Abstract
Background & Aims
The appropriate alanine aminotransferase (ALT) threshold value to use for diagnosis of chronic liver disease in children is unknown. We sought to develop sex-specific, biology-based, pediatric ALT thresholds.
Methods
The screening ALT for elevation in today’s youth (SAFETY) study collected observational data from acute care children’s hospitals, the national health and nutrition examination survey (NHANES, 1999–2006), overweight children with and without non-alcoholic fatty liver disease (NAFLD), and children with chronic Hepatitis B virus (HBV) or Hepatitis C virus (HCV) infections. The study compared the sensitivity and specificity of ALT thresholds currently used by children’s hospitals versus study-derived, sex-specific, biology-based, ALT thresholds for detecting children with NAFLD, HCV, or HBV.
Results
The median upper limit of ALT at children’s hospitals was 53 U/L (range, 30–90). The 95th percentile levels for ALT in healthy weight, metabolically normal, liver disease-free, NHANES pediatric participants were 25.8 U/L (boys) and 22.1 U/L (girls). The concordance statistics of these NHANES-derived thresholds for liver disease detection were 0.85 (95% confidence interval [CI] 0.74–0.96) in boys and 0.91 (95% CI 0.83–0.99) in girls for NAFLD, 0.80 (95% CI 0.70–0.91) in boys and 0.79 (95% CI 0.69–0.89) in girls for HBV, and 0.86 (95% CI 0.77–0.95) in boys and 0.84 (95% CI 0.75–0.93) in girls for HCV. Using current children’s hospitals ALT thresholds, the median sensitivity for detection of NAFLD, HBV, and HCV ranged from 32% to 48%; median specificity was 92% (boys) and 96% (girls). Using NHANES-derived thresholds, the sensitivities were 72% (boys) and 82% (girls); specificities were 79% (boys) and 85% (girls).
Conclusions
The upper limit of ALT used in children’s hospitals varies widely and is set too high to reliably detect chronic liver disease. Biology-based thresholds provide higher sensitivity and only slightly less specificity. Clinical guidelines for use of screening ALT and exclusion criteria for clinical trials should be modified.
Keywords: obesity, hepatitis, nonalcoholic fatty liver disease, patient safety, clinical trials
Introduction
Chronic liver disease is an increasingly important clinical problem in children and adolescents.1 Screening for chronic liver disease is most commonly done using serum alanine aminotransferase (ALT) activity. Pediatric clinical trials use ALT to exclude potential subjects with liver disease. Numerous national guidelines recommend the use of ALT to screen for nonalcoholic fatty liver disease (NAFLD) in overweight and obese children.2-4
Despite the widespread use of ALT in pediatrics, the threshold value for detecting liver disease in children is unknown and the proper interpretation of ALT assays performed in children is unclear. Due to these uncertainties, laboratories establish their own normal ranges for ALT based on analyses of their local populations. Laboratories, however, do not exclude children with NAFLD from these population samples. Therefore the distribution of ALT values may be skewed upwards, and the upper limit of normal (ULN) will be set falsely high. In adults, there has been an increasing acceptance that the clinically relevant ULN for ALT is lower than frequently used,5 and it is likely the same is true for children. If so, many children with potentially treatable liver disease may be missed or exposed to unnecessary risk by taking potentially hepatotoxic medicines. Moreover, clinical trials commonly use a cutoff of 2 to 3 times the ULN as a means to exclude subjects with liver disease. As the ULN value is not standardized, the ALT threshold used to protect against including pediatric subjects with occult liver disease may vary widely across hospitals, research centers, and geographic locations.
It is important to have a uniform standard for normal values of ALT in children. The liver SAFETY (Screening ALT For Elevation in Today’s Youth) study was conducted to help develop such a standard and had the following aims: 1) to determine the ALT thresholds used currently in acute care children’s hospitals in the United States, 2) to develop new gender-specific biologically-based ALT thresholds derived from a national population sample of children free of known liver disease or established risk factors for liver disease, and 3) to compare the sensitivity and specificity of the ALT thresholds in current use with the study-derived ALT thresholds for classifying children as having or not having 3 common forms of chronic liver disease (Hepatitis B virus (HBV), Hepatitis C virus (HCV), and NAFLD).
METHODS
Aim 1: Determination of ALT thresholds in current use
We surveyed all freestanding acute care children’s hospitals in the United States (n = 43), (see Appendix 1 for list). Clinical laboratories provided the ALT reference range used at their respective center for boys and girls between the ages of 2 through 17 years. Data on how each institution determined its normal range was not provided. However because of strong data supporting a gender-specific difference in the normal threshold value of ALT in adults, information was obtained regarding the use of gender-specific normal values.
