Abstract
The aryl hydrocarbon receptor (AhR) is a basic helix-loop-helix protein that belongs to the superfamily of environment-sensing PAS (Per-ARNT-Sim) proteins. A large number of ligands have been described to bind AhR and promote its nuclear translocation. In the nucleus, the AhR and its dimerization partner the AhR nuclear translocase (ARNT), also known as HIF1β, form a DNA-binding complex that acts as a transcriptional regulator. Animal and human data suggest that, beyond its mediating responses to xenobiotic and/or unknown endogenous ligands, the AhR has a role, although as yet undefined, in the regulation of cell cycle and inflammation. The AhR also appears to regulate the hematopoietic and immune systems during development and adult life in a cell-specific manner. While accidental exposure to xenobiotic AhR ligands has been associated with leukemia in humans, the specific mechanisms of AhR involvement are still not completely understood. However, recent data are consistent with a functional role of the AhR in the maintenance of hematopoietic stem and/or progenitor cells (HSCs/HPCs). Studies highlighting AhR-regulation of HSCs/HPCs provide a rational framework to understand their biology, a role of the AhR in hematopoietic diseases, and a means to develop interventions for these diseases.
Keywords: aryl hydrocarbon receptor, hematopoiesis, hematopoietic stem cells, leukemia, dioxins
INTRODUCTION
Unlike more differentiated populations of cells in the blood, HSCs are functionally defined by their ability to self-renew, differentiate into multiple cell lineages and reconstitute the hematopoietic system of lethally irradiated hosts. Surface markers, dye exclusion and in vitro cultures are also used to phenotypically characterize HSCs [1, 2]. LSK (Lin−Sca-1+c-Kit+) bone marrow cells that are enriched for HSCs [3] lack the expression of cell surface proteins characteristic of lineage committed cells (B220, CD3, Gr-1, Ter119, Mac-1) and express the Stem cell antigen-1 (Sca-1) and CD117 (also c-Kit) cell surface proteins. Sca-1 is a glycosyl phosphatidylinositol-anchored protein that regulates self-renewal of HSCs [4]. c-Kit is a tyrosine kinase receptor for stem cell factor which is a cytokine that promotes self-renewal while preventing apoptosis in HSCs [5]. Additional markers can be used to further phenotypically define functionally different sub-populations of HSCs contained within the LSK population [6].
Adult HSCs are able to maintain homeostasis of hematopoiesis through their ability to self-renew and differentiate into HPCs while maintaining a pool of HSCs lasting throughout a lifetime. The critical function of the numerically rare HSC is exquisitely regulated by multiple intrinsic and extrinsic mechanisms [7]. HSC maintenance is intrinsically regulated by restricting proliferation [8-10] and promoting self-renewal. Self-renewal of HSCs is linked to the pathways that contribute to the commitment of cell types during development. Many of these developmental regulators act as ligands that lead to the activation of transcription factors such as GATA2, p53, retinoic acid receptor among others [11]. Stem cells are also contained within physical and chemical environments known as microenvironments or niches [12] that extrinsically regulate HSC function by releasing soluble factors [13, 14], activating intermediate cells [15-17], or by direct cell-cell interactions [18, 19].
Thus, HSCs have evolved multiple and overlapping levels of control to maintain homeostasis of the hematopoietic and immune system. Disruption of any of these can lead to multiple hematological disorders, including leukemia. In this review, we discuss the hypothesis that the aryl hydrocarbon receptor (AhR) is a transcription factor regulating hematopoiesis and that inappropriate signaling may lead to hematopoietic disease.
THE AHR IS A UBIQUITIOUS LIGAND-ACTIVATED TRANSCRIPTION FACTOR
The AhR is a basic helix-loop-helix protein, and a member of the PAS (Per-ARNT-Sim) superfamily of proteins. Physiologically, many of these proteins act by sensing molecules and stimuli from the cellular/tissue environment, and initiating signaling cascades to elicit the appropriate cellular responses. Other PAS proteins include sensors of oxygen availability like hypoxia-inducible factor-1 alpha (HIF-1α) [20], and regulators of circadian rhythms such as brain muscle Arnt-like protein 1 (BMAL1) [21].
