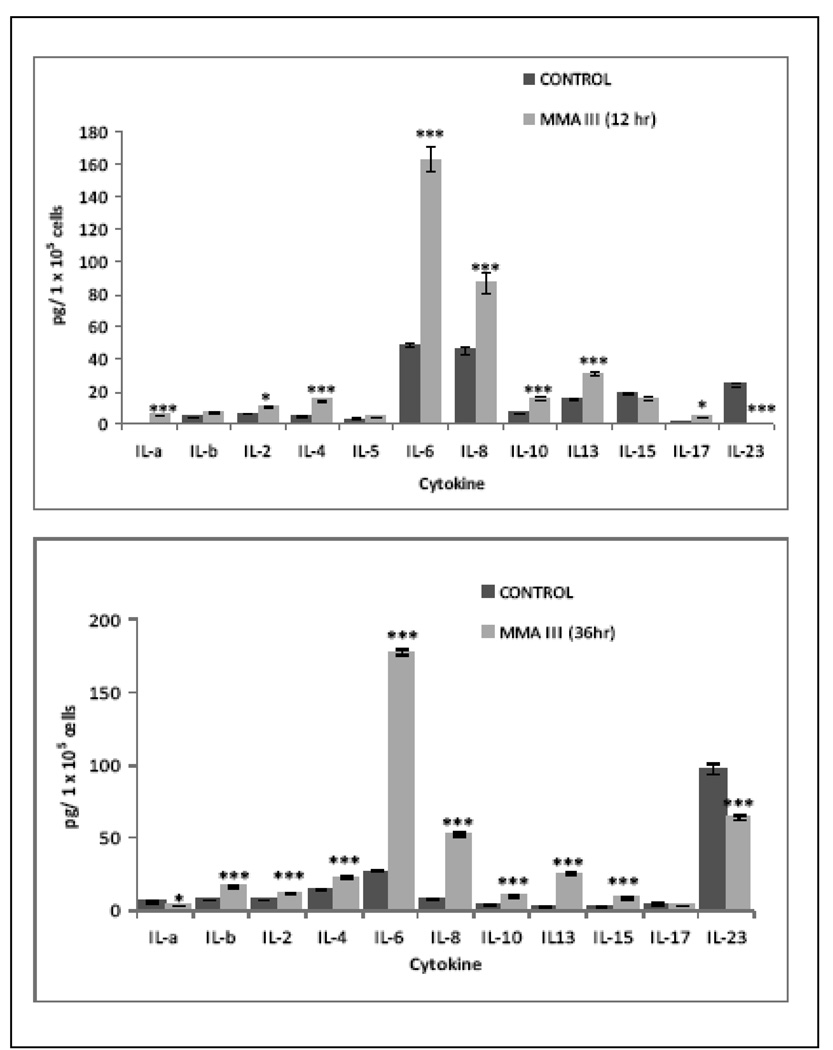
Acute Exposure to MMA(III): The exposure of UROtsa cells to MMA(III) for 12 and 36 h leads to an overproduction of different cytokines, some of those were better expressed after 12 or 36 h (Figure 1A and 1B). The acute exposure led to an over production of inflammatory (IL-1, IL-2, IL-4, IL-6, IL-8, IL-15 and 17) as well as anti-inflammatory cytokines (IL-10, IL-13) at 12 and 36 h compared with non treated cells. However the acute exposure to MMA(III) lead to an down regulation in the production of cytokine IL-23.
Figure 1.
Acute exposure to 50 nM MMA(III) leads to over-production of inflammatory cytokines in UROtsa cells. Cytokine production profile in UROtsa cells after 12 (A) and 36 (B) hr of exposure to MMA(III). Cells were exposed to 50 nM MMA(III) for 12 and 36 hr and the supernatant were recovered and analyzed for inflammatory cytokines by 16-plex ELISA array. Graph represents the mean of three independent experiments ± SD of the exposed cells. Asterisks indicate a significant difference [(*), p<0.05, (***) p<0.001] compared with none exposed cells as indicated by Bontferroni ‘s post two way ANOVA test.
3.2 Chronic Exposure to MMA(III) – sustained expression of cytokines
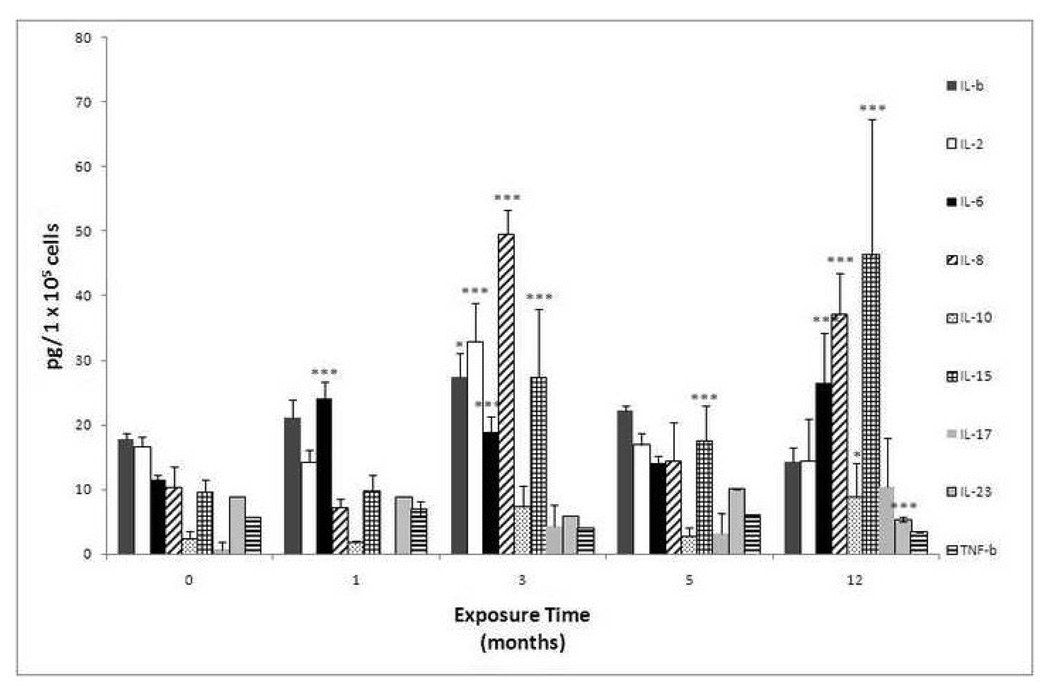
To determine the sustained production of inflammatory cytokines, MMA(III)-chronically treated UROtsa cells were cultured in absence of the arsenical for 48–72 h (until confluent). 1, 3, 5, or 12 mo-exposed UROtsa cells demonstrated an over-expression of selected pro-inflammatory as well as anti-inflammatory cytokines. Over-expression of pro-inflammatory cytokines was more prominent and was comparable to the response seen after acute exposure. The 1 mo-exposed UROtsa cells showed a significant over-expression in IL-6, but the 3 and 12 mo-exposed cells showed the most prominent cytokine over-production. The over-produced cytokines in 3 mo-exposed cells were IL-1β, IL-2, IL-6, IL-8, IL-15 as well as TNF-β and TNF-α; while in 12 mo exposed cells (MSC52 cells) IL-6, IL-8, IL-10, IL-15, and IL-17 were over-produced (Figure 2) . The most relevant cytokines which were over-produced in chronically exposed cells that could account for the MMA(III)-induced transformation process in UROtsa cells are IL-1β, IL-2, IL-6, IL-8, and IL-15 . Interestingly, the same cytokines were also over-produced following just acute exposure to MMA(III) as soon as 12 h after exposure to MMA(III).
Figure 2.
Chronic exposure to 50 nM of MMA(III) leads to a sustained over-production of inflammatory cytokines associated with cell proliferation and survival. The figure shows the cytokine production profile in UROtsa cells exposed to 50 nM MMA(III) for 1, 3, 5 or 12 mo. The bars represented the mean of three independent experiments ± SD. Asterisk indicate a significant difference [(***) P< 0.001, (*) P< 0.05 ] compared with non exposed cells as indicated by Bontferroni ‘s post two way ANOVA test.
3.3 MIF expression
Macrophage inhibitory factor (MIF), a key regulator of immune and inflammatory responses, plays a critical role in inflammation-associated cancer due the fact that MIF induces activation of the ERK-1/2 and AKT pathways (Hagemann et al., 2007) and is involved in the regulation of JAB1, p53, and HIF-1 (Bucala et al., 2007). Many cytokines including IL-6, IL-8 and IL-17 are regulated by MIF production through the activation of p-38, ERK 1/2, and STAT3, however, MIF seems to be regulated by IL-1 production (Stojanović et al., 2009). To know if this cytokine could be the responsible for the cytokines over-production in acute and chronic exposure to MMA(III) the MIF production in chronic exposed UROtsa cells to 50 nM MMA(III) was assessed.