Aim 2: Population-based determination of ALT threshold
In order to determine the distribution of ALT in a nationally representative sample, we included participants of the National Health and Nutrition Examination Survey (NHANES) 1999 to 2006 between ages of 12 through 17 who had ALT measured. In order to define a group of children without liver disease or known risk factors for liver disease, numerous exclusions were made. The product inserts of all medications reported by NHANES participants were reviewed; 90 medications had an association with hepatotoxicity and/or ALT elevation (see Appendix 2). We excluded participants who used any of these 90 medications. Fatty liver is associated with both underweight and overweight. Therefore we excluded participants who were underweight, overweight or obese as defined by having a BMI percentile outside the normal range of the 5th percentile to < 85th percentile.2
We also excluded subjects with viral hepatitis, HIV, or iron overload. Hepatitis B surface antigen was tested using the Auszyme Monoclonal test (Abbot Diagnostics). All specimens considered reactive initially were repeat-tested in duplicate using the same procedure. If either of the repeat tests were reactive, the specimens were considered positive and subjects were excluded. Hepatitis C antibody was measured using the direct solid-phase enzyme immunoassay. Positive specimens were tested using the Chiron RIBA 3.0 Strip Immunoblot Assay (Chiron Corporation, Inc). Subjects with a positive or indeterminate RIBA result were excluded. HIV antibody was tested using the Synthetic Peptide Enzyme Immunoassay (Bio-Rad Laboratories, Redmond, WA) to HIV-1 or HIV-2. Positive specimens were confirmed using the Cambridge Biotech HIV Western Blot Kit (Calypte Biomedical Corporation, Rockville, MD). Iron overload was inferred if transferrin saturation >= 50%.
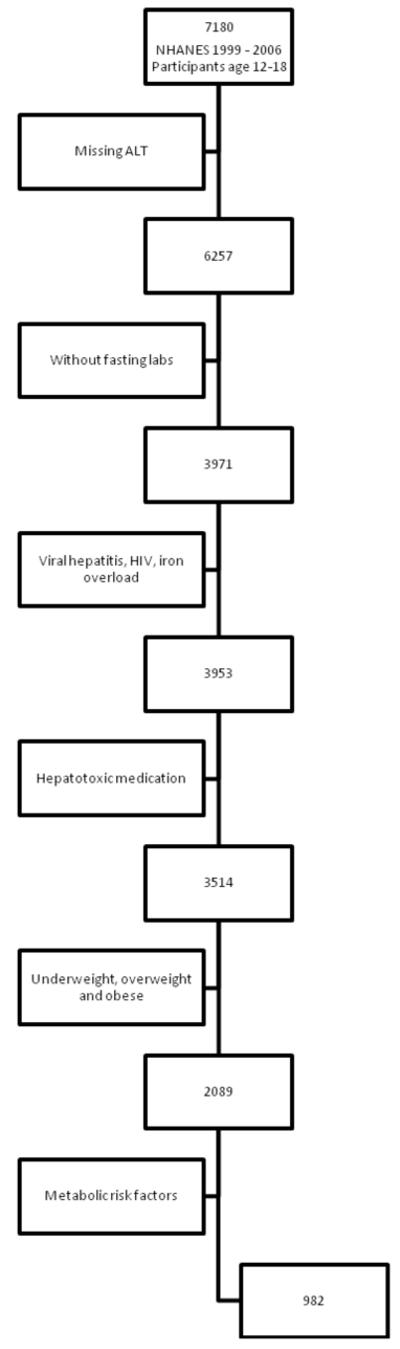
Because of the association between fatty liver and insulin resistance6 we excluded those participants who were missing fasting laboratory data as well as those with any of the following metabolic risk factors for NAFLD: elevated serum triglyceride( >150 mg/dL), low serum HDL-cholesterol (<40 mg/dL for boys or < 50 mg/dL for girls), or impaired fasting glucose ( ≥ 100 mg/dL).7 Table 1 shows the characteristics of the sample with fasting labs before and after exclusions. Figure 1 shows the flow of inclusion and exclusion.