When in the cytoplasm, AhR forms a complex with the chaperone protein heat-shock protein (Hsp)90, co-chaperone p23, and an immunophilin-like protein (AIP or XAP-2) [22]. Following ligand binding [23], the AhR amino (N)-terminal nuclear localization signal is activated directing it to translocate to the nucleus. Once in the nucleus, AhR forms a heterodimeric complex with the aryl hydrocarbon nuclear translocator (ARNT; also known as HIF-1β). The AhR-ARNT complex binds to a consensus core DNA recognition sequence 5′-TNGCGTG-3′ known as the AhR responsive element (AhRE) [24]. The AhR also contains a glutamine-rich transactivation domain in its carboxy (C)-terminal region which participates in the AhR-ARNT mediated recruitment of coactivators, co-repressors and basal transcription factors [25]. The AhR-ARNT heterodimer has been shown to regulate the expression of several batteries of genes involved in diverse signaling pathways including phase I/II metabolism, inflammation, and cell cycling [26]. However, the exact normal function of this transcription factor has not yet been defined.
The AhR was discovered as the mediator of the toxic responses of halogenated aromatic hydrocarbons and polycyclic aromatic hydrocarbons (PAHs), including the most potent 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). However, it is now known that a range of structurally diverse xenobiotic chemicals can bind the AhR with different affinities [24, 27]. Most of these compounds are potent inducers of several drug-metabolizing enzymes – particularly, CYP1a1, CYP1a2 and CYP1b1 – glutathione-S-transferase Ya and NAD(P)H-quinone oxidoreductase. Interestingly, there are several endogenous compounds capable of AhR-mediated induction of these genes such as tryptamine, indole acetic acid, bilirubin, biliverdin, 2-(10H-indole-30-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), tryptophan metabolites including the photoproduct 6-formylindolo[3,2-b]carbazole (FICZ), lipoxin A4 [27, 28], leukotriene A4 derivatives [29] and the intracellular second messenger cAMP [30]. However, the true endogenous ligands have not yet been clearly identified.
Due to the discovery of the AhR in association with the toxic dioxins [31], and the limited information about the existence or identity of AhR endogenous ligands [22], much of what is known about AhR biology is based on activation of the receptor using exogenous chemicals. TCDD, the most potent exogenous AhR ligand, and other dioxins are by-products of the chemical synthesis of herbicides popularly used between 1960 and 1980. These chemicals are also contaminants formed during several manufacturing processes and during the incineration and combustion of waste materials. As such, they are still relatively ubiquitous, but most often found at higher concentrations in areas near manufacturing plants and incineration sites where they could be released. Because they are highly lipophilic and bioaccumulate; dioxins are environmental pollutants found in the human food chain [32]. As endocrine disruptors, dioxins have been found to alter reproductive behavior in wildlife and laboratory animals [33], and have been shown to be potent carcinogens for multiple end-points [34]. In humans, dioxins are carcinogenic [34] and immunotoxic [35]. Benzo[a]pyrene (BP), another PAH and AhR ligand, is also found in tobacco and coal smoke. However, metabolites of BP, produced by enzymes transcriptionally regulated by the AhR, can also form DNA-binding adducts and cause mutagenesis and other effects by non-AhR pathways [36].