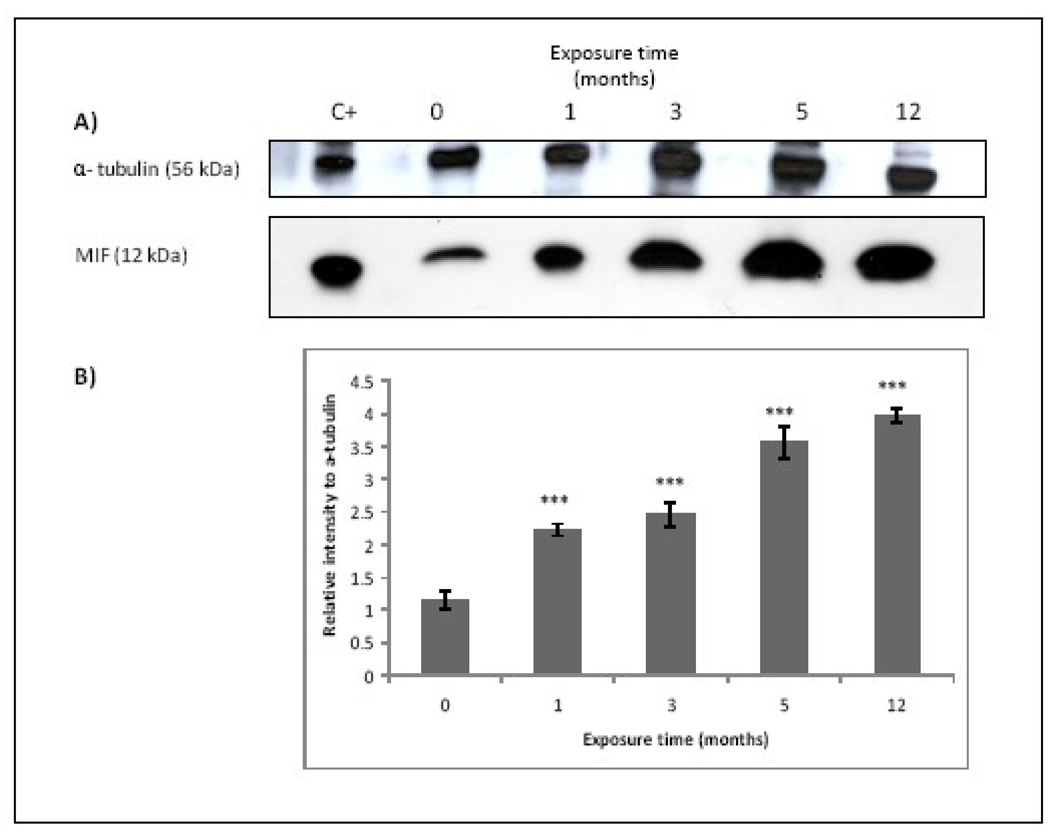
A significant over-production of MIF was found in chronic exposed cells in a time-dependent fashion compared with non-treated cells and in accordance with the cytokine over-production found previously (Figure 3). However when the production of MIF was assessed in cells acutely exposed to MMA(III) the results showed that MIF over-production does not take place in the first 24 h (data not shown). These results suggest that MIF is not responsible of the MMA(III)-induced acute over-expression of the pro-inflammatory cytokines the first time that UROtsa cells are exposed to MMA(III). However since MIF is over-produced during chronic exposure, it is possible that MIF could participate in the sustained inflammatory state in chronic MMA(III) exposure.
Figure 3.
MIF is over-produced after chronic exposure to MMA(III) in a time dependent fashion. (A) Representative Western Blot of 12 kDa monomeric MIF band (N≥3). HL-60 cells whole lysate was used as positive control. (B) Histogram of MIF densitometric analysis (mean ± SD). Asterisks indicate a significant difference [(***) P<0.001 compared to non treated cells) as indicated by one way analysis of variance and Dunnett's Multiple Comparison as post-Test analysis.
3.4 NFкβ sustained activation causes cytokine over-production in MMA(III) chronically exposed UROtsa cells
NFкβ is a key transcriptional regulator of most inflammatory genes and recently it has been identified as a key element in cancer development (Pikarsky et al., 2004). NFкβ regulates the expression of IL-6, IL-1, IL-8, IL-15, and TNFα. Recently Veillat et al. (2009) identified NFκβ binding sites in the MIF gene promoter, suggesting that MIF is also under the regulation of this transcription factor. Since MMA(III) induced the over-production of pro-inflammatory cytokines in acute as well as chronic exposure in UROtsa cells, the next experiments were designed to demonstrate if there is sustained NFкB activation in chronically exposed UROtsa cells.
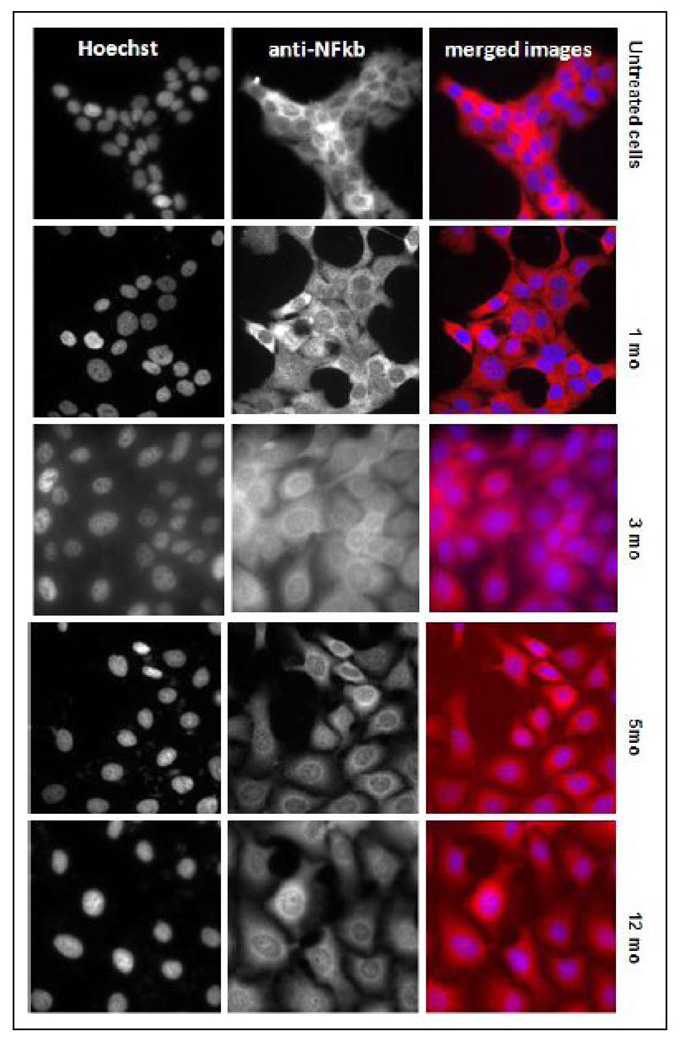
The chronically exposed UROtas cells were grow in the absence of the arsenical for 48 to 72 h, plated and cultured an additional 24 h. The NFкβ translocation into the nucleus was assayed by immunodetection and confirmed with a specific transcription factor ELISA assay. All the chronically MMA(III) exposed cells showed substantial constitutive translocation of NFкβ to the nucleus compared with non-exposed cells, and the intensity of the translocation was time-dependent (Figure 4). The NFKB translocation into the nucleus in MMA(III)-exposed cells was not complete, since some NFkb remained in the cytoplasm. When this transcription factor was assayed by an specific ELISA using nuclear proteins, its activation also showed a time-dependent increase beginning at 1 mo exposure and progressively increasing throughout the 12 mo exposure, confirming previous results (Figure 5). The results to this point showed that important pro-inflammatory cytokines are produced in chronically exposed cells to MMA(III) as a consequence of a continued NFкβ activation.
Figure 4.
NFKb is constitutively translocated into the nucleus in 50 nM MMA(III) chronically exposed UROtsa cells. Chronically exposed UROtsa cells were cultured in a 96 well plate in a density of 5 × 105 cells/well. After 24 hr, the cells were fixed and permeabilized, specific antibodies against the p65 subunit of NFКβ were added to the plate. The secondary antibody labeled with Daylight-549 (Thermo Scientific) leads the cell to fluoresce in red. Hoechst was used to stain the nuclei; when NFКβ (red) is translocated into the nucleus (blue) the nucleus color looks pink-purple. The images were obtained using a Delta Vision deconvolution inverted microscope.