Table 1. Demographic and metabolic characteristics NHANES reference sample.
| Total Sample | Final Sample | |||
|---|---|---|---|---|
| Characteristic | Boys (N = 2029) |
Girls (N = 1942) |
Boys (N = 548) |
Girls (N = 434) |
| Age, mean (SD), y | 14.5 (1.7) | 14.6 (1.7) | 14.5 (1.8) | 15.1 (1.8) |
| Race/Ethnicity, N (%) | ||||
| African American, non-Hispanic | 300 (14.8) | 255 (13.1) | 100 (18.4) | 61 (14.0) |
| Hispanic | 371 (18.3) | 365 (18.8) | 95 (17.3) | 78 (18.0) |
| White, non-Hispanic | 1244 (61.3) | 1196 (61.6) | 302 (55.0) | 254 (58.6) |
| Other, non-Hispanic | 114 (5.6) | 126 (6.5) | 51 (9.3) | 41 (9.4) |
| Triglyceride mean (SD), mg/dL | 91 (83) | 85 (43) | 68 (26) | 66 (23) |
| HDL-Cholesterol, mean (SD), mg/dL | 49 (12) | 53 (12) | 54 (10) | 62 (10) |
| Glucose, mean (SD), mg/dL | 95 (17 ) | 90 (10) | 91 (10) | 87 (6) |
| ALT, mean (SD), U/L | 21 (12) | 16 (6) | 18 (5) | 15(5) |
Figure 1. Inclusion and Exclusion for NHANES Reference Population.
Aim 3: Clinically characterized samples with and without chronic liver disease
In order to test the sensitivity and specificity of ALT thresholds, we assembled a group of children with normal livers and 3 groups of children with liver disease: HBV, HCV, and NAFLD. The demographics of these groups are shown in Table 2. The protocol was approved by the institutional review boards of the UC San Diego (La Jolla, CA) and Mount Sinai Medical Center (New York, NY). The parent(s) of all subjects < 18 years of age provided written informed consent for their children. Written assent was obtained from all children age 7 to 17.
Table 2. Demographics of clinically characterized children with and without liver disease.
| Normal | HBV | HCV | NAFLD | |
|---|---|---|---|---|
| Age, mean (SD), y | 12.7 (2.9) | 9.3 (5.9) | 10.3 (5.4) | 13.2 (2.7) |
| Sex, N (%) | ||||
| Boys | 24 (48) | 47 (47) | 48 (48) | 25 (50) |
| Girls | 26 (52) | 53 (53) | 52 (52) | 25 (50) |
| ALT, mean (SD), U/L | 22 (11) | 61 (95) | 55 (49) | 50 (39) |
Normal livers
The challenge was to identify children with normal livers without selecting for serum ALT activity. Therefore we screened children with magnetic resonance imaging (MRI) to determine the absence of fatty liver. Additional requirements to assure the presence of a normal liver were no use of potentially hepatotoxic medications (see Appendix 2), absence of known chronic liver disease, absence of chronic alcohol consumption, normal laboratory studies (complete blood count, CRP, negative HCV antibody, negative HBsAg). There is controversy regarding the amount of liver fat, as measured by MRI, which can be considered normal. Because MRI-estimated liver fat fractions as small as 3% may be abnormal, we defined normal as a liver fat fraction between 0 and 2%.8
NAFLD
Because children are typically referred for NAFLD based upon serum ALT elevation, we sought to identify children with NAFLD without regard for serum ALT activity. Obese children with unknown liver status were recruited for MRI. In order to be included in the NAFLD group, participants needed to be free from any potential reason for hepatic steatosis other than NAFLD including medication (amiodarone, glucocorticoids, L-asparaginase, valproic acid), chronic disease (cystic fibrosis, HIV, hepatitis C, Wilson’s disease, celiac disease, type 1 diabetes mellitus), and chronic alcohol consumption. Liver fat fraction ≥ 5% was defined as fatty liver.9
Viral Hepatitis
The HBV and HCV groups were identified from children referred to a pediatric liver clinic. Children with viral hepatitis were not included nor excluded on the basis of ALT. Children co-infected with both hepatitis B and C, or with HIV were excluded. A diagnosis of chronic HBV was based upon: 1) persistently positive HBsAg for > 6 months, and 2) elevated serum HBV DNA. A diagnosis of chronic hepatitis C virus infection was based upon: 1) age > 18 months, 2) positive anti-hepatitis C antibody titer, and 3) detectable hepatic C virus by serum PCR.
MRI Protocol
Hepatic fat fraction was measured non-invasively using MRI with a modified Dixon technique.10 Subjects were scanned at 1.5T (Siemens Symphony, Siemens Medical Systems, Erlangen, Germany). Multi-slice two-dimensional spoiled gradient recalled echo images were obtained in the axial plane. To minimize T1 effects, a low flip angle (10°) was used with a repetition time of 122 msec.11-13 To assess fat-water signal interference effects and correct for T2* effects,11, 13, 14 six echoes were obtained at serial out-of-phase and in-phase echo times (2.3, 4.6, 6.9, 9.2, 11.5, 13.8 msec) during a single breath-hold.
