Abstract
Curcumin, a component of turmeric (Curcuma longa), exhibits anti-inflammatory and anti-proliferative activities through the generation of reactive oxygen species (ROS). Curcumin (diferuloylemethane) contains two hydroxyl, two methoxy and two phenyl groups but how these groups contribute to its activity is poorly understood. We synthesized analogues that varied in inclusion of these groups and compared their activity. We found that bisdemethylcurcumin (BDC) was more potent than curcumin as an anti-inflammatory agent as indicated by suppression of TNF-induced NF-κB activation, more potent as an anti-proliferative agent, and more potent in inducing ROS. Hispolon, which lacks one aromatic unit in relation to curcumin, also exhibited enhanced anti-inflammatory and anti-proliferative activities. When synthetic curcumin (Cur-S) was compared with bisdemethylcurcumin (BDC), hispolon, hispolon methyl ether (HME), dehydroxy hispolon (DH), hydroxy hispolon (HH), methoxy hispolon methyl ether (MHME), and methoxy hispolon (MH), we found that following order of anti-inflammatory activity: BDC=Hispolon>HME> HH>Cur-S>MHME>MH>DH; for anti-proliferative Hispolon> BDC>MHME> Cur-S>MH>HME=HH>DH; and for prooxidant BDC>Cur-S=MHME>HH>MH+HME>DH (254-1414 mean fluorescence intensity). Thus dehydroxyhispolon was least potent for all three activities. Overall the results indicates that the substitution of a hydroxyl group for a methoxy group at the meta positions of the phenyl rings in curcumin significantly enhanced the anti-inflammatory activity, and the removal of phenyl ring at the 7th position of the heptadiene back bone and addition of hydroxyl group significantly increased the anti-proliferative activity of curcumin.
Keywords: Curcumin, hispolon, NF-κB, TNF, anti-proliferation
1. Introduction
Curcumin, 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione, is the primary bioactive compound isolated from turmeric, the dietary spice made from the rhizome of Curcuma longa. One of the most important aspects of curcumin is its effectiveness against various types of cancer, having both chemopreventive and chemotherapeutic properties [1]. Curcumin mediates anti-inflammatory effects through the downregulation of transcription factor nuclear factor-κB (NF-κB) [2], tumor necrosis factor (TNF)-α [3, 4], interleukin (IL)-6 [5], IL-8 [6], adhesion molecules [7], inducible nitric oxide synthase (iNOS) [3], matrix metalloproteinase-9 (MMP-9) [8], cyclooxygenase-2 (COX-2) [9], 5-lipoxygenase (5-LOX) [10] and chemokine receptor CXCR4 [11]. Central to the wide range of effects, curcumin exerts its down-regulation of the transcription factor NF-κB. Curcumin is a potent blocker of NF-κB activation, which has been linked with proliferation, invasion, and angiogenesis as well as induction of apoptosis [7].
Besides being a potent anti-inflammatory agent, curcumin is also a potent anti-proliferative agent [12-14]. No cancer cell type has yet been found where curcumin lacks anti-proliferative effects, and this effect is selective towards tumor cells, it has minimum effect on normal cells. How curcumin selectively manifests its effects towards tumor cells has been discussed recently [15]. In addition, curcumin acts as an antioxidant at low doses and prooxidant at high doses [16]. Both anti-inflammatory and anti-proliferative activities of curcumin have been shown to be mediated through the prooxidant mechanism [17].
Because of these anti-inflammatory and anti-cancer activities, there has been lot of interests in the origin of these activities from within the curcumin molecule. Indeed, the interest has persisted ever since curcumin was first synthesized by Lampe in 1918. The entire molecule can be divided into halves that are mirror images of each other. Besides a β-diketone, it consists of two phenyl, two methoxy, and two hydroxyl groups. Besides natural analogues (e.g., demthoxycurcumin and bisdemethoxycurcumin), numerous analogues have been synthesized in an attempt to find “super curcumin” [13]. In the current report, we describe certain analogues of curcumin that are more potent than curcumin as anti-inflammatory and anti-proliferative agents against various tumor cells including cancers of the colorectum, prostate, and breast, and against human myeloid leukemia and multiple myeloma cells.
2. Materials and Methods
2.1. Materials
Synthetic Curcumin, bisdemethylcurcumin and structurally related hispolon analogues of curcumin were synthesized as described [18]. A series of hispolon analogues of curcumin were synthesized through the condensation of appropriately protected hydroxybenzaldehydes with acetylacetone, as described below.
2.1.1. Synthesis of hispolon methyl ether (HME)
To a solution of acetylacetone (335 mL, 3.28 mol, 5 eq) in ethyl acetate was added boric anhydride (32 g, 0.459 mol, 0.7 eq) and stirred for 30 minutes at 70°C. To the above solution was added vanillin (100 g, 0.657 mol, 1 eq) and tributyl borate (177 mL, 0.657 mol, 1 eq) and stirred for 30 min at 70°C. After 30 min the temperature was raised to 85°C and n-butyl amine (64.9 mL, 0.657 mol, 1 eq) in ethyl acetate was added drop-wise and the stirring was continued for 1 h at 100°C. The reaction mixture was cooled to 50°C and hydrolyzed by adding 1N HCl (200 mL) and stirred for 30 min at 30°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic layer was washed with water until neutral and dried over sodium sulphate. After removal of the solvent in vacuum the crude product obtained was purified by column chromatography using chloroform as eluent followed by recrystallization from ethyl acetate and hexane to obtain hispolon monomethyl ether (26% yield, HPLC purity: 99%). M.p. 140 – 144.5°C, 1H NMR (d6-acetone, 300 MHz): δ 15.74 (1H, brs), 8.12 (1H, brs), 7.52 (1H, d, J=15.8 Hz), 7.29 (1H, d, J=2.0 Hz), 7.11 (1H, d, J=8.2 Hz), 6.85 (1H, d, J=8.2 Hz), 6.57 (1H, d, J=15.8 Hz), 5.75 (1H, s), 3.89 (3H, s), 2.09 (3H, s); Mass: m/z 235 (M+H)+; 257 (M+Na)+; 273 (M+K)+.