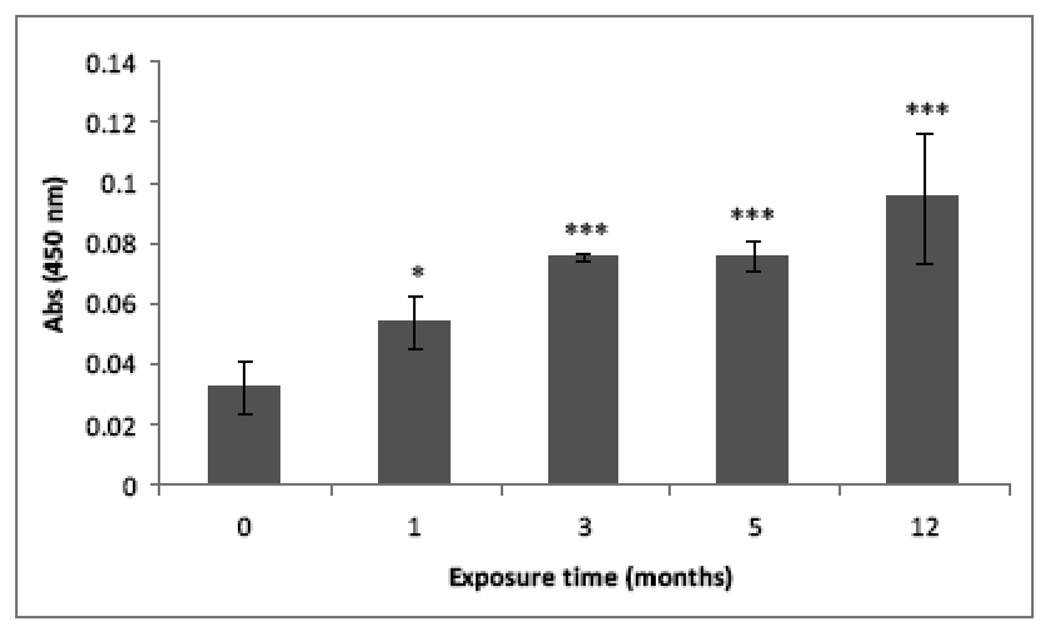
Figure 5.
NFКβ activation in chronically exposed cells to MMA(III). The NFКβ activation assessed by ELISA was two times higher in UROtsa cells exposed for 1, 3, 5, or 12 mo to 50 nM MMA(III). The chronically exposed cells were cultured in 100 mm flasks at 90% confluence, the nuclear proteins were recovered from the cells, quantified and adjusted to assay NFКβ activation using an ELISA based system specific for the phosphorylated p65 subunit. Asterisks indicate a significant difference [(***) P<0.001 or 0.05 (*)] compared to non treated cells as indicated by one way analysis of variance and Dunnett's Multiple Comparison as post-Test analysis.
3.5 Association between sustained inflammatory state and the activation of key inflammatory signal molecules and transcriptional factors associated with proliferation and cell survival in MMA(III)-chronically exposed UROtsa cells
It is well known that many cytokines induce the activation of c-Jun/AP-1 pathway through phosphorylation and its subsequent translocation in to the nucleus, where it activates the transcription of different genes associated with cell growth, proliferation, survival, and invasion. IL-1β for example, promotes cell migration trough MAPKs / AP-1 and NFкβ activation in A594 cell line (Lin et al., 2009) and IL-6 have been associated with the ability of the cell to grow without anchorage to a matrix in colorectal carcinoma cell lines (Hsu and Chung, 2006). The activation of p38 MAPK pathway which in turns activates c-Jun/AP-1 it is associated with this ability (Meng et al, 2006). Accordingly, the next experiments were designed to determine whether the cytokine over-expression in UROtsa cells exposed to MMA(III) is associated with a constitutive activation of c-Jun in cells, which could explain the cell hyper proliferation by 3 mo of MMA(III) exposure (Bredfeldt et al., 2006) and eventually the cell transformation.
3.5.1 The constitutive activation of c-Jun is associated with pro-inflammatory cytokines over-production in MMA(III) chronically exposed UROtsa cells
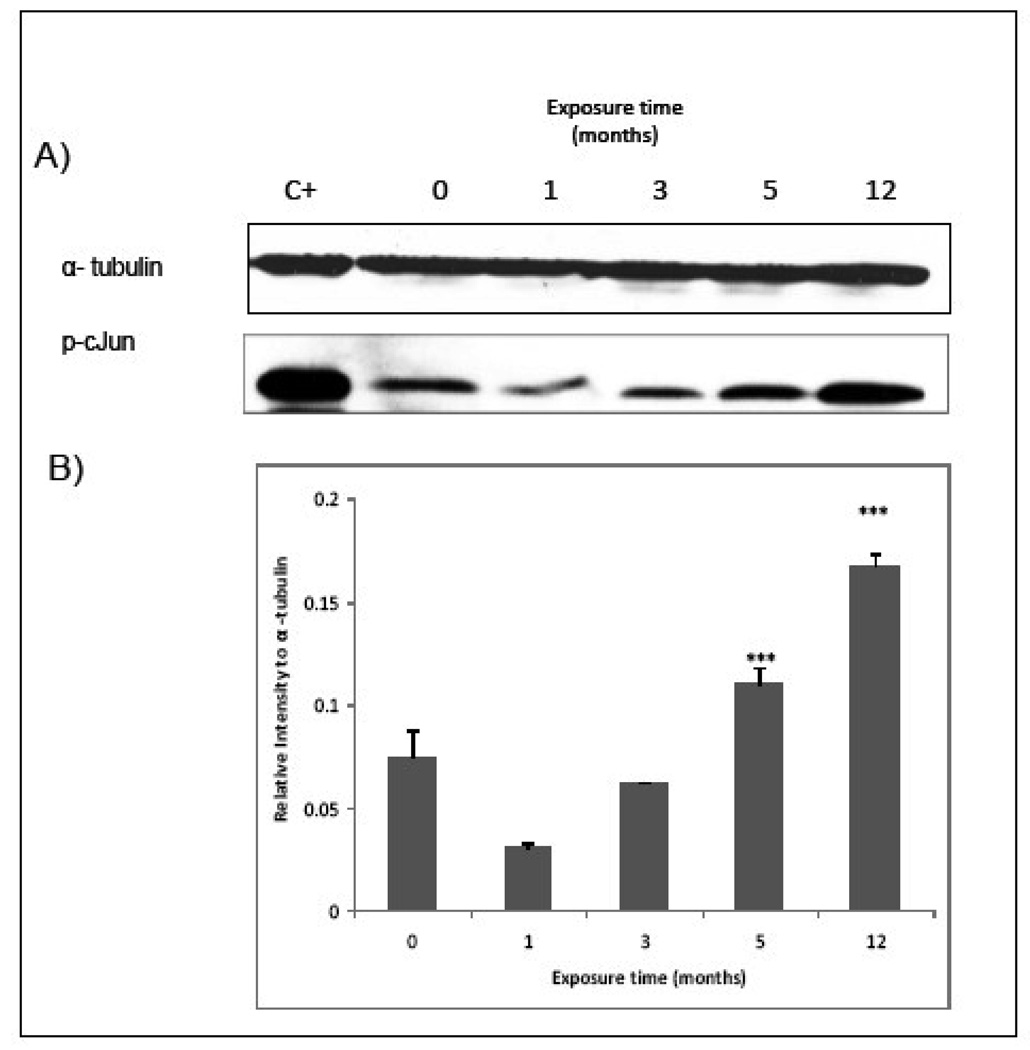
Chronically MMA(III) exposed UROtsa cells had increased expression of phospho c-Jun transcription factor by 3 mo exposure and further increase levels at 5 and 12 mo exposure (Figure 6) with localization of the transcription factor in the nucleus (Figure 7).
Figure 6.
UROtsa cells exposed for 3, 5, or 12 mo showed a higher c-Jun phosphorylation than non-exposed cells. MMA(III) chronically exposed UROtsa cells were allowed to reach 80% confluence and then were serum starved for 24h. Cell proteins were recovered, quantified and adjusted to assay c-Jun activation trough the use of specific antibodies against phosho-c/Jun by Western Blot. A) Representative blot shows higher c-Jun phosphorylation in chronically exposed cells (N≥3). NIH/3T3 cell whole lysate was used as positive control. B) Histogram of densitometric analysis using imageJ software. Asterisks indicate a significant difference [(***) P<0.001 compared to non treated cells) as indicated by one way analysis of variance and Dunnett's Multiple Comparison as post-Test analysis.
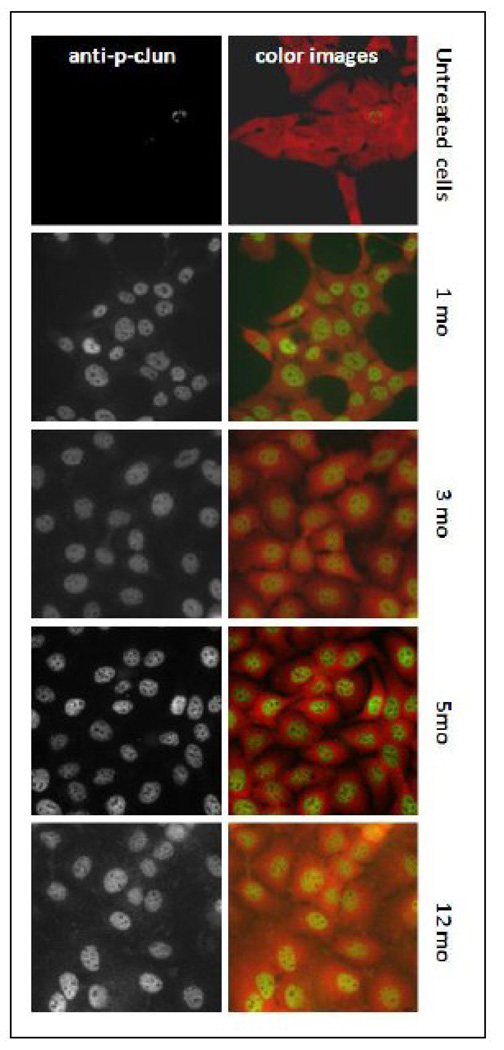
Figure 7.