For each subject, images at each of the six echo times were reviewed on a diagnostic-quality digital monitor. Avoiding organ boundaries, imaging artifacts, major vessels and bile ducts, a single elliptical region of interest, approximately 300-400 mm2, was manually selected within a representative slice of liver parenchyma. The mean signal intensity within each ROI was measured and recorded. As described by Bydder, the measured signal intensity variation was modeled as a function of echo time.13 This method models the interference between fat-water and fat-fat while also correcting for exponential T2* decay and computes the fat fraction (defined as [fat/(fat+water)]) in the liver region of interest.
Data Analysis
Data were expressed as means ± standard deviations or medians with inter-quartile ranges. NHANES 1999 – 2006 participants without risk factors for liver disease were used to derive a new ALT cut point. NHANES was based on a complex, multistage, stratified, clustered probability sampling which could not be treated as a simple random sample. The SUDAAN 9.0 module (Research Triangle Institute, Research Triangle Park, NC) was used to incorporate primary sampling unit, strata and weighting into the analysis15. The 95th percentile of serum ALT was determined separately for boys and girls and was used to define new gender-specific ALT thresholds which could be tested in the subsequent analyses.
To perform sensitivity and specificity calculations, clinically characterized samples with normal livers were combined with those participants with HBV, HCV, and NAFLD. The ROC curve of various ALT cut points was plotted for each disease and gender. The sensitivity and [1-specificity] of ALT cut points currently utilized by the children’s hospitals were plotted on the ROC curves. The sensitivity and specificity of each hospital cut-point and the NHANES-derived proposed cut-point were recorded. The concordance statistics and 95 percent confidence interval of serum ALT in detecting HBV, HCV, and NAFLD was calculated using Delong’s method.
RESULTS
Normal values in current use
The median and range for the ULN used for serum ALT activity by children’s hospitals was 53 U/L (30 to 90). Gender-specific normal values were used at 44% (19/43) of children’s hospitals. When restricted to hospitals using gender-specific cut-points the median ULN for boys was 50 U/L (30 to 70) and for girls was 40 U/L (29 to 65).
NHANES derived normal values
Among all NHANES participants age 12 to 17 years, before excluding those with risk factors for liver disease, the 95th percentile for ALT was 37.2 U/L in boys and 26.0 U/L in girls. The flow chart in figure 1 shows how the NHANES reference sample was developed. After all exclusions were applied 982 participants remained (548 boys and 434 girls). The 95th percentile for ALT in this reference sample was 25.8 U/L in boys and 22.1 U/L in girls. Using ALT >25 U/L for boys and ALT > 22 U/L for girls as the threshold for abnormal ALT, 5.3% of boys and 4.4% of girls in the reference sample had elevated serum ALT activity.
Application of Existing and Newly Proposed Cut-points
Prevalence of abnormal ALT in United States
Among all adolescents in NHANES that had ALT available (n = 6257), using the median cutoffs currently used by children’s hospitals, 2.4% (95% CI 1.7% - 3.4%) of boys and 1.3% (95% CI 0.9% – 2.0%) of girls had abnormal ALT. Using the threshold values defined by the healthy weight, metabolically normal, liver disease-free reference sample, 15.0% (95% CI 13.3% - 16.9%) of boys and 8.6% (95% CI 7.2% - 10.2%) of girls had abnormal ALT. Based on the weighting of the NHANES design, approximately 1,631,000 boys and 787,000 girls age 12 – 17 in the United States would be classified as normal using the median ALT threshold of children’s hospitals but abnormal according to the threshold defined by the reference sample of healthy subjects.
Detection of Chronic Liver Disease
ROC curves were developed separately for boys and girls (figures 2 and 3). Plotted on these figures are the sensitivity and [1-specificity] for each form of chronic liver disease (HBV, HCV, and NAFLD) of each ALT threshold value in current use by a children’s hospital in the United States. The concordance statistics for detection of liver disease was 0.80 (95% CI 0.70 – 0.91) for HBV, 0.86 (95%CI 0.77 – 0.95) for HCV, and 0.85 (95%CI 0.74 – 0.96) for NAFLD. In girls, the concordance statistics were 0.79 (95%CI 0.69 – 0.89) for HBV, 0.84 (95%CI 0.75 – 0.93) for HCV, and 0.91 (95%CI 0.83 – 0.99) for NAFLD.
Figure 2. Receiver Operating Characteristic (ROC) curves for ALT in Boys and Girls.