Besides the transcriptional activation through AhRE DNA binding, there is also evidence to suggest that ligand binding to AhR can elicit biological responses through AhR interactions with other transcription factors or cofactors. In an in vivo ischemia-induced model, treatment with BP was shown to impair angiogenesis by a molecular mechanism that results in induced vascular endothelial growth factor (VEGF). The proposed mechanism suggests that after ligand binding the formation of the AhR-ARNT complex resulted in lower levels of ARNT bound to its other partner HIF-1α whose signaling resulted in upregulation of VEGF [36]. More recently, AhR deficiency has also been shown to modulate angiogenesis by a mechanism involving VEGF depletion in endothelial cells and overexpression of TGF-β in stromal cells [37]. In mammary tumor cells, the association between the RelA subunit of NFκB and AhR induced cellular proliferation by activation of the oncogene c-myc [38, 39]. Also, interactions between AhR and the RelB subunit of NFκB induced chemokine production in leukemic cells [40]. In the lung, AhR appears to have a role in regulating inflammatory responses by maintaining RelB expression in fibroblasts [41]. AhR interaction with Rb has been shown to suppress progression of the S-phase of the cell cycle, but this interaction is restricted to the hypophosphorylated form of Rb found in quiescent cells, suggesting that the AhR is a negative regulator of proliferation [42, 43]. Alternatively, the AhR-Rb interaction has been proposed to coactivate the transcription of genes encoding proteins that suppress cell cycle progression in hepatoma cells [44]. Together these data suggest that while there are multiple mechanisms for AhR regulation of several signaling pathways, in particular those involving the cell cycle, the responses are specific to the type and cycling status of the cell.
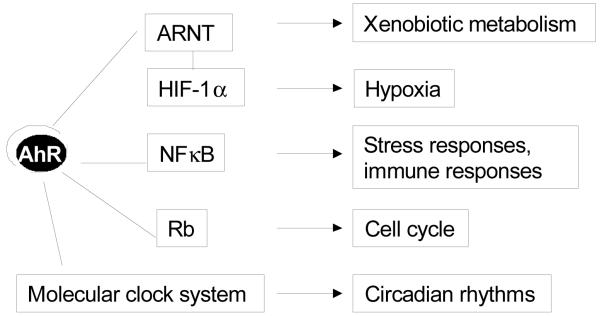
AhR-mediated responses to cellular stress by mechanisms specific to cell type have also been proposed. Part of the short- and long-term cellular stress responses of skin to ultraviolet light, including altered cell cycling and inflammatory reactions, may be mediated by AhR ligands generated as photoproducts [45]. Acute-phase response genes regulated by NF-κB are expressed in hepatocytes as an early reaction to cellular stress, and AhR ligands have been shown to repress this response in murine and human hepatocytes by a mechanism that requires AhR-ARNT nuclear translocation but not AhRE binding [46]. In response to light, TCDD also alters expression of the circadian regulators Per1 and Bmal1 genes and changes the phase shifts of circadian-regulated responses [47]. AhR and cyp1a1 genes have been shown to have a circadian expression in hepatocytes, brain cells [47] and immune cells [48]. These data support the concept that the AhR participates in the regulation of responses to environmental stimuli. Figure 1 illustrates these mechanisms emphasizing that AhR actions involve direct interaction of the AhR-ARNT complex with DNA through AhREs and/or interactions of the AhR with other transcription factors. Note that the ultimate responses observed following altered AhR activity may not be mutually exclusive to specific mechanisms. For example, the involvement of the AhR to regulate cell cycle may occur by mechanisms other than its ability to interact with Rb.
Figure 1.
Multiple molecular interactions of the AhR with transcription factors involved in cellular responses to environmental stimuli. AhR, aryl hydrocarbon receptor; ARNT, Aryl hydrocarbon nuclear translocator; Rb, retinoblastoma tumor suppressor protein; HIF1α, hypoxia-inducible factor-1 alpha.
After nuclear translocation, the AhR is targeted for degradation via the ubiquitin-proteosome pathway [49]. AhR activity in the cell is also regulated by the presence of the AhR Repressor protein whose expression is regulated by the AhR [50]. The AhR signaling pathway regulates the metabolism of its own exogenous and likely endogenous ligands by up-regulating cytochrome P-450 proteins [27]. These multiple mechanisms to regulate AhR expression and activity suggest an evolutionary pressure to maintain and finely regulate homeostatic activity of this protein, further suggesting a physiological role for AhR [26] and that its dysregulation, absence or chronic activation may lead to disease.