2.1.2. Synthesis of hispolon
Vanillin (70 g, 0.46 mol, 1 eq.) and ethylene dichloride (1400 mL, 20 Vol.) were taken in a 5 L RB flask at room temperature and cooled slowly to 0-5°C and added aluminum chloride (153.3 g, 1.15 mol, 2.5 eq.) lot wise followed by pyridine (349 mL, 4.324 mol, 9.4 eq.) drop-wise for 15 min. The temperature of the reaction was raised to reflux and maintained for 2 h under reflux. After completion of the reaction (progress of the reaction was monitored by TLC), the contents of the flask were cooled to 0-5°C and added aq. HCl (20%) drop-wise maintaining the temperature below 20°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate (four times) and the combined organic layer was washed with water (thrice), dried over anhydrous sodium sulphate. The solvent was removed and the crude product obtained was recrystllized from ethyl acetate and hexane to obtain 3, 4-Dihydroxybenzaldehyde (53.0 g, yield: 83%, HPLC purity: 98.5%).
To a solution of acetylacetone (9.6 mL, 0.094 mol, 5 eq) in ethyl acetate was added tributyl borate (25 mL, 0.09 mol, 5 eq) and stirred for 30 min at 70°C. To the reaction mixture, was added 3,4-dihydroxybenzaldehyde (2.6 g, 0.01 mol, 1 eq) and stirred for 30 minutes at 70°C. After 30 min, the temperature was raised to 85°C and n-butyl amine ( 1.8 mL, 0.01 mol, 1 eq) in ethyl acetate was added drop-wise and the stirring was continued for 1 h at 100°C and boric anhydride (0.9 g, 0.013 mol, 0.7 eq). The mixture was cooled to 50°C and hydrolyzed by adding 1N HCl and stirred for 30 min at 30°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined layers were washed with water until neutral and dried over sodium sulphate. After removal of the solvent in vacuum the crude product was purified by column chromatography using chloroform as eluent followed by recrystallization with ethyl acetate and hexane to obtain hispolon monomethyl ether ( 1 g, yield : 24%, HPLC purity: 99%).
2.1.3. Synthesis of hispolon (Alternative method)
To an ice cold solution of hispolon methylether (11 g, 0.04 mol, 1 eq) in ethylacetate was added aluminum chloride (15 g, 0.11 mol, 2.3 eq) followed by the drop-wise addition of pyridine (35 mL, 0.43 mol, 9.26 eq) for 15 min and the reaction mixture was heated under reflux for 7 h at 65°C. After completion of the reaction, the reaction mixture was cooled to 10°C and added cold HCl (20%) to decompose aluminum chloride complex and extracted with ethyl acetate. The combined ethyl acetate layer was washed with water, brine and dried over anhydrous sodium sulphate. The solvent was evaporated and the crude product obtained was purified by column chromatography using chloroform as eluent followed by recrystallization from a mixture of ethyl acetate and hexane to obtain hispolon (5.3 , yield : 51%, HPLC purity: 99%). M.p. 150.0-151.9°C, 1H NMR (d6-acetone, 300 MHz): δ 15.75 (1H, brs), 8.47 (1H, brs), 8.14 (1H, brs), 7.46 (1H, d, J=15.9 Hz), 7.13 (1H, d, J=2.0 Hz), 7.02 (1H, d, J=8.2, 2.0 Hz), 6.84 (1H, d, J=8.2 Hz), 6.46 (1H, d, J=15.9 Hz), 5.76 (1H, s), 2.09 (3H, s); Mass: m/z 221 (M+H)+; 243 (M+Na)+; 219 (M-H)−.
2.1.4. Synthesis of methoxyhispolon methyl ether (MHME)
To a solution of acetylacetone (69.9 mL, 0.68 mol, 5 eq) in ethyl acetate was added boric anhydride (6.68 g, 0.09 mol, 0.7 eq) and stirred for 30 min at 70°C. To the above solution was added syringaldehyde (25 g, 0.13 mol, 1 eq) and tributyl borate (37 mL, 0.13 mol, 1 eq) and stirred for 30 minutes at 70°C. After 30 minutes the temperature was raised to 85°C and n-butyl amine (13.5 mL, 0.13 mol, 1 eq) in ethyl acetate was added drop-wise and the stirring was continued for 1 h at 100°C. The mixture was cooled to 50°C and hydrolyzed by adding 1N HCl and stirred for 30 min at 50°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined layers were washed with water until neutral and dried over sodium sulphate. After removal of the solvent in vacuum the crude product was purified by column chromatography using chloroform as eluent followed by recrystallization with ethyl acetate and hexane to obtain Methoxy hispolon methyl ether (11 g, yield : 30%, HPLC purity: 99%). M.p. 118-120°C, 1H NMR (d6-acetone, 300 MHz): δ 15.45 (1H, brs), 7.51 (1H, d, J=15.8 Hz), 6.76 (2H, s), 6.32 (1H, d, J=15.8 Hz), 5.75 (1H, s), 5.64 (1H, s), 3.93 (6H, s), 2.16 (3H, s); Mass: m/z 265 (M+H)+; 287 (M+Na)+.
2.1.5 .Synthesis of methoxyhispolon (MH) and hydroxyhispolon (HH)
N,N-Dimethylaniline (34.5 mL, 0.27 mol, 12 eq) was taken in a flask at room temperature and slowly the temperature was raised to 40°C and aluminium chloride (36.3 g, 0.27 mol, 5 eq) was added lot wise to the N,N-Dimethylaniline and stirred for 30 min at 60°C. After 30 min ethyl acetate was added and stirred for 30 min later solution of methoxy hispolon methylether (6 g, 0.022 mol, 1 eq) in ethylacetate was added to the above mixture and stirred for 17 h at 80°C. After 12 h the reaction mixture was hydrolyzed with aqueous HCl and ethyl acetate layer was separated and the aqueous layer was extracted with ethyl acetate. The combined ethyl acetate layer was washed with water, brine and dried over anhydrous sodium sulphate. The solvent was filtered and evaporated the solvent. The crude product was purified by column chromatography using chloroform as eluent followed by recrystallization with ethyl acetate and hexane to obtain Methoxy hispolon (0.45 g, HPLC purity 99%). M.p. 150.1-153.9°C, 1H NMR (d6-acetone, 300 MHz): δ 15.70 (1H, brs), 7.87 (2H, brs), 7.45 (1H, d, J=15.8 Hz), 6.86 (1H, s), 6.82 (1H, s), 6.52 (1H, d, J=15.8 Hz), 5.76 (1H, s), 3.85 (3H, s) 2.09 (3H, s); Mass: m/z 251 (M+H)+; 273 (M+Na)+; 289 (M+K)+; 249 (M-H)-; and hydroxyhispolon (0.2 g, HPLC purity: 99%). M.p. 194 – 197.9° C, 1H NMR (d6-acetone, 300 MHz): δ 15.73 (1H, brs), 8.10 (2H, s), 7.86 (1H, s), 7.38 (1H, d, J=15.8 Hz), 6.76 (2H, s), 6.40 (1H, d, J=15.8 Hz), 5.76 (1H, s), 2.08 (3H, s); Mass: m/z 237 (M+H)+; 259 (M+Na)+; 275 (M+K)+; 235 (M-H)−.