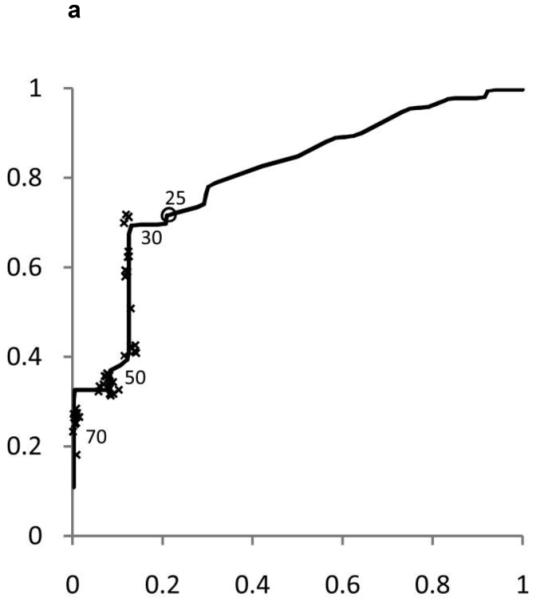
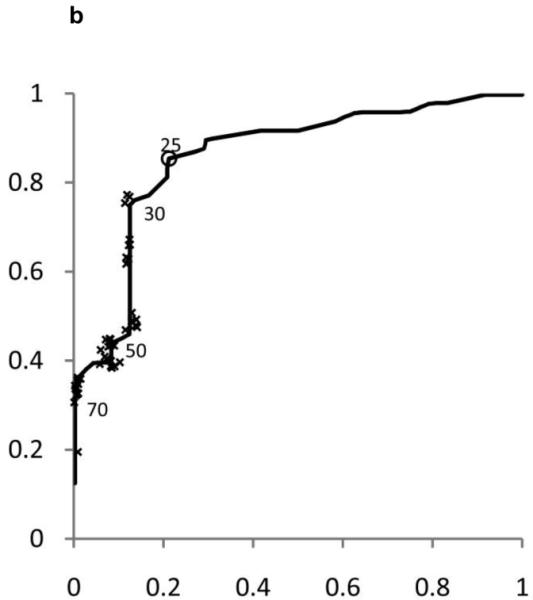
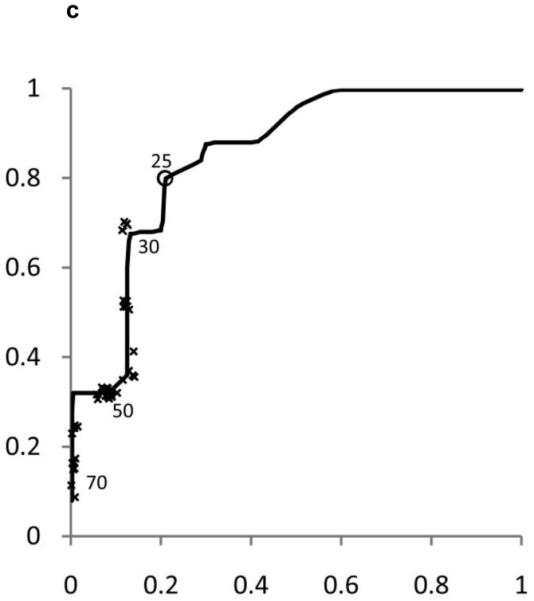
Shown in Figure 2 for boys and Figure 3 for girls are the sensitivity and [1-specificity] of ALT in detecting Hepatitis B Viral Infection (Figure 2A, 3A), Hepatitis C Viral Infection (Figure 2B, 3B), and Nonalcoholic Fatty Liver Disease (Figure 2C, 3C). Plotted along the ROC curves are the ALT thresholds currently utilized by children’s hospitals (marked with: x) and the biologically-derived ALT threshold (marked with: o). The numbers on the ROC curves represent the median and interquartile range of ALT thresholds currently used by children’s hospitals, as well as the biologically- derived ALT threshold.
Figure 3. Receiver Operating Characteristic (ROC) curves for ALT in Boys and Girls.