Chronic exposure to 50 nM MMA(III) induces the sustained c-Jun translocation into the nucleus in UROtsa cells. Chronically exposed UROtsa cells were cultured in a 96 well plate in a density of 5 × 103 cells/well. After 24 hr the cells were serum starved for additional 24 h, then fixed and permeabilized. Specific antibodies against phospho c-Jun were added to the plate. The secondary antibody labeled with Daylight-488 leads the cells nuclei to fluoresce in green-yellow when reached the target. Daylight-549 antibodies against total NFКβ were used for contrast in color images. The images were obtained using a deconvolution inverted microscope.
3.5.2 Association between cytokine alteration and kinase activation in UROtsa chronically exposed to MMA(III)
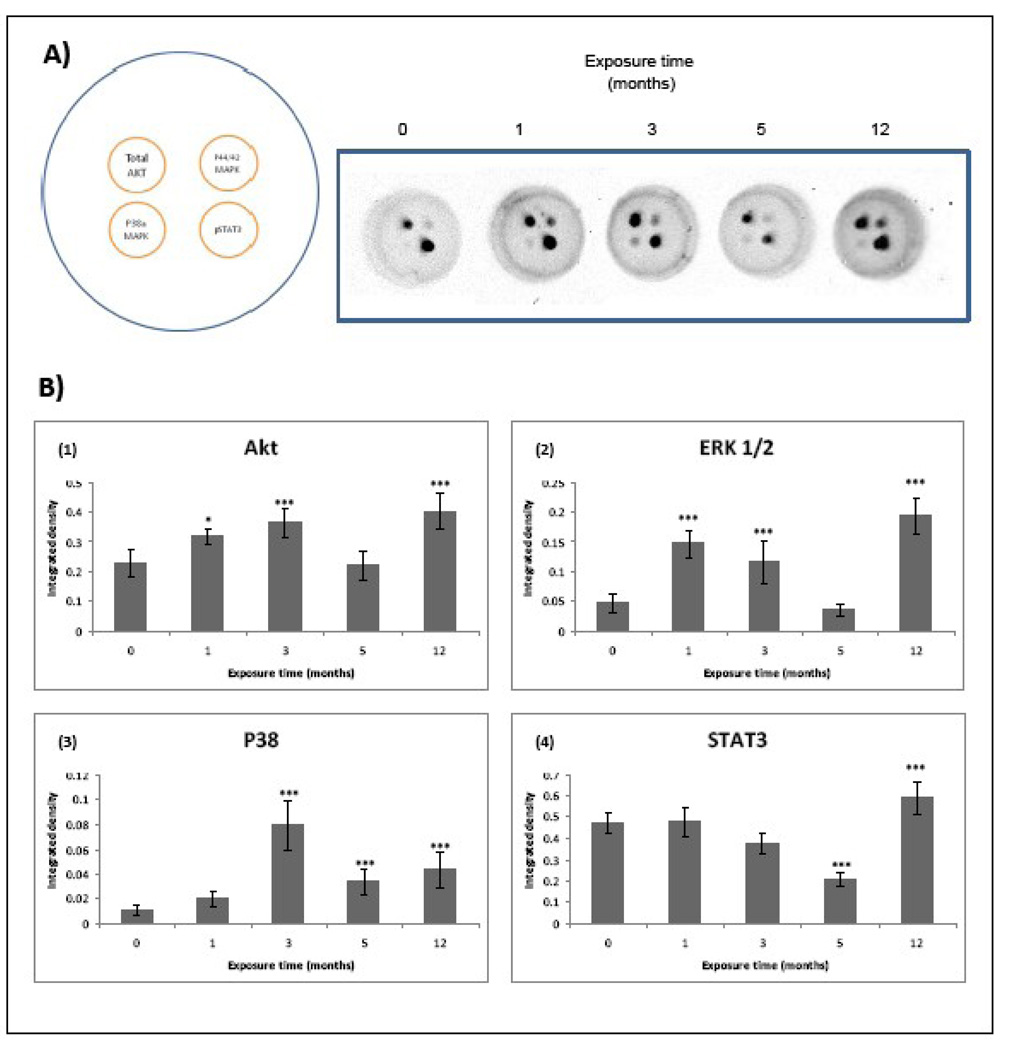
Cytokine signaling is initiated after specific binding to cell surface receptors followed for activation of intracellular kinases, such as Janus activated kinase (JAK), phosphatidylinositol-3-kinase (PI3/K)/Akt, IKK, and MAP kinases. This activation leads to a subsequent activation of different transcription factors, predominantly STAT3, NFκβ, and AP-1. The constitutive activation of these factors has been found in different kind of cancers. The Ras/Raf/MEK/ERK and PI3K/PTEN/AKT signaling cascades play critical roles in signal transmission from growth factor receptors and cytokines to regulate gene expression and prevent apoptosis. Importantly, components of these pathways are mutated or aberrantly expressed in human cancer (McCubrey et al., 2006). Our next studies determined the production of total AKT, p-ERK 1/2, p-p38 MAPK and p-STAT3 to know the inflammatory transduction pathways involved in MMA (III)-induced cell transformation (Figure 8).
Figure 8.
Inflammatory cell signaling is over activated in UROtsa cells chronically exposed to 50 nM MMA(III). The proteins from chronically exposed cells were recovered when the cells reached 90% confluence and then adjusted to assay an inflammation 4-plex signaling array (Quansys) A) The left image depicts the target localization in each well of the 96 well plate and the right image are representative images showing the correspondent results after chemiluminescent reaction (N≥3) . B) Histograms of densitometric analysis. Cells exposed to 1, 3, or 12 mo show and over activation in AKT p38 and p44/42 (Erk 1/2) MAPKs, while 12 mo exposed cells also showed an increase in total AKT and phospho STAT3. Cells exposed for 5 mo show a decrease in the activation of P38 MAPK and STAT3.
3.5.3 Total AKT
Cells exposed for 1, 3, and 12 mo to MMA(III) showed an increase in total AKT production, being more prominent in 12 mo-exposed cells (Figure 8B–Figure 1).
3.5.4 p44/42 MAPK (Erk1/2)
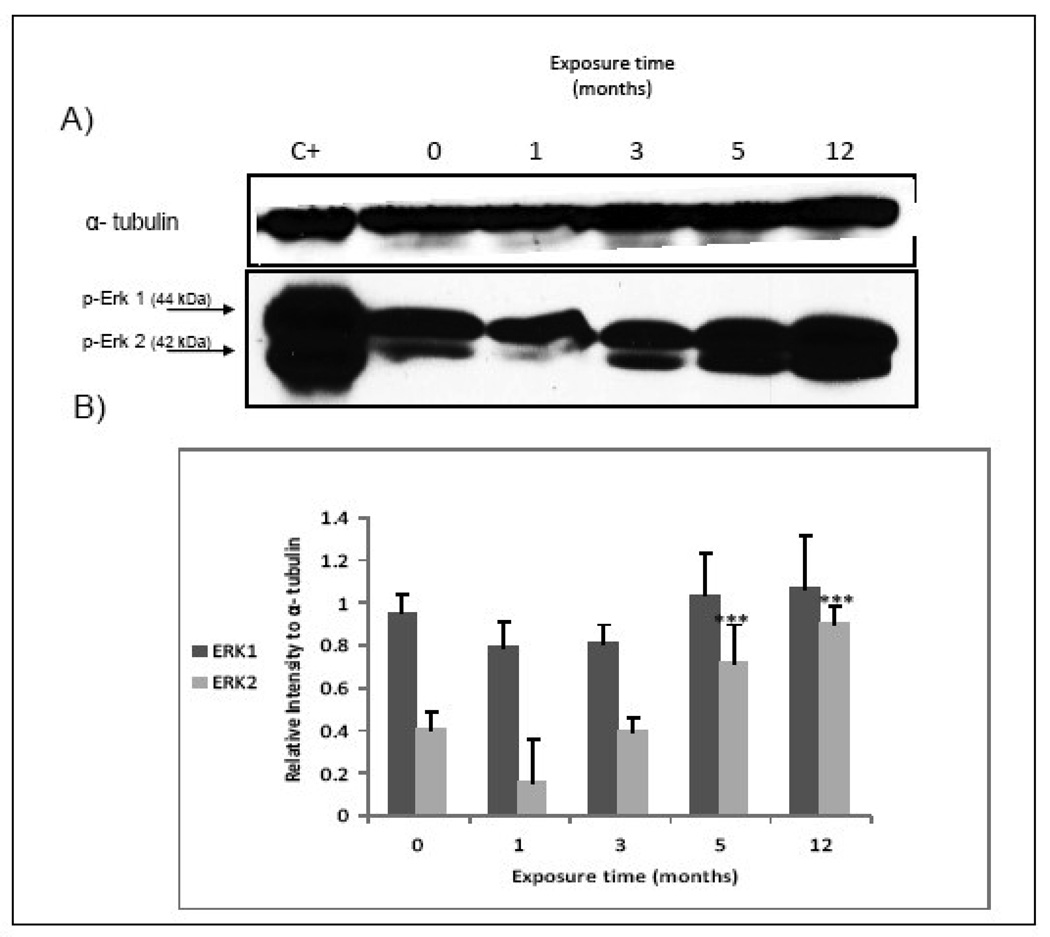
Simeonova et al. (2002) demonstrated that As(III) activates EGR via src activation and subsequent phosphorylation of ERK 1 and 2. Over-activation of ERK2 was evident in UROtsa cells exposed for 1, 3 and 12 mo to MMA(III)(Figure 8B–Figure 2). These results were confirmed with western blot analysis. In the blot a significant increase of ERK 2 was found in cells exposed for 5 and 12 mo to MMA (III), although ERK 1 seems not to change over this time period (Figure 9).