2.1.6. Synthesis of methoxyhispolon (MH) (Alternative method)
Syringaldehyde (10 g, 0.0548 mol, 1 eq.) and ethylene dichloride (200 mL, 20 Vol.) were taken in a 1 L RB flask at room temperature and cooled slowly to 0-5°C and added aluminum chloride (36.6 g, 0.2744 mol, 5 eq.) lot wise followed by addition of pyridine (44 mL, 0.5489 mol, 10 eq.) drop-wise for 30 min. The temperature of the reaction was raised to reflux and maintained for 1 h under reflux. After completion of the reaction (progress of the reaction was monitored by TLC), the contents of the flask were cooled to −10°C and added aq. HCl (20%) drop-wise. The organic layer was separated and the aqueous layer was extracted with ethyl acetate (four times) and the combined organic layer was washed with water (thrice), dried over anhydrous sodium sulphate. The solvent was distilled and a light brown solid was obtained. (8.8 g, yield: 95%, HPLC purity: 98.5%).
To a solution of acetylacetone (103 mL, 1.01 mol, 5 eq) in ethyl acetate was added boric anhydride (9.8 g, 0.141 mol, 0.7 eq) and stirred for 30 min at 70°C. To the above solution was added 3,4-Dihydroxybenzaldehyde (34 g, 0.20 mol, 1 eq) and tributyl borate (54.5 mL, 0.20 mol, 1 eq) and stirred for 30 min at 70°C. After 30 min the temperature was raised to 85°C and n-butyl amine (19.98 mL, 0.2 mol, 1 eq) in ethyl acetate was added drop-wise and the stirring was continued for 1 h at 100°C. The mixture was cooled to 50°C and hydrolyzed by adding 1N HCl and stirred for 30 min at 30°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined layers were washed with water until neutral and dried over sodium sulphate. After removal of the solvent in vacuum the crude product was purified by column chromatography using chloroform as eluent followed by recrystallization with ethyl acetate and hexane to obtain Methoxy hispolon (13.6 g, yield : 26%, HPLC purity: 98%).
2.1.7. Synthesis of hydroxyhispolon (HH): Alternative method 1
Syringaldehyde (25 g, 0.1372 mol, 1 eq.) and Toluene (200 mL, 8 Vol.) were taken in a 2 L RB flask at room temperature and cooled slowly to 0-5°C and added aluminum chloride (137 g, 1.029 mol, 7.5 eq.) lot wise and raised the temperature to 50-60°C and added pyridine (166 mL, 2.058 mol, 15 eq.) drop-wise for 30 min. The temperature of the reaction was raised to reflux and maintained for 6 h under reflux. After completion of the reaction (progress of the reaction was monitored by TLC), the contents of the flask were cooled to 50°C and added aq. HCl (50%) drop-wise and stirred for 30 min. The organic layer was separated and the aqueous layer was extracted with ethyl acetate (four times) and the combined organic layer was washed with water (thrice), dried over anhydrous sodium sulphate. The solvent was removed and the crude product obtained was washed with toluene and filtered the solid 3, 4,5-Trihydroxybenzaldehyde (21.0 g, yield: 64%, HPLC purity: 98.5%).
To a solution of acetylacetone (33 mL, 0.32 mol, 5 eq) in ethyl acetate was added boric anhydride (3.16 g, 0.045 mol, 0.7 eq) and stirred for 30 min at 70°C. To the above solution was added 3,4-Dihydroxybenzaldehyde (10 g, 0.064 mol, 1 eq) and tributyl borate (84.5 mL, 0.32 mol, 5 eq) and stirred for 30 min at 70°C. After 30 min the temperature was raised to 85°C and n-butyl amine (6.4 mL, 0.064 mol, 1 eq) in ethyl acetate was added drop-wise and the stirring was continued for 1 h at 100°C. The mixture was cooled to 50°C and hydrolyzed by adding 1N HCl and stirred for 30 min at 30°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined layers were washed with water until neutral and dried over sodium sulphate. After removal of the solvent in vacuum the crude product was purified by column chromatography using chloroform as eluent followed by recrystallization with ethyl acetate and hexane to obtain Methoxy hispolon (1 g, yield : 6%, HPLC purity: 98%).
2.1.8. Synthesis of hydroxyhispolon (HH): Alternative method 2
Methoxy hispolon (1 g, 0.003 mol, 1 eq.) and ethylene dichloride (50 mL, 50 Vol.) were taken in a 250 mL RB flask at room temperature and cooled slowly to 0-5°C and added aluminum chloride (3.9 g, 0.029 mol, 7.5 eq.) lot wise followed by addition of pyridine (4.8 mL, 0.059 mol, 15 eq.) drop-wise for 30 min. The temperature of the reaction was raised to reflux and maintained for 16 h under reflux. The reaction did not progress for completion (progress of the reaction was monitored by TLC), the contents of the flask were cooled to −10°C and added aq. HCl (20%) drop-wise. The organic layer was separated and the aqueous layer was extracted with ethyl acetate (four times) and the combined organic layer was washed with water (thrice), dried over anhydrous sodium sulphate. The solvent was distilled and purified through column chromatography using chloroform as eluent followed by recrystallization with ethyl acetate and hexane to obtain hydroxy hispolon. (0.1 g, yield: 10%, HPLC purity: 98%).
2.1.9. Synthesis of dehydroxyhispolon (DH)
To a solution of acetylacetone (25.5 mL, 5 eq) in ethyl acetate was added boric anhydride (2.4 g, 0.7 eq) and stirred for 30 min at 70°C. To the above solution was added p-hydroxybenzaldehyde (6.1 g, 1 eq) and tributyl borate (12.7 mL, 1 eq) and stirred for 30 min at 70°C. After 30 min the temperature was raised to 85°C and n-butyl amine (4.9 mL, 1 eq) in ethyl acetate was added drop-wise and the stirring was continued for 1 h at 100°C. The mixture was cooled to 50°C and hydrolyzed by adding 1N HCl and stirred for 30 min at 30°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined layers were washed with water until neutral and dried over sodium sulphate. After removal of the solvent in vacuum the crude product was purified by column chromatography using chloroform as eluent followed by recrystallization with ethyl acetate and hexane to obtain dehydroxy hispolon (11% yield, HPLC purity: 99%). M.p. 146.0-149.7°C, 1H NMR (d6-acetone, 300 MHz): δ 15.75 (1H, brs), 8.87 (1H, brs), 7.52 (2H, d, J=8.7 Hz), 6.86 (2H, d, J=8.7 Hz), 6.69 (1H, d, J=15.9 Hz), 6.53 (1H, d, J=15.9 Hz), 5.77 (1H, s), 2.09 (3H, s); Mass: m/z 205 (M+H)+; 227 (M+Na)+; 203 (M-H)−.