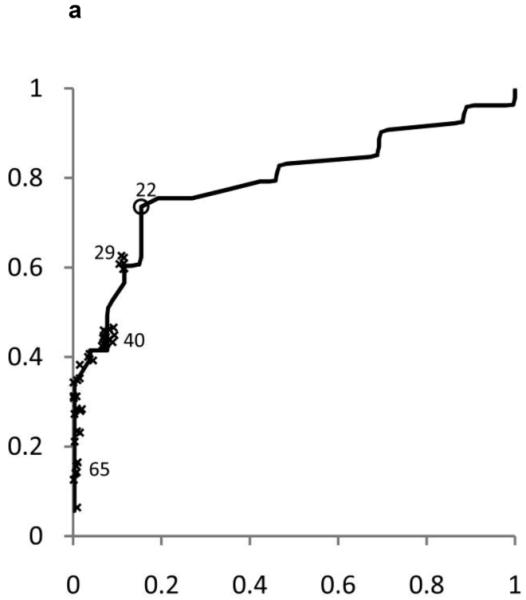
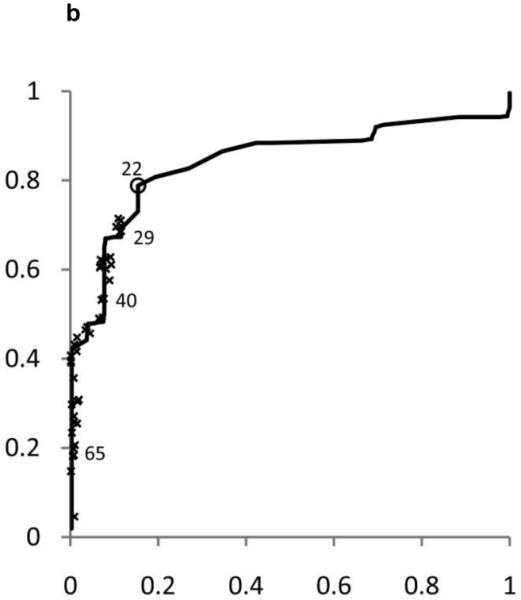
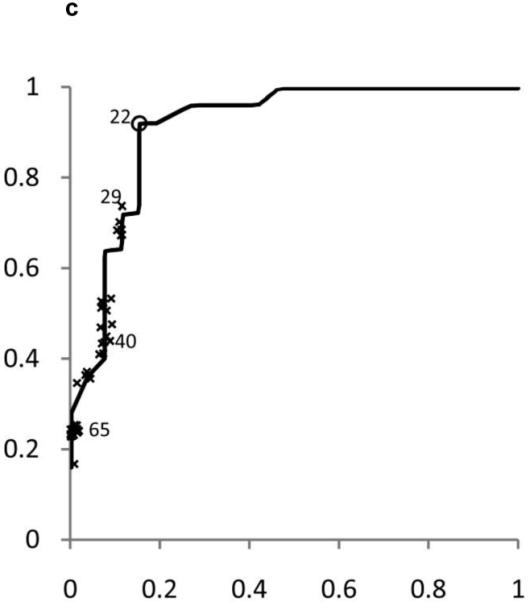
Shown in Figure 2 for boys and Figure 3 for girls are the sensitivity and [1-specificity] of ALT in detecting Hepatitis B Viral Infection (Figure 2A, 3A), Hepatitis C Viral Infection (Figure 2B, 3B), and Nonalcoholic Fatty Liver Disease (Figure 2C, 3C). Plotted along the ROC curves are the ALT thresholds currently utilized by children’s hospitals (marked with: x) and the biologically-derived ALT threshold (marked with: o). The numbers on the ROC curves represent the median and interquartile range of ALT thresholds currently used by children’s hospitals, as well as the biologically- derived ALT threshold.
Table 3 shows the sensitivity and specificity for the ALT thresholds used in children’s hospitals and for the NHANES-derived threshold. For the children’s hospitals ALT thresholds, sensitivity was low for HBV, HCV, and NAFLD in boys and girls, with median sensitivities ranging from 32% to 48%. By comparison, the median specificities were high: 92% in boys and 96% in girls. When the NHANES-derived threshold was applied for boys the sensitivity increased to 72% for HBV, 85% for HCV, and 80% for NAFLD. The specificity decreased to 79%. For girls, using the NHANES-derived ALT cut-point of >22 U/L improved sensitivity to 74% for HBV, 79% for HCV, and 92% for NAFLD. The specificity decreased to 85%.