Figure 9.
Erk 2 is over expressed in chronically exposed cells to MMA(III). A) Representative western blot show a higher phosphorylation in Erk 2 in cells exposed chronically to 50 nM MMA(III) (N≥3). AKLL myocites treated with insulin whole cell lysate was used as positive control. B) Histogram of densitometric analysis. Asterisks indicate a significant difference [(***) P<0.001) compared to non treated cells as indicated by one way analysis of variance and Dunnett's Multiple Comparison as post-Test analysis.
3.5.5 p-38 MAPK
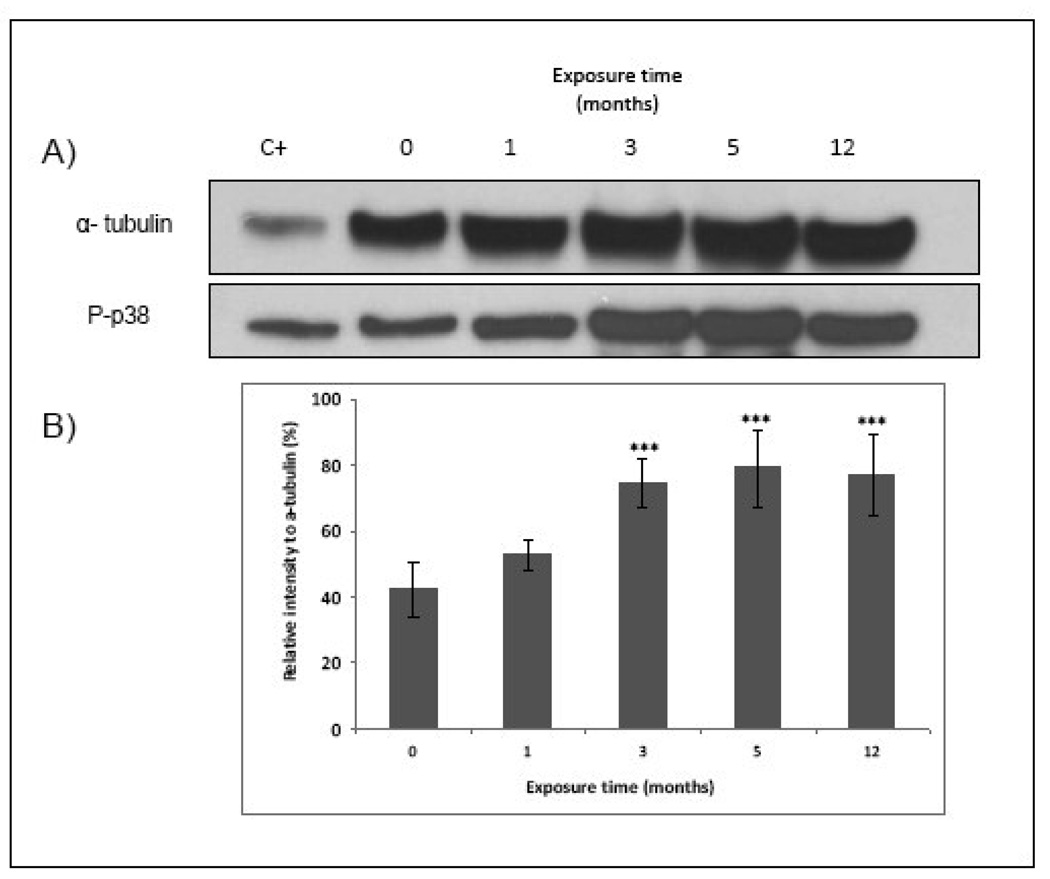
MAPK was found over-expressed in 1, 3 and 12 mo exposed cells, being more prominent in cells exposed for 3 and 12 mo to 50 nM of MMA(III) (Figure 8B–Figure 3). These results were confirmed by western blot analysis (Figure 10).
Figure 10.
Chronic exposure of UROtsa cells to MMA(III) over-expresses p-P38 MAPK. A) Representative blot shows higher phosphorylation of p-38 MAPK in UROtsa cells exposed chronically for 3, 5 and 12 mo to 50 nM MMA(III). (N≥3). HeLa plus heat shock whole cell lysate was used as positive control . B) Histogram of densitometric analysis. Asterisks indicate a significant difference [(***) P<0.001) compared to non treated cells as indicated by one way analysis of variance and Dunnett's Multiple Comparison as post-Test analysis.
3.5.6 p-STAT3
STAT3 activation was only increased after 12 mo MMA(III) exposure, but was significantly decreased in cells exposed for 5 mo to the toxic (Figure 8B–Figure 4).
2 Discussion
Chronic Inflammation is a key factor for the development of many cancers, including bladder cancer. Previous studies have shown that chronic exposure to 50 nM MMA (III) leads to a malignant transformation of the non-tumorigenic, immortalized urothelium cell line (UROtsa cells), thus this investigation examined if an inflammatory response plays a role in this MMA(III)-induced transformation. Inflammatory cytokines were profiled in cells exposed acutely and chronically to the MMA(III). When UROtsa cells are acutely exposed to MMA(III), many pro-inflammatory as well as anti-inflammatory cytokines are released in the cell culture media as soon as 12 hr after exposure. These cytokines include IL-1α and β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, and L-15 as well as a decrease in the antitumor cytokine IL-23. These results are coincident with those of Vega et al. (2001) showing a significant increase in IL-6, after 24 hr treatment of human epidermal keratinocytes using concentrations ranging from 1 to 50 nM methyl arsine oxide.
In chronic exposure to MMA(III), the most prominent cytokine over production occurred in cells exposed for 3 mo. These cells are significantly over expressing mainly pro-inflammatory cytokines like IL-6, IL-8, IL-17, TNF-α and TNF-β, as well as IL-2 and IL-15 which are anti-apoptotic cytokines and activators of NK cells. MIF, which was already over-produced in cells exposed for 1 mo, is still overproduced at 3 mo. Interestingly, the findings in this phase are coincident with the appearance of new characteristics in the cells including the cell ability to grow faster and an increase in cell size (Bredfeldt et al., 2006). In this sense, many pro-inflammatory cytokines induce cell proliferation, for example, IL-6 modulates the expression of genes involved in cell cycle progression, primarily through the JAK/STAT signaling (Lin and Karin, 2007) and IL-1α promotes the expression of genes involved in cell survival, proliferation and angiogenesis trough NFКβ and AP-1 activation (Wolf et al., 2001). Such results suggest that the increased and sustained production of pro-inflammatory cytokines in cells exposed for 3 mo to 50 nM MMA (III) could be responsible of the shortening in cells doubling time and are associated in some way to the increment in their size.
After five mo of exposure to MMA (III), the cells appear to go into a stationary phase in terms of cytokine production, showing a cytokine production profile similar to the non exposed cells, with exception of IL-10 which is two times over-expressed in this phase. However, the 12 mo exposed cells (the transformed cells) show an increased production of IL-6, IL-8, IL-15, and a decreased expression of IL-23. Somewhere between 5 and 12 mo of exposure the cells are altered in a way that leads them to reach a stable transformation state and the increase in the inflammatory response seems to be associated with it.
All of the over-expressed cytokines in our model have been found in increased quantities in other cancers. Bladder cancer studies have shown that tumor cells over-express many cytokines, growth factors and their receptors, for example TGFβ, IL1, IL-2, IL-6, IL-8, IL-10, IL-12, IFN-γ, and TNF-α (Cai et al., 2007). Our results with chronically MMA(III)-exposed UROtsa cells support the notion that MMA(III) is favoring the development of a sustained inflammatory state which is clearly associated with the progress of cell transformation in this study.
Interleukin-6, one of the over-expressed cytokines in our model, is a multifunctional cytokine that participates in inflammation-associated carcinogenesis (Rose-John and Schooltink, 2007), Hodgkin’s lymphoma (Cozen et al.,2004), and gastric cancers (Kai et al., 2005). As was mentioned above, IL-6 modulates the expression cell cycle progression and in apoptosis inhibition genes (Lin and Karin, 2007). It has been also demonstrated that IL-6 is associated with the cell ability to grow in soft agar (anchorage independent) in human colon carcinomas (Schneider et al., 2000). In our studies, this cytokine was over expressed all the time during cell transformation and its over-production in conjunction with other pro-inflammatory cytokines is coincident with the cells ability to growth in anchorage independency after 3 mo exposure to MMA (III).