2.2. Reagents
Antibodies against cyclin D1, matrix metalloproteinase (MMP)-9, cyclin-D1 and bcl-xL were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibody against vascular endothelial growth factor (VEGF) was obtained from Thermo Scientific (Fremont, CA). Penicillin, streptomycin, Iscove's modified Dulbecco's medium (IMDM), RPMI-1640 and Dulbecco's modified Eagle's medium (DMEM) medium were purchased from Invitrogen (Grand Island, NY). Fetal bovine serum was obtained from Atlanta biologicals (Lawrenceville, GA). All other chemicals were obtained from Sigma Chemicals (St. Louis, MO) unless otherwise stated.
2.3. Cell lines
Human myeloid leukemic cell line KBM-5, human prostate cancer PC-3 cells, human multiple myeloma U266, human colorectal cancer cell line HCT 116, and human breast cancer MCF-7 cell line were obtained from the American Type Culture Collection (Manassas, VA). KBM-5 cells were cultured in IMDM with 15% FBS. MCF-7 and HCT 116 cells were cultured in DMEM with 10% FBS. PC-3 and U266 were cultured in RPMI 1640 medium with 10% FBS. Culture media were also supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin.
2.4. Electrophoretic mobility shift assay for NF-κB
To measure NF-κB activation, we performed electrophoretic mobility shift assay as described previously [19]. Briefly, nuclear extracts prepared from treated KBM-5 cells were incubated with 32P-end-labeled, 45mer double stranded NF-κB oligonucleotides (15 μg of protein with 16 fmol of DNA) from the human immunodeficiency virus long terminal repeat (5’- TTGTTACAAGGGACTTTCC GCTGGGGAC TTTCCAGGG AGGCGTGG-3’; bold face indicates NF-κB-binding sites) for 30 min at 37°C. The DNA-protein complex formed was separated from free oligonucleotide on 6.6% native polyacrylamide gels. The dried gels were visualized, and radioactive bands were quantified with a PhosphorImager using ImageQuant software (GE Healthcare, Piscataway, NJ).
2.5.Cytotoxicity assay
The cytotoxic effects of synthetic curcumin and hispolon analogues were determined by the 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) uptake method as described previously [17]. Briefly, the cells were incubated medium in triplicate in a 96-well plate and then treated with the indicated concentrations of curcumin or analogues at 37 °C for 24 h. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution was added to each well and incubated at 37 °C for 2 h. An extraction buffer (20 % SDS, 50 % dimethylformamide) was added, and the cells were incubated at 37 °C overnight. The absorbance was measured at 570 nm using a 96-well multiscanner (MRX Revelation; Dynex Technologies, Chantilly, VA)
2.6.Western blot analysis
To determine the levels of protein expression, we prepared whole-cell extracts from treated KBM-5 cells [20] and fractionated them by SDS-PAGE. After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, blotted with the appropriate antibodies against cyclin D1, MMP-9, cyclin-D1, bcl-xL and VEGF and detected by enhanced chemiluminescence (GE Healthcare). The protein in the bands was quantified using ImageQuant software (GE Healthcare).
2.7. Measurement of reactive oxygen species (ROS)
To detect intracellular ROS generated by curcumin and hispolon analogues, KBM-5 cells were pre-incubated with 20 μM DCF-DA for 15 min at 37°C before being treated with 25 μM concentrations of the compounds. After 1 h of incubation, the increase in fluorescence resulting from oxidation of DCF-DA to DCF was measured by flow cytometry [16]. The mean fluorescence intensity was calculated. Data were collected from at least 10,000 cells at a flow rate of 250–300 cells/sec.
3. Results
The objective of the present study was to determine whether bisdemethylcurcumin (BDC) and different synthetic analogues of hispolon differ from that of curcumin in their ability to suppress cellular proliferation and inflammation. Inflammatory effects were measured by measuring TNF-induced NF-κB activation and NF-κB-regulated production of gene products. For most experiments human myeloid KBM-5 cells were used as these cells are very well characterized in our laboratory for cytotoxicity and for NF-κB activation, convenient to culture and express both the receptors for TNF. The structures of curcumin and various hispolon analogues examined are shown in Fig. 1. We used both natural curcumin (curcumin-N) and synthetic curcumin (curcumin-S) for comparison. Curcumin-N consists of approximately 80% diferuloylmethane, 18% bisdemethoxycurcumin and 2% bisdemethoxycurcumin.
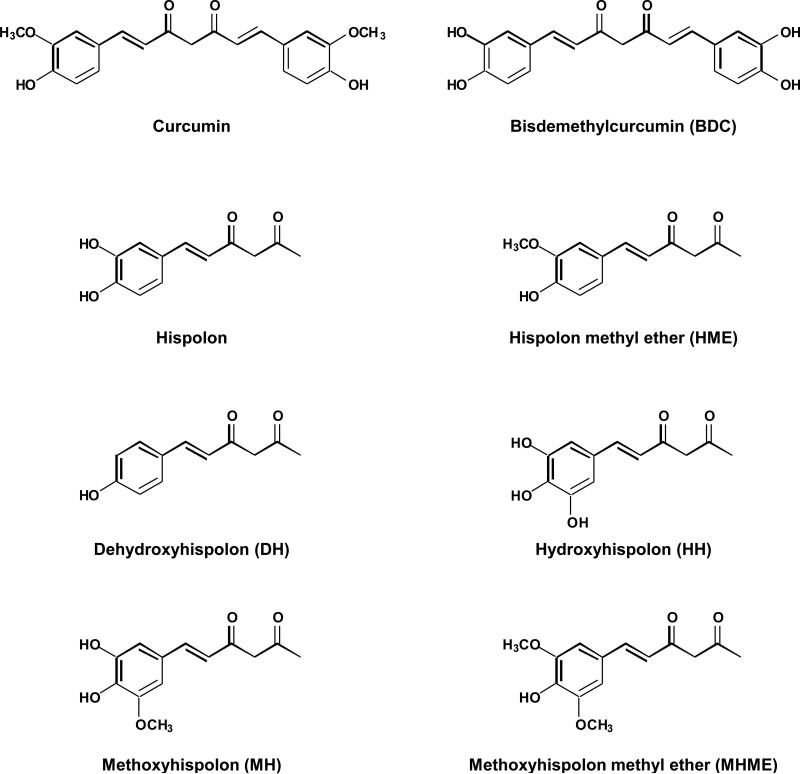
Figure 1.