Table 3. Sensitivity and Specificity of ALT.
| Sensitivity | ||||
|---|---|---|---|---|
| ALT Threshold Used | HBV | HCV | NAFLD | Specificity |
| Children’s Hospitals | ||||
| Boys, median (IQR) | 35 (28 – 41) | 44 (35 –48) | 32 (24 – 40) | 92 (88–100) |
| Girls, median (IQR) | 41 (33 – 63) | 48 (40 – 67) | 36 (24 – 52) | 96 (92 – 100) |
| NHANES derived | ||||
| Boys | 72 | 85 | 80 | 79 |
| Girls | 74 | 79 | 92 | 85 |
DISCUSSION
We used a multi-modal approach to characterize ALT thresholds in children. The survey of children’s hospitals in the United States showed that laboratories vary widely in the ULN value used for ALT, most laboratories use values that are falsely high, and fewer than half of hospitals use gender-specific values. The analysis of a large population-based sample restricted to metabolically normal children showed that the statistically defined ULN for ALT was less than half of the median value currently used for children in the United States. The analysis of diagnostic performance in children with and without liver disease showed that ALT thresholds in current use had low sensitivity for detection of liver disease. The gender-specific thresholds derived from the analysis of the NHANES population doubled the sensitivity with only a small reduction in specificity.
The wide variability in ULN of ALT at children’s hospitals is not attributable to the equipment or laboratory used to measure ALT activity. As part of the laboratory accreditation program, the College of American Pathologists sends standardized samples to clinical laboratories for assessment of ALT activity. As shown by Tetri et al., standardized samples analyzed in different laboratories with different analyzers from different manufacturers had only a modest difference in measured ALT activity; the standard deviation between laboratories was 2 U/L.18 Moreover, many laboratories use populations as small as 100 people to set their normal range for an assay such as ALT. Therefore the widely divergent ALT ULN values used by laboratories are likely related to the characteristics of the populations used by individual laboratories to define their own reference ranges. If potentially silent liver disease such as NAFLD is not excluded, then the distribution of ALT would be expected to be falsely skewed upwards.
The large variability in the ULN values used in children’s hospitals has broad implications for patient care and patient safety. Millions of children take medication for chronic diseases. Biochemical monitoring for hepatoxicity is important for many such medications and requires reliable interpretation of assays including ALT.19 However, in current practice, a child may or may not have a drug reaction identified, depending upon where he or she lives. Another example of the problem with locally defined ULN is the impact upon whether or not a child with HBV infection is considered eligible for treatment. Guidelines currently recommend treatment only for those patients with chronic HBV infection who have an ALT > 2 times the ULN.20 The current data demonstrate that this threshold could be interpreted as being anywhere from 44 U/L (2 times the proposed threshold for girls) to 180 U/L (2 times the highest ULN in use) with the median ULN in current use producing a required value of 106 U/L. Thus whether a child is treated or not and potentially whether the treatment is covered by insurance is based upon a flawed construct.
The current study explains existing limitations in the utility of ALT as a screening tool for chronic liver disease and offers meaningful improvements. We incorporated the methodology of Prati et al.5 who calculated a statistically-defined ULN of normal in Italian adult blood donors without viral hepatitis or known risk factors for liver disease, applied this to children, and provided data on sensitivity and specificity. In current practice ALT has sensitivities for the detection of chronic hepatitis that range from 30 to 40%. Using a biologically-determined, gender-specific threshold substantially improved the sensitivity into the 70-80% range, while only modestly reducing the specificity from about 90% to about 80%. Thus, using this new threshold, ALT has utility as an important component in screening for liver disease, but it should not be considered a definitive test. These data are particularly important because of the increase in screening for liver disease in children based upon recent recommendations to screen for NAFLD in overweight and obese children.3,4 Several authors have shown that children may have NAFLD with ALT values that were perceived to be normal. Notably, when the threshold of ALT > 25 for boys and > 22 for girls is applied to the entire NHANES sample, the prevalence of elevated ALT yields an estimate for NAFLD that is consistent with the prevalence of pediatric fatty liver disease as estimated by a population-based autopsy study.16
Hy Zimmerman observed that substantial hepatotoxicity could be defined as ALT activity ≥ 3 times the ULN plus serum bilirubin concentration ≥ 2 times the ULN.23 The Food and Drug Administration advocated the use of Hy’s rule in the assessment of the hepatotoxicity of newly developed drugs.21, 22 Treatment trials are to be stopped if these criteria are fulfilled. Because this definition relies upon the local laboratory definition for ULN, the threshold for ALT applied in children’s hospitals could range from 90 to 270 U/L. Such a large discrepancy is driven by outdated logistics rather than biology. Moreover, although Hy’s rule was intended as stop point and not as a means of detecting chronic liver disease, clinical trials commonly use a baseline ALT value of 3 times the ULN as the means to exclude participants with liver disease. As the data from the current study show, this criterion will fail to exclude the majority of children with chronic hepatitis. Thus if pediatric clinical trials are to appropriately exclude children with liver disease and help assure safety of trial participants, a major revision in standard operating procedure is required.