Interestingly the cells exposed for 5 mo, which over-produced IL-10, do not present increased quantities of IL-6 compared with non-exposed cells. IL-10 is an anti-inflammatory cytokine that has been associated to cancer development since it can down-regulate the cell mediate immunity through stimulation of Th2 cells, resulting in improved tumor immune escape (Fortis et al., 1996., Merville et al., 1992).
Other key cytokine in cancer development is IL-8 (Xie, 2001). The over-expression of this cytokine has been correlated with tumor stage and disease progression in different neoplasias, including bladder cancer (Black et al., 2007). When this cytokine is transfected into non tumorigenic 253J–P cell line it leads the changed to a tumorigenic cell line and developed the ability of spontaneous metastases in an orthotopic bladder cancer xenograft model (Inoue et al., 2000). This cytokine was also over-expressed following both acute and chronic exposed to MMA(III) in our model, mainly after 36 hr in acute exposure and after 3 mo of MMA(III) chronic exposure. The transformed cells MSC52 (or 12 mo cells) also showed a constitutive over-expression of this cytokine being consistent with other studies (Mian et al., 2003).
MIF, a highly conserved immune mediator with pleiotropic effects, functionally belongs to the inflammatory cytokines. Its function is fundamental in tumorigenesis since it induces proliferation, apoptosis evasion, angiogenesis, and invasion (Bifulco et al., 2008). In addition, the angiogenic function (Xu et al., 2008) involves the ERK 1/2 and AKT pathways activation and the regulation of p53 and HIF-1 (Bifulco et al., 2008). The over-expression of MIF has been reported in many tumors such as prostate and breast cancer, melanomas, colon carcinomas, hepatocellular carcinoma, gastric cancer, and gliomas (Xu et al. 2008). In our studies MIF was over expressed in chronically exposed UROtsa cells in a time-dependent fashion, but in acute exposure to MMA(III) the production of this cytokine was not altered, which suggest that it is not responsible of the early pro-inflammatory cytokines over-production induced by MMA(III) but could be involved in the next phases of cell transformation.
Other interesting observation from our study was the decrease in IL-23 after acute exposure of UROtsa cells to MMA(III) and the same decrease found in MMA-induced transformed cells (12 mo exposed cells). IL-23 is one of the newly reported cytokines with anti-tumor activity (Reay et al., 2009). When melanoma cell lines are transfected with IL-23 gene, potent anti-tumor and anti-metastatic effects are expressed when injected in mice. In the transformation process this cytokine seems to be produced in a normal quantity in chronically exposed cells; however the transformed cells (exposed for 12 mo, MSC52) show a significant decrease in this cytokine production. This suggests that in this stage this cytokine do not longer confers enough anti-tumor protection to the cells.
Since the most of the over produced cytokines in this study are under the regulation of NFκβ, we decided to determine the activation and translocation of this factor following acute and chronic exposure to MMA(III). NFκB is known to be rapidly induced under cellular stress conditions and is very sensitive to arsenic-induced cellular oxidative status (Bode and Dong, 2000, 2002). Although involvement of NFκB in As-induced toxicity has been reported in other cells and organs, there are no reports about the role of its metabolite MMA (III) in urothelial cells. Our results clearly demonstrated that MMA(III) exposure leads to a significant increase and sustained NFκB p65 phosphorylation and translocation to the nucleus in chronically MMA (III)-exposed UROtsa cells. The sustained NFκβ activation may be responsible of for the over-expression of TNF, IL-1, IL-6 and MIF pro-inflammatory cytokines after chronic exposure to 50 nM MMA(III), while the observed over activation of c-Jun could account for UROtsa cells hyperproliferation after MMA (III) chronic exposure.
It has been reported that NFκB can serve as a target of MAP kinases (p38, ERK1/2 and JNK) (Cowan and Storey, 2003; Yang et al., 2003) and these MAPK have been shown to play important roles in the pathogenesis of many kind of cancers (Wagner et al., 2009, Wu et al., 2007). We investigated the changes in the levels of ERK1/2 and p38 by 4-plex ELISA array and immunoblot analyses. We observed a marked increase in protein content of phosphorylated p38 MAPK. It was also demonstrated that the treatment leads to an increased and sustained phosphorilation of ERK 2, in UROtsa cells. These results are coincident with studies from Ghosh et al. (2009) who demonstrated the activation of NFkb (p65) and p38 in cardiomyocites treated with 5 µM sodium arsenite for 5 wk and with those of Huang et al. (1999) who demonstrate that sodium arsenite in concentrations ranging from 0.8 to 200 µM induce cell transformation in mouse epidermal cell JB6 CI41 cells through the activation of ERK.
Previous work have demonstrate that 50 nM MMA(III) induces COX-2 expression in both short and chronic exposure in UROtsa cells via src activation, and the phosphorylation of ERK 1/2 and PI3K . The ERK 1/2 phosphorylation was demonstrated as early as 15 min after 50 nM MMA(III) treatment (Eblin et al., 2007. MMA(III). In this work we demonstrated a time-dependent increase in p38 and ERK 1/2 phosphorylation in cells exposed chronically to 50 nM MMA(III).
Inflammatory cytokines as IL-1 and IL-8 could be the responsible for such changes in acute and chronic exposed UROtsa cells. For example, the pro-inflammatory protein IL-1 has been reported to increase the expression of COX2 in human pulmonary epithelial cells line A549 and in other cells (Lin et al., 2000., Tsuzaki et al., 2003., Kuwano et al., 2004). On the other hand, IL-8 signaling promotes activation of the primary effectors PI3K or PLC, promoting the activation of Akt, PKC, calcium mobilization and/or MAPK signaling cascades (Waugh et al., 2008). IL-8 induce the activation of the classic MAPK signaling cascade (Raf-1/MAP/ERK 1 kinase/Erk) with downstream phosphorylation of ERK 1/2 in cancer cells (MacManus et al., 2007., Venkatakrishnan et al., 2000., Luppi et al., 2007) and p38 MAPK phosphorylation (Murphy et al., 2005). Interestingly using a keratinocytes cell line (HaCat) Ouyang et al. (2006) demonstrated that arsenite triggers the P13K/Akt/IKKbeta/NFКβ signal cascade which in turn induces the expression of cyclin D1 and MacManus et al. (2007) showed that IL-8 signaling regulates the cyclin D1 expression in human prostate cancer cells.
In previous work the treatment of UROtsa cells with 50 nM MMA(III) stimulate the phosphorylation of ErbB2 in an autophosphorylation site after 30 min of exposure to the toxic. Interestingly studies conducted in ovarian and lung cancer cells have shown that IL-8 signaling transactivates ErbB2 (epidermal growth factor receptor, EGFR), promoting in this way the downstream activation of MAPK signaling (Venkatakrishnan et al., 2000., Luppi et al., 2007) and finally inducing the cell to proliferate.
Other transcription factors that were found to be over activated in MMA(III)-chronically exposed UROtsa cells were c-Jun and STAT3. In addition to being a transcription factor involved in inflammatory responses in immune and non immune cells, c-Jun over-expression also has been associated with anchorage independent growth in human lung cancer (Maeno et al., 2006). Additionally, in the last few years different studies also have demonstrated the critical role of STAT3 in malignant transformation and tumorigenesis and it is accepted as a target for anti-cancer treatment (Siddiquee and Turkson, 2008).
Recently Hester et al. (2009) demonstrate that 24 hr exposure of UROTsa/F35 to 1 µM inorganic arsenic leads to a significant alteration in different cluster of genes related to chromosomal stability, DNA synthesis, and cell cycle. In this work 38% of genes associated with p38-MAPK and other MAPK cascade as well as 41% of and cell cycle G1/S transition regulation were modified after 24 hr exposure to arsenite. These results are consistent with the observations of our past and present studies with MMA(III)-chronically exposed UROtsa cells.
As we demonstrate here, 3 mo of exposure of UROtsa cells to 50 nM of MMA(III) seems to be a key phase for cellular transformation which involves: 1)pro inflammatory cytokine over-production including IL-1β, IL-6, IL-8, TNFα and TNFβ, and the anti apoptotic cytokines IL-2 and IL-15, 2) sustained NFКβ and c-Jun activation, 3) over activation of MAPK p38 and Erk2.
Other interesting observation in these cells was done by Wnek et al., (2009) who has demonstrated a sustained increase in DNA damage, mainly as single strand breaks, following 3 mo exposure to MMA(III) which reduced by using ROS scavengers. In this context, it is important to remember that during an inflammatory state the sources of ROS and reactive nitrogen species (RNS) can be different but they are mainly produced under the stimulus of pro-inflammatory cytokines like TNF and IL-1 in phagocytic as well as in non-phagocytic cells (Federico et al., 2007). These molecules and their derivative products could also activate transcription factors such as NFКβ, inducing the production of a new amount of inflammatory cytokines, enhancing inflammation and leading to more ROS and RSN production, self perpetuating oxidative stress in the cell. In chronic inflammation the ROS and RSN mediated carcinogenesis can be propagated through direct the DNA oxidation or nitration.