Chemical structure of curcumin and analogues.
3.1. Hispolon and bisdemethylcurcumin are most potent as anti-proliferative agents
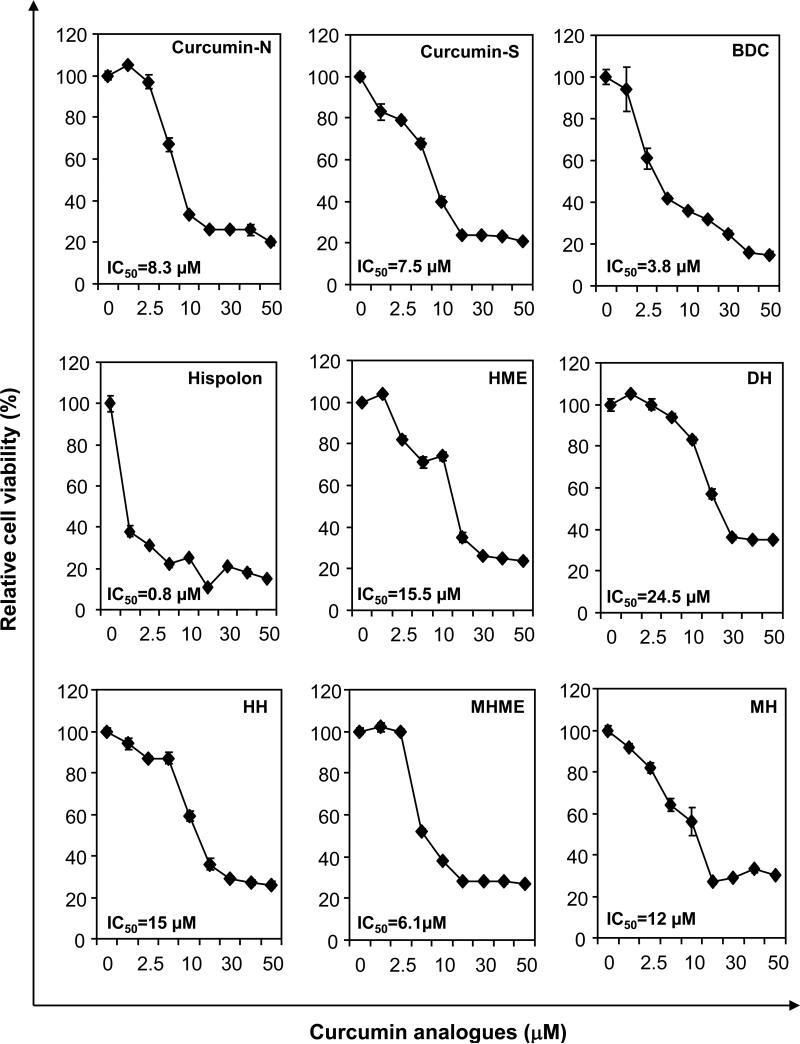
We investigated the ability of curcumin, BDC, hispolon and its analogues to inhibit the proliferation of human leukemia cell line KBM-5. All the compounds inhibited the proliferation of KBM-5 cells but with various potencies (Fig. 2A). Hispolon was the most cytotoxic, with an IC50 of 0.8 μM. Fig. 2A summarizes the relative cell viability of KBM-5 cells treated with curcumin analogues. The order of potency of the analogues was as follows; hispolon > BDC > methoxy hispolon methyl ether (MHME) > curcumin-S > curcumin-N > methoxy hispolon (MH) > hydroxy hispolon (HH) > hispolon methyl ether (MHE) > dehydroxy hispolon (DH) (Fig. 2B).
Figure 2.
Effect of curcumin and analogues on cell proliferation. Five thousand human myeloid leukemic (KBM-5) cells per well were seeded in triplicate onto 96-well plates; treated with the each compound at 1, 2.5, 5, 10, 25, 50 μM for 72 h; cell viability measured by the MTT method, and percent cell viability calculated.
3.2. Hispolon analogues suppresses proliferation of a variety of cancer cells
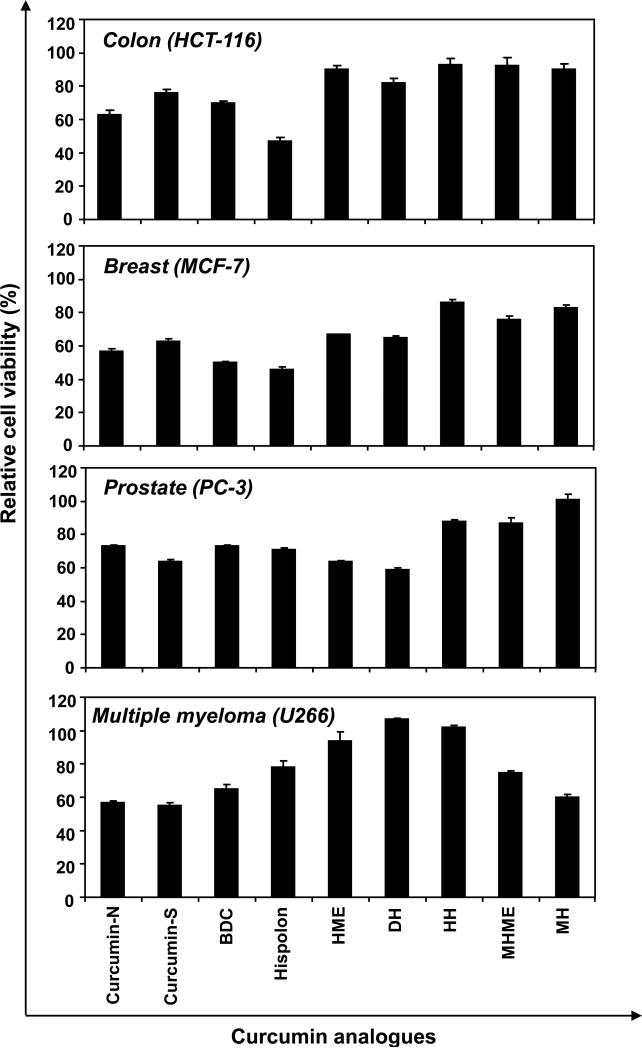
We also investigated the ability of these agents to inhibit the proliferation of other tumor cells, such as colorectal (HCT-116), breast (MCF-7), and prostrate (PC-3) cancer and multiple myeloma (U266). Hispolon analogues showed differential levels of suppression of proliferation in the cell lines. When exposed at 10 μM dose for 72 h, hispolon was the most cytotoxic in HCT-116 and MCF-7 cells. Dehydroxy hispolon (DH) showed maximum cytotoxicity in PC-3 cells, and curcumin-S was most cytotoxic in U266 cells (Fig. 3).