THE AHR HAS A PHYSIOLOGICAL ROLE IN THE HEMATOPOIETIC AND IMMUNE SYSTEM
The existence of AhR homologs in vertebrates and invertebrates and their involvement in the development of hepatic, reproductive, cardiovascular and immune system [51] support a physiological role of the AhR. Further support is provided by studies using AhR null-allele (AhR-/-) mice, which show defects in the development of the hematopoietic, cardiovascular and immune system [52, 53]. There has been some discrepancy regarding the immune phenotype of young AhR-/- mice because several of these phenotypes are most evident only under the context of specific immune responses [26]. Absence of AhR results in impaired differentiation of naïve T cells into T-helper 2 cells in a model of allergic sensitization [54], and AhR-/- mice also have shown delayed kinetics of T-helper 17 (Th17)-mediated autoimmune disease [55]. These mice exhibit constitutive accumulation of lymphocytes in the spleen and lymph nodes, along with being more susceptible to Listeria monocytogenes infection [56]. Also, AhR-/- mice were more sensitive to lipopolysaccharide (LPS)-induced lethal shock than wild type controls due to a higher production of IL6 and TNF-α by activated macrophages [57]. Transgenic mice with a mutation leading to the inability of the AhR to bind the AhRE (Ahrdbd) also showed defects in cardiovascular, splenic and hepatic development [58]. Characterization of a transgenic mouse expressing constitutively active AhR (CA-AhR) showed increased organ weights for heart, liver and kidney but decreased weight of the immune-related organs thymus and spleen [59]. Table 1 summarizes the phenotypes observed in hematopoietic and immune cells for the different animal models to study AhR. These models suggest that the AhR plays an important role during development and maintenance of the hematopoietic and immune system.
Table 1.
Hematopoietic and immune phenotypes in different animal models used for mechanistic studies of the AhR.
| Animal model | Phenotype observed | References |
|---|---|---|
| TCDD-treated | • Increased numbers of HSC/HPCs and decreased competitive repopulation of the bone marrow |
6, 77 |
| • Multiple and complex effects on innate and adaptive immune responses |
32 | |
|
| ||
| AhR-/- 1 | • Abnormal accumulation of lymphocytes | 52, 53 |
| • Sub-optimal innate and adaptive immune responses | 54-57 | |
|
| ||
| AhRdbd 2 | • Abnormal accumulation of lymphocytes | 58 |
|
| ||
| CA-AhR 3 | • Abnormal accumulation of lymphocytes | 59 |
AhR-/-, AhR null-allele mice
AhRdbd, transgenic mice unable to bind to the AhRE binding site of DNA
CA-AhR, transgenic mice with a constitutively active AhR.
Functional AhR is expressed by different hematopoietic and immune populations. In B-cells, the constitutive low levels of AhR mRNA and protein expression are highly upregulated after activation of resting cells [60, 6]. Western blots of human monocytes and CD34+ progenitors showed constitutive levels of the protein [61, 62]. When CD4+ T-cells become activated, the level of AhR message is upregulated in Th17 populations [55, 63]. AhR expression can also be detected in certain bone marrow stromal cell lines [64] and some cell lines derived from leukemic cells [65]. AhR message has been detected in murine LSK cells [6, 66].
Despite AhR being ubiquitous in the hematopoietic and immune system, the type and the magnitude of the responses initiated by AhR-activation appears to be cell-specific [67]. In macrophages, AhR interacts with signal transducer and activator of transcription 1 (Stat1) and NFκB to regulate proinflammatory innate immune responses elicited by LPS [57]. Dendritic cells that present antigen to naïve T cells in the periphery are reduced in numbers after AhR activation by the low-molecular-weight compound VAF347, and this appears to be due to the AhR-dependent decreased expression of IL6, IL10 and TGFβ [68]. During Th17 cell development, AhR regulates differentiation via Stat