It is also interesting to note that these results suggest that 5 mo exposed cells seem to go into a quiescent state in terms of inflammatory response in cytokine as well as in cellular pathways; the cells in this state of transformation are over-producing the anti-inflammatory cytokine IL-10. However in the laboratory this cells shown all the characteristics of a transformation cell with hyper proliferation, anchorage independent growth and the ability to form tumors in nude mice, so, other experiments need to be conducted to know what is going on in this phase and the changes that finally lead to the cells to being transformed.
Taken together these findings show that MMA(III)-induced in vitro UROtsa cell transformation is associated with the promotion of a chronic inflammatory state with prevalence of cytokines associated with cell growth, proliferation, spread and survival, suggesting an important role of inflammation in MMA(III)-induced cell transformation. Other studies are being conducted by our group to determine the mechanisms by which the over-expressed inflammatory cytokines could be participating in such cell transformation.
ACKNOWLEDGMENTS
The authors would like to thanks Drs. Donald and Mary Ann Sens and Dr. Scott Garret for the UROtsa cells donation and assistance with culturing conditions, Dr. David Elliott (research microscopy core service) for his help in image analysis. These studies were supported by the NIEHS Superfund Basic Research Program (ES 04940) and the Southwest Environmental Health Sciences Center (ES 06694). MCG academic stay was funded through the Binational Center, University of Arizona, and CEL is funded by a CONACYT Fellowship (91679).
The abbreviations used are
- ErbB2
epidermal growth factor receptor B2
- Erk2
extracellular signal-regulated kinase 2
- HIF-a
hypoxia factor 1-α
- iNOS
inducible nitric oxide sintetase
- IL-
Interleukin
- MAPK
mitogen-activted protein kinases
- MIP-2
macrophage inflammatory protein- 2
- MIF
macrophage inhibitory factor
- MMA (III)
monomethylarsenous acid
- COX 1–2
ciclooxigenases 1–2
- NFКβ
nuclear factor Кβ
- PKC
protein kinase C
- PLC
phospholipase C
- PI3K
Phosphoinositide 3-kinases
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- STAT3
signal transducers and activator of transcription-3
- TNF-α
tumor necrosis factor-aplha.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aguirre-Bañuelos P, Escudero-Lourdes C, Sanchez-Peña LC, Del Razo LM, Perez-Urizar JT. Inorganic arsenic exposure affects pain behavior and inflammatory response in rat. Toxicol Appl Pharmacol. 2008;229(3):374–85. doi: 10.1016/j.taap.2008.01.029. 15. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts and Figures 2009. Atlanta, (GA): American Cancer Society; 2009. [Google Scholar]
- Ariztia EV, Lee CJ, Gogoi R, Fishman DA. The tumor microenvironment: key to early detection. Crit Rev Clin Lab Sci. 2006;43(5–6):393–425. doi: 10.1080/10408360600778836. [DOI] [PubMed] [Google Scholar]
- Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Cogliano V. Carcinogenicity of some aromatic amines, organic dyes, and related exposures. Lancet Oncol. 2008;9(4):322–3. doi: 10.1016/S1470-2045(08)70089-5. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. 17. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003;15(1):49–55. doi: 10.1016/s1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- Bifulco C, McDaniel K, Leng L, Bucala R. Tumor growth-promoting properties of macrophage migration inhibitory factor. Curr Pharm Des. 2008;14(36):3790–801. doi: 10.2174/138161208786898608. [DOI] [PubMed] [Google Scholar]
- Black PC, Dinney CP. Bladder cancer angiogenesis and metastasis--translation from murine model to clinical trial. Cancer Metastasis Rev. 2007;26(3–4):623–34. doi: 10.1007/s10555-007-9084-9. [DOI] [PubMed] [Google Scholar]
- Boffetta P. Tobacco smoking and risk of bladder cancer. Scand J Urol Nephrol Suppl. 2008 Sep;218:45–54. doi: 10.1080/03008880802283664. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Kopplin MJ, Gandolfi AJ. Effects of arsenite on UROtsa cells: low-level arsenite causes accumulation of ubiquitinated proteins that is enhanced by reduction in cellular glutathione levels. Toxicol Appl Pharmacol. 2004;198(3):412–8. doi: 10.1016/j.taap.2003.10.013. 1. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Jagadish B, Eblin KE, Mash EA, Gandolfi AJ. Monomethylarsonous acid induces transformation of human bladder cells. Toxicol Appl Pharmacol. 2006;216(1):69–79. doi: 10.1016/j.taap.2006.04.011. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode AM, Dong Z. The paradox of arsenic: molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol Hematol. 2002;42(1):5–24. doi: 10.1016/s1040-8428(01)00215-3. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. Signal transduction pathways: targets for chemoprevention of skin cancer. Lancet Oncol. 2000;1:181–8. doi: 10.1016/s1470-2045(00)00029-2. [DOI] [PubMed] [Google Scholar]
- Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity. 2007;26(3):281–285. doi: 10.1016/j.immuni.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol. 2004;201:32–39. doi: 10.1016/j.taap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Coimbra M, Kuijpers SA, van Seters SP, Storm G, Schiffelers RM. Targeted delivery of anti-inflammatory agents to tumors. Curr Pharm Des. 2009;15(16):1825–1843. doi: 10.2174/138161209788453220. [DOI] [PubMed] [Google Scholar]
- Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206(Pt 7):1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- Chow WH, Lindblad P, Gridley G, Nyrén O, McLaughlin JK, Linet MS, Pennello GA, Adami HO, Fraumeni JF., Jr Risk of urinary tract cancers following kidney or ureter stones. J Natl Cancer Inst. 1997;89(19):1453–1457. doi: 10.1093/jnci/89.19.1453. [DOI] [PubMed] [Google Scholar]
- Cozen W, Gill PS, Ingles SA, Masood R, Martínez-Maza O, Cockburn MG, Gauderman WJ, Pike MC, Bernstein L, Nathwani BN, Salam MT, Danley KL, Wang W, Gage J, Gundell-Miller S, Mack TM. IL-6 levels and genotype are associated with risk of young adult Hodgkin lymphoma. Blood. 2004;103(8):3216–3221. doi: 10.1182/blood-2003-08-2860. [DOI] [PubMed] [Google Scholar]
- Drobná Z, Jaspers I, Thomas DJ, Stýblo M. Differential activation of AP-1 in human bladder epithelial cells by inorganic and methylated arsenicals. FASEB J. 2003;17(1):67–69. doi: 10.1096/fj.02-0287fje. [DOI] [PubMed] [Google Scholar]
- Eblin KE, Bredfeldt TG, Buffington S, Gandolfi AJ. Mitogenic, pro-inflammatory signal transduction caused by monomethylarsonous acid in UROtsa cells. Tox Sci. 2007;95:321–330. doi: 10.1093/toxsci/kfl160. [DOI] [PubMed] [Google Scholar]
- Eblin KE, Jensen TJ, Wnek SM, Buffington SE, Futscher BW, Gandolfi AJ. Reactive oxygen species regulate properties of transformation in UROtsa cells exposed to monomethylarsonous acid by modulating MAPK signaling. Toxicology. 2009;255(1–2):107–114. doi: 10.1016/j.tox.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121(11):2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- Fortis C, Foppoli M, Gianott i L, Galli L, Citterio G, Consogno G. Increased interleukin-10 serum levels in patients with solid tumors. Cancer Lett. 1996;104:1–5. doi: 10.1016/0304-3835(96)04213-9. [DOI] [PubMed] [Google Scholar]
- Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S, Mahidol C, Ruchirawat M, Samson LD. Activation of Inflammation/NF-kappaB Signaling in Infants Born to Arsenic-Exposed Mothers. PLoS Genet. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J, Das J, Manna P, Sil PC. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappaB, p38 and JNK MAPK pathway. Toxicol Appl Pharmacol. 2009;240(1):73–87. doi: 10.1016/j.taap.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Guo HR, Chiang HS, Hu H, Lipsitz SR, Monson RR. Arsenic in drinking water and incidence of urinary cancers. Epidemiology. 1997;8(5):545–555. doi: 10.1097/00001648-199709000-00012. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, Balkwill FR. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol. Cancer Ther. 2007;6(7):1993–2002. doi: 10.1158/1535-7163.MCT-07-0118. [DOI] [PubMed] [Google Scholar]
- Hester S, Drobná Z, Andrews D, Liu J, Waalkes M, Thomas DJ, Styblo M. Expression of AS3MT alters transcriptional profiles in human urothelial cells exposed to arsenite. Hum Exp Toxicol. 2009;28(1):49–61. doi: 10.1177/0960327109102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Chung YC. Influence of interleukin-6 on the invasiveness of human colorectal carcinoma. Anticancer Res. 2006;26(6B):4607–4614. [PubMed] [Google Scholar]
- Huang C, Ma WY, Li J, Goranson A, Dong Z. Requirement of Erk, but not JNK, for arsenite-induced cell transformation. J Biol Chem. 1999;274(21):14595–14601. doi: 10.1074/jbc.274.21.14595. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- Inoue K, Slaton JW, Kim SJ, Perrotte P, Eve BY, Bar-Eli M, Radinsky R, Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000;60(8):2290–2299. 15. [PubMed] [Google Scholar]