Figure 3.
Effect of curcumin and analogues on cell proliferation in different tumor cells. Five thousand human colon cancer (HCT-116), breast (MCF-7), prostrate (PC-3) and multiple myeloma (U266) cells, per well were seeded in triplicate onto 96-well plates; treated with the each compound at 10 μM for 72 h, cell viability measured by the MTT method and percent cell viability calculated.
3.3. Curcumin and hispolon analogues differentially suppress TNF-induced NF-κB activation
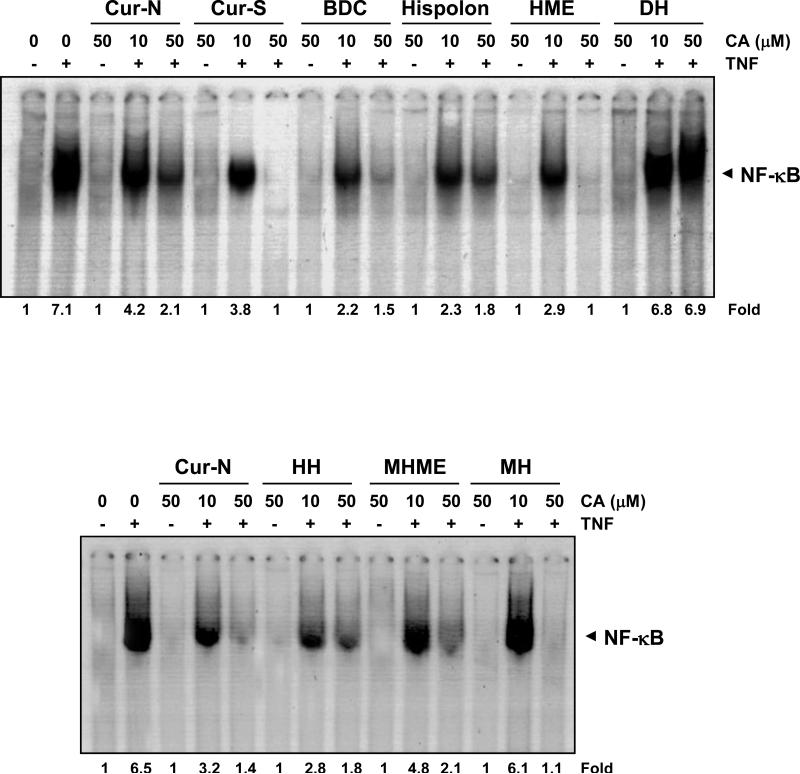
Both TNF and NF-κB are major mediators of inflammation; TNF mediates its inflammatory effects through the activation of NF-κB. Whether curcumin and different analogues modulate TNF-induced NF-κB activation to a similar extent was investigated. Results in Fig. 4 indicate that curcumin-N, curcumin-S, BDC, hispolon, HME, HH, MHME and MH inhibited TNF-induced NF-κB activation in KBM-5 cells in a dose-dependent manner, but the potency was varied. Curcumin-S, HME and HH completely inhibited; curcumin-N, BDC, hispolon and MHME inhibited 70-80%; and DH did not inhibit the TNF-induced NF-κB activation significantly. At 10 μM, BDC and hispolon were most effective in blocking NF-κB activation, whereas DH was least effective.
Figure 4.
Dose-dependent inhibition of TNF-induced NF-κB activation by curcumin and analogues. KBM-5 cells were pre-incubated with 10 and 50 μM concentrations of each compound for 4 h, treated with 0.1 nM TNF for 30 min and subjected to electrophoretic mobility shift assay of NF-κB activation. Suppression of the TNF-induced NF-κB was calculated as fold control.
3.4. Curcumin and hispolon analogues differ in their ability to generate ROS and induce death receptors
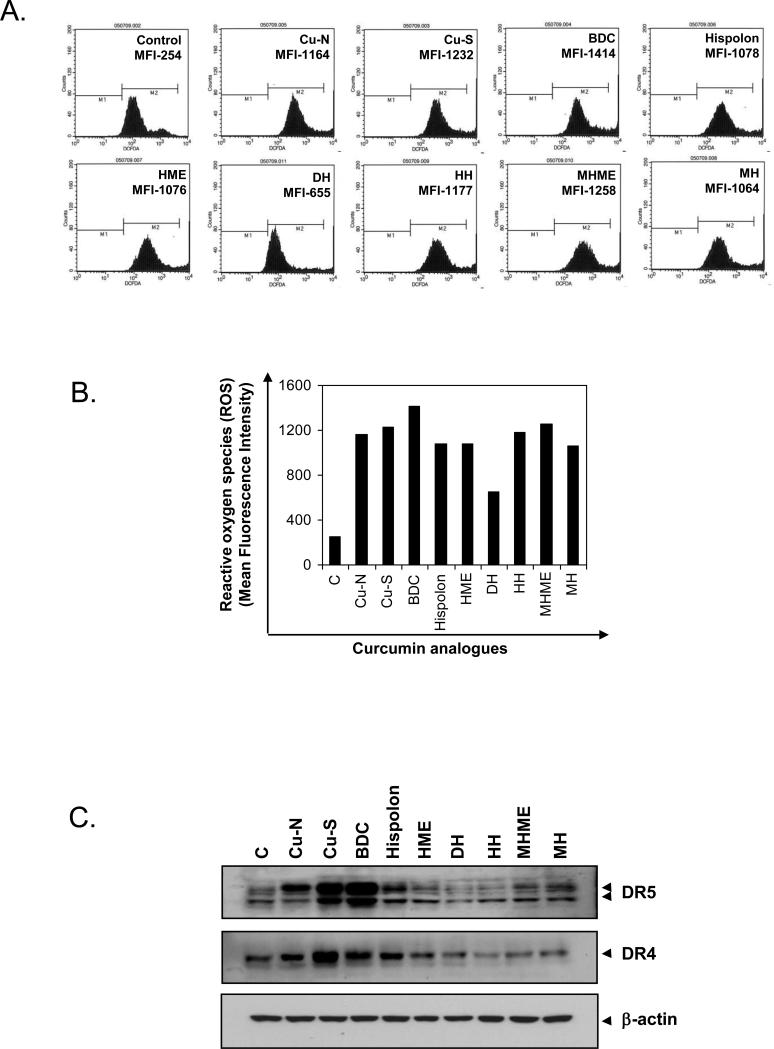
Whether curcumin and hispolon analogues differ in their ability to generate ROS in KBM-5 cells was examined using DCF-DA as a probe to measure the increase in ROS levels inside cells. Cells were treated with 25 μM curcumin or its analogues for 1 h and then was measured ROS by FACS. Fig. 5A shows histograms of flow cytometric fluorescence intensity patterns of ROS produced by cells on treatment with each compound. All the compounds generated ROS, but with varying capacity. BDC produced the maximum, i.e., 6 fold of control, and DH did the minimum (Fig. 5B).
Figure 5.
Effect of curcumin and hispolon analogues on production of cellular ROS and death receptor induction. (A) KBM-5 cells were labeled with DCF-DA, treated with 25 μM concentrations of each compound for 1 h, and examined for ROS production by flow cytometry. Quantitation of ROS production is shown as histogram (B) Graphical representation of mean fluorescence intensity. (C) Induction of death receptors DR5 and DR4 by curcumin and hispolon analogues.
Through ROS production, curcumin has been shown to induce the death receptors (DR)-4 and DR5 [21]. Whether curcumin and its analogues differ in their capacity to induce these death receptors was examined. BDC produced the maximum DR5 induction and dehydrohispolon, the minimum. In contrast to this, synthetic curcumin showed the maximum DR4 induction (Fig. 5C). These results correlate with ROS production.
3.5. Curcumin and hispolon analogues differ in their ability to suppress the expression of NF-κB–dependent gene products
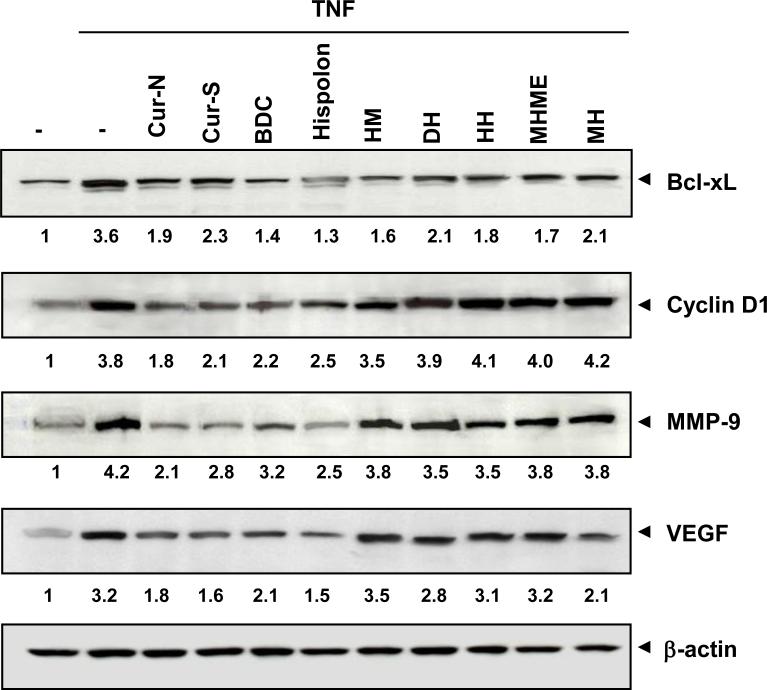
NF-κB activation has been linked with the regulation of the expression of survival (bcl-xL), proliferative (cyclin D1), invasion (MMP-9), and angiogenic (VEGF) gene products. As shown in Fig. 6, expression of bcl-xL, cyclin D1, MMP-9, and VEGF was induced by TNF treatment. Treatments with 25 μM compound for 4 h reduced the TNF-induced expression of these gene products. Curcumin-N, curcumin-S, BDC and hispolon significant inhibited TNF-induced MMP-9, bclxL, cyclin D1 and VEGF expression. Other hispolon analogues, such as HM, DH, HH, MHME, and MH, significantly inhibited the expression of bcl-xL. But hispolon analogues showed minimum inhibition of the TNF-induced expression of cyclin D1, MMP-9 and VEGF.
Figure 6.
Effect of curcumin and analogues on NF-κB-dependent gene expression. KBM-5 cells were incubated with 25 μM concentrations of the each compound for 4 h, treated with 1 nM TNF for 24 h, whole-cell extracts prepared and western blot analysis performed using the relevant antibodies. β-actin was used as protein loading control.
4. Discussion
Curcumin has been identified as the active principle in turmeric; chemically, it is a bis-α, β-unsaturated β-diketone that exhibits keto-enol tautomerism. It has been shown to exhibit anti-inflammatory and anti-carcinogenic activities. This polyphenol also exhibits hepatoprotective and nephroprotective activities, suppresses thrombosis, protects against myocardial infarction, and has hypoglycemic and antirheumatic properties. Moreover, this yellow dye has been shown in various animal models and human studies to be extremely safe even at very high doses [1, 14, 22-26]. Because of all these activities there has been intense interest in this molecule. During the last decade, synthetic modifications of curcumin aimed at enhancing its bioactivities, have been intensively studied [13]. Here we have analyzed synthetic curcumin, bisdemethylcurcumin (BDC) and a series of hispolon analogues for their biological activities for anti-inflammatory and anticancer potential.
In this study we have compared the biological activities of curcumin, bisdemethylcurcumin (BDC) and hispolon analogues. These include hispolon, hispolon methyl ether (HME), dehydroxy hispolon (DH), hydroxy hispolon (HH), methoxy hispolon methyl ether (MHME) and methoxy hispolon (MH). A comparison of cytotoxicity, relative NF-κB inhibition and NF-κB-regulated gene products by curcumin analogues was given in Table 1.
Table 1.
Cytotoxicity and relative NF-κB inhibition and NF-κB-regulated gene products by curcumin and hispolon anlogues in human myeloid leukemic KBM-5 cells
| Cytotoxicity (IC50) (μM) | NF-κB ↓ (%) | MMP-9↓ (%) | cyclinD1↓ (%) | VEGF↓ (%) | bcl-xL↓ (%) | |
|---|---|---|---|---|---|---|
| Cur-N | 8.3 | 41 | 50 | 53 | 44 | 47 |
| Cur-S | 7.5 | 46 | 33 | 45 | 50 | 36 |
| BDC | 3.8 | 69 | 24 | 42 | 34 | 6 |
| Hispolon | 0.8 | 68 | 40 | 34 | 53 | 64 |
| HME | 15.5 | 59 | 10 | 8 | 0 | 56 |
| DH | 24.5 | 4 | 0 | 0 | 13 | 4.2 |
| HH | 15.0 | 52 | 17 | 0 | 3 | 50 |
| MHME | 6.1 | 26 | 10 | 0 | 0 | 53 |
| MH | 12.0 | 6 | 10 | 0 | 34 | 42 |
Cur-N, natural curcumin; Cur-S, synthetic curcumin; BDC, bisdemethyl curcumin; HME, hispolon methyl ether; DH, dehydroxy hispolon; HH, hydroxyl hispolon; MHME, methoxy hispolon methyl ether; MH methoxy hispolon.
Among these compounds hispolon was the most cytotoxic to human leukemic cell line KBM-5, with an IC50 of 0.8 μM. Hispolon is known to induce apoptosis in human gastric cancer cell through a ROS-mediated mitochondrial pathway [27]. Hispolon showed a dose-dependent inhibition of human epidermoid KB cell proliferation and induced the death of KB cells through a mitochondria-mediated apoptotic pathway [28]. The anti-proliferative effects of curcumin and analogues are not cell specific. Curcumin is known to inhibit cell proliferation in a variety of cell types, such as U937, KBM-5, Jurkat, H1299, Calu-6, A549, SCC-4, Panc-1, MCF-7 and DU145 cells [17]. We found that hispolon analogues also inhibited cell proliferation in different cell types. Hispolon remained the most cytotoxic in colorectal (HCT-116) and breast cancer (MCF-7) cells. Why the different cell lines exhibited different sensitivity in terms of suppression of proliferation by hispolon analogues is unclear. However, it suggests the critical role of hydroxyl groups in the cytotoxic effects of hispolon.
Even though natural curcumin (IC50-8.3 μM) and synthetic curcumin (IC50-7.5 μM) did not vary much in their cytotoxic profile, synthetic curcumin was more effective in inhibiting TNF-induced NF-κB activation. At 50 μM, synthetic curcumin completely abolished the NF-κB activation and curcumin-N produced 70% inhibition. Complete inhibition of NF-κB was observed with HME and MH treatments. The TNF-induced NF-κB activation was significantly inhibited by BDC, hispolon, HH and MHME. DH appeared to have no NF-κB suppressive activity.
The production of ROS has been linked to the anti-proliferative effects of most agents. In our study, curcumin and hispolon analogues produced ROS, but the highest level of ROS was induced by BDC. The level of ROS production by various analogues correlated with neither their suppression of NF-κB activation nor with their inhibition of cell proliferation. At equimolar concentrations, the analogues varied in their ability to generate ROS. Induction of death receptors DR5 and DR4 was correlated with ability of ROS generation, as the BDC showed the maximum ROS generation, which corresponds with its ability to induce death receptors, DH generated the least ROS and did not induce death receptors.
The suppression of NF-κB correlated with the inhibition of NF-κB-regulated gene products MMP-9, cyclin D1, bcl-xL and VEGF. We found that curcumin-N was most effective in inhibiting MMP-9 and cyclin D1. These results are in line with earlier reports [17]. Curcumin-S, BDC and hispolon were moderately effective in inhibiting MMP-9 and cyclin D1. HME, DH, HH, MHME and MH do not show any significant inhibition of MMP-9 and cyclin D1. Our results indicate that hispolon was most effective in inhibiting VEGF, whereas curcumin-S, curcumin-N, and BDC were moderately effective. HME, DH, HH, MHME and MH did not significantly inhibit VEGF. Curcumin, bisdemethylcurcumin and all hispolon analogues were found to be effective in inhibiting the anti-apoptotic protein, bcl-xL. The clinical application and development of curcumin have been limited by its instability and poor metabolic property. In this paper, we present a series of structurally related analogues of curcumin with enhanced biological activity. Subsequently, the cytotoxic activities of analogues against seven tumor cells were evaluated by MTT method. Our results found the ability of different analogues to suppress NF-κB activation and NF-κB-regulated gene products. Our results indicate a lack of a direct relationship between suppression of cyclin D1 and cell proliferation, implying that there are other factors involved in cell proliferation. Our data provide a few novel leading compounds for the development of structurally related and biologically active curcumin analogues.
Thus overall our results suggest the critical role of hydroxyl groups in the anti-proliferative and anti-inflammatory effects of curcumin. The role of methoxy groups in the action of curcumin appears optional. Our results also suggest an optional role of the second phenyl ring in the action of curcumin.
5. Acknowledgments
We thank Walter Pagel for editing the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and grant from Center for Targeted Therapy of M.D. Anderson Cancer Center. The authors thank Dr C Satyanarayana, CEO, Aptuit Laurus for his encouragement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Current problems in cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. The Journal of biological chemistry. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 3.Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochemical pharmacology. 1998;55:1955–62. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- 4.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Annals of the New York Academy of Sciences. 2005;1056:206–17. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 5.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 6.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxidants & redox signaling. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21:251–62. doi: 10.1016/s0945-053x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 9.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 10.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer research. 1991;51:813–9. [PubMed] [Google Scholar]
- 11.Skommer J, Wlodkowic D, Pelkonen J. Gene-expression profiling during curcumin-induced apoptosis reveals downregulation of CXCR4. Experimental hematology. 2007;35:84–95. doi: 10.1016/j.exphem.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? The AAPS journal. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochemical pharmacology. 2008;76:1590–611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer letters. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends in pharmacological sciences. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, et al. Role of prooxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free radical biology & medicine. 2007;43:568–80. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 18.Venkateswarlu S, Ramachandra MS, Subbaraju GV. Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorganic & medicinal chemistry. 2005;13:6374–80. doi: 10.1016/j.bmc.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods in enzymology. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 20.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. The Journal of biological chemistry. 2003;278:24233–41. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 21.Jung EM, Park JW, Choi KS, Park JW, Lee HI, Lee KS, et al. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006;27:2008–17. doi: 10.1093/carcin/bgl026. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer research. 2003;23:363–98. [PubMed] [Google Scholar]
- 23.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. Journal of clinical immunology. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Advances in experimental medicine and biology. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 25.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Molecular pharmaceutics. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. The international journal of biochemistry & cell biology. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Zhao Z, Li L, Wu B, Chen SF, Zhou H, et al. Hispolon induces apoptosis in human gastric cancer cells through a ROS-mediated mitochondrial pathway. Free radical biology & medicine. 2008;45:60–72. doi: 10.1016/j.freeradbiomed.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, He FY, Li YQ. The apoptosis effect of hispolon from Phellinus linteus (Berkeley & Curtis) Teng on human epidermoid KB cells. Journal of ethnopharmacology. 2006;105:280–5. doi: 10.1016/j.jep.2006.01.026. [DOI] [PubMed] [Google Scholar]