The use of a nationally representative sample allowed for these results to be generalized to children in the United States and allowed for the control of BMI percentile, medications, and laboratory parameters. The study was further strengthened by the use of a multi-center sample of children with clinical liver disease. In addition, a state of the art MRI technique was used as an objective method to demonstrate normal livers and to identify fatty livers. A limitation of the study was that ALT measurements were made at a single point in time. Thus intra-individual variability in ALT with repeated measurement was not assessed. Another limitation was the absence of histological assessment of disease severity. In addition there were not data to determine whether there was any effect of pubertal status on the appropriate threshold value for ALT. However, it would impractical to require Tanner staging for the interpretation of a common laboratory test such as ALT. A final limitation is that positive and negative predictive values could not be determined because these are population specific.
In conclusion, ULN values widely used for ALT in children are too high to reliably detect chronic liver disease. There is considerable hospital-to-hospital variability that is not based upon biology nor assay characteristics. This creates randomness in whether children with chronic liver disease are identified. Moreover, these findings have important ramifications for pediatric clinical trials and implications for medical management of children with chronic disease.27 We propose that: 1) there be a re-examination of laboratory thresholds used for children, 2) exclusion criteria for clinical trials should be modified to properly identify children with liver disease, and 3) physicians consider using the values derived from this study to identify children with possible liver disease. Studies in varied populations will be needed to prospectively test these values for the ability to determine the presence and absence of liver disease.
Supplementary Material
Acknowledgments
Funding: This work was funded in part by grants from the National Institutes of Health including R21 DK71486 from the National Institute of Diabetes and Digestive and Kidney Diseases, P60 MD00220 for the San Diego EXPORT Center from the National Center of Minority Health and Health Disparities, M01 RR000827 from the National Center for Research Resources for the General Clinical Research Center at UCSD, DK080506 for the UCSD Digestive Diseases Research Development Center and NIH NRSA grant T32 DK07202. The funders did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ALT
alanine aminotransferase
- BMI
body mass index
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- NHANES
National Health and Nutrition Examination Survey
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- ULN
upper limit of normal
- FDA
United States Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: no conflicts of interest exist.
REFERENCES
- 1.Jonas MM. Children with hepatitis C. Hepatology. 2002;36:S173–178. doi: 10.1053/jhep.2002.36799. [DOI] [PubMed] [Google Scholar]
- 2.Barlow S, the Expert Committee Expert Committee Recommendations on the Assessment, Prevention, and Treatment of Child and Adolescent Overweight and Obesity, Summary Report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 3.August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93:4576–4599. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau DC. Synopsis of the 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ. 2007;176:1103–1106. doi: 10.1503/cmaj.070306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–283. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Celedon MA, Lavine JE, et al. Heritability of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 11.Hussain HK, Chenevert TL, Londy FJ, et al. Hepatic fat fraction: MR imaging for quantitative measurement and display--early experience. Radiology. 2005;237:1048–1055. doi: 10.1148/radiol.2373041639. [DOI] [PubMed] [Google Scholar]
- 12.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 13.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26(3):347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153–1161. doi: 10.1002/jmri.21090. [DOI] [PubMed] [Google Scholar]
- 15.Uygun A, Kadayifci A, Yesilova Z, et al. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000;95:3584–3589. doi: 10.1111/j.1572-0241.2000.03297.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 17.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983–988. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuschwander-Tetri BA, Unalp A, Creer MH. Influence of local reference populations on upper limits of normal for serum alanine aminotransferase levels. Arch Intern Med. 2008;168:663–666. doi: 10.1001/archinternmed.2007.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta NK, Lewis JH. Review article: The use of potentially hepatotoxic drugs in patients with liver disease. Aliment Pharmacol Ther. 2008;28:1021–1041. doi: 10.1111/j.1365-2036.2008.03822.x. [DOI] [PubMed] [Google Scholar]
- 20.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 21.Davidson CS, Leevy CM, Chamberlayne EC. Guidelines for Detection of Hepatotoxicity Due to Drugs and Chemicals. Fogarthy Conference,1978; Washington, DC. 1979; NIH Publication no. 79-313. [Google Scholar]
- 22.Senior JR. Drug hepatotoxicity from a regulatory perspective. Clin Liver Dis. 2007;11:507–524. vi. doi: 10.1016/j.cld.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. Appleton-Century-Crofts; New York: 1978. [Google Scholar]
- 24.Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 25.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 26.Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43:618–631. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 27.Cox ER, Halloran DR, Homan SM, Welliver S, Mager DE. Trends in the prevalence of chronic medication use in children: 2002-2005. Pediatrics. 2008;122:e1053–1061. doi: 10.1542/peds.2008-0214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