- Kai H, Kitadai Y, Kodama M, Cho S, Kuroda T, Ito M, Tanaka S, Ohmoto Y, Chayama K. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005;25(2A):709–713. [PubMed] [Google Scholar]
- Khan MA, Travis LB, Lynch CF, Soini Y, Hruszkewycz AM, Delgado RM, Holowaty EJ, van Leeuwen FE, Glimelius B, Stovall M, Boice JD, Jr, Tarone RE, Bennett WP. p53 mutations in cyclophosphamide-associated bladder cancer. Cancer Epidemiol Biomarkers Prev. 1998;7(5):397–403. [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Kosugi A, Hayashi F, Liddicoat DR, Yasuda K, Saitoh S, Hamaoka T. A pivotal role of cysteine 3 of Lck tyrosine kinase for localization to glycolipid-enriched microdomains and T cell activation. Immunol. Lett. 2001;76:133–138. doi: 10.1016/s0165-2478(01)00174-2. [DOI] [PubMed] [Google Scholar]
- Kuwano T, Nakao S, Yamamoto H, Tsuneyoshi M, Yamamoto T, Kuwano M, Ono M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004;18(2):300–310. doi: 10.1096/fj.03-0473com. [DOI] [PubMed] [Google Scholar]
- Lantz RC, Hays AM. Role of oxidative stress in arsenic-induced toxicity. Drug Metab Rev. 2006;38(4):791–804. doi: 10.1080/03602530600980108. [DOI] [PubMed] [Google Scholar]
- Lin CC, Kuo CT, Cheng CY, Wu CY, Lee CW, Hsieh HL, Lee IT, Yang CM. IL-1 beta promotes A549 cell migration via MAPKs/AP-1- and NF-kappaB-dependent matrix metalloproteinase-9 expression. Cell Signal. Nov. 2009;21(11):1652–1662. doi: 10.1016/j.cellsig.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Lin CH, Sheu SY, Lee HM, Ho YS, Lee WS, Ko WC, Sheu JR. Involvement of protein kinase C-gamma in IL-1beta-induced cyclooxygenase-2 expression in human pulmonary epithelial cells. Mol Pharmacol. 2000;57(1):36–43. [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007 May;117(5):1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CF, Cohen MB. Urinary system. Cancer. 1995 Jan 1;75(1 Suppl):316–329. doi: 10.1002/1097-0142(19950101)75:1+<316::aid-cncr2820751314>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007. 2007;56(1):25–33. doi: 10.1016/j.lungcan.2006.11.014. [DOI] [PubMed] [Google Scholar]
- MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S, Purcell C, McGurk M, Johnston PG, Waugh DJ. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res. 2007;5(7):737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- Maeno K, Masuda A, Yanagisawa K, Konishi H, Osada H, Saito T, Ueda R, Takahashi T. Altered regulation of c-jun and its involvement in anchorage-independent growth of human lung cancers. Oncogene. 2006;25(2):271–277. doi: 10.1038/sj.onc.1209018. 12. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. 24. [DOI] [PubMed] [Google Scholar]
- Matthews CP, Colburn NH, Young MR. AP-1 a target for cancer prevention. Curr Cancer Drug Targets. 2007;7:317–324. doi: 10.2174/156800907780809723. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Yamagiwa Y, Ueno Y, Patel T. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol. 2006;44(6):1055–1065. doi: 10.1016/j.jhep.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merville P, Rousset F, Banchereau J, Klein B, Betaille R. Serum interleukin-10 in early stage multiple myeloma. Lancet. 1992;340:1544–1545. doi: 10.1016/0140-6736(92)92795-h. [DOI] [PubMed] [Google Scholar]
- Mian BM, Dinney CP, Bermejo CE, Sweeney P, Tellez C, Yang XD, Gudas JM, McConkey DJ, Bar-Eli M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res. 2003;9(8):3167–3175. [PubMed] [Google Scholar]
- Millar IT, Heany H, Heinekey DM, Fernelius WC. Methyliiodoarsine. Inorg. Synth. 1960;6:113–115. [Google Scholar]
- Mommsen S, Sell A. Prostatic hypertrophy and venereal disease as possible risk factors in the development of bladder cancer. Urol Res. 1983;11(2):49–52. doi: 10.1007/BF00256946. [DOI] [PubMed] [Google Scholar]
- Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, Montironi R, Waugh DJ. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005;11(11):4117–4127. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Li J, Ma Q, Huang C. Essential roles of PI-3K/Akt/IKKbeta/NFkappaB pathway in cyclin D1 inducton by arsenite in JB6 C141 cells. Carcinogenesis. 2006;27(4):864–873. doi: 10.1093/carcin/bgi321. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004 Sep 23;431(7007):461–466. doi: 10.1038/nature02924. 2004. [DOI] [PubMed] [Google Scholar]
- Reay J, Kim SH, Lockhart E, Kolls J, Robbins PD. Adenoviral-mediated, intratumor gene transfer of interleukin 23 induces a therapeutic antitumor response. Cancer Gene Ther. 2009 doi: 10.1038/cgt.2009.27. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S, Schooltink H. Cytokines are a therapeutic target for the prevention of inflammation-induced cancers. Recent Results Cancer Res. 2007;174:57–66. doi: 10.1007/978-3-540-37696-5_5. [DOI] [PubMed] [Google Scholar]
- Shen S, Lee J, Weinfeld M, Le XC. Attenuation of DNA damage-induced p53 expression by arsenic: a possible mechanism for arsenic co-carcinogenesis. Mol Carcinog. 2008;47(7):508–518. doi: 10.1002/mc.20406. [DOI] [PubMed] [Google Scholar]
- Siddiquee KA, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18(2):254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DT, Hartge P, Morrison AS, Devesa SS. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 1992;6(1):1–30. [PubMed] [Google Scholar]
- Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(−/−) mice. Environ Health Perspect. 2003;111(14):1744–1748. doi: 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Hoeflich A, Fischer JR, Wolf E, Sordat B, Lahm H. Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett. 2000;151(1):31–38. doi: 10.1016/s0304-3835(99)00401-2. [DOI] [PubMed] [Google Scholar]
- Stojanović I, Cvjetićanin T, Lazaroski S, Stosić-Grujicić S, Miljković D. Macrophage migration inhibitory factor stimulates interleukin-17 expression and production in lymph node cells. Immunology. 2009;126(1):74–83. doi: 10.1111/j.1365-2567.2008.02879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouba KJ, Germolec DR. Micromolar concentrations of sodium arsenite induce cyclooxygenase-2 expression and stimulate p42/44 mitogen-activated protein kinase phosphorylation in normal human epidermal keratinocytes. Toxicol Sci. Jun. 2004;79(2):248–257. doi: 10.1093/toxsci/kfh132. [DOI] [PubMed] [Google Scholar]
- Tsai SH, Liang YC, Chen L, Ho FM, Hsieh MS, Lin JK. Arsenite stimulates cyclooxygenase-2 expression through activating IkappaB kinase and nuclear factor kappaB in primary and ECV304 endothelial cells. J Cell Biochem. 2002;84:750–758. doi: 10.1002/jcb.10096. [DOI] [PubMed] [Google Scholar]
- Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. IL-1 beta induces COX2, MMP-1, −3 and −13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21(2):256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Vega L, Styblo M, Patterson R, Cullen W, Wang C, Germolec D. Differential effects of trivalent and pentavalent arsenicals on cell proliferation and cytokine secretion in normal human epidermal keratinocytes. Toxicol Appl Pharmacol. 2001;172(3):225–232. doi: 10.1006/taap.2001.9152. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000;275(10):6868–6875. doi: 10.1074/jbc.275.10.6868. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9(8):537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wnek SM, Medeiros MM, Eblin KE, Gandolfi AJ. Persistence of DNA damage following exposure of human bladder cells to chronic monomethylasonous acid. Tox Appl. Pharm. 2009 doi: 10.1016/j.taap.2009.08.016. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JS, Chen Z, Dong G, Sunwoo JB, Bancroft CC, Capo DE, Yeh NT, Mukaida N, Van Waes C. IL-(Interleukin) 1-alpha promotes nuclear factor-Кβ and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin. Cancer Res. 2001:1812–1820. [PubMed] [Google Scholar]
- Wu GS. Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev. 2007;26(3–4):579–585. doi: 10.1007/s10555-007-9079-6. [DOI] [PubMed] [Google Scholar]
- Wu J, Liu J, Waalkers MP, Chen ML, Li L, Li CX, Yang Q. High dietary fat exacerbates arsenic induced liver fibrosis in mice. Exp. Biol. Med. 2008;233:377–384. doi: 10.3181/0710-RM-269. [DOI] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12(4):375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008;261(2):147–157. doi: 10.1016/j.canlet.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Hirano S, Vogel CF, Cui X, Matsumura F. Selective activation of NFKB and E2F by low concentration of arsenite in U937 human monocytic leukemia cells. J Biochem Mol Toxicol. 2008;22(2):136–146. doi: 10.1002/jbt.20222. [DOI] [PubMed] [Google Scholar]
- Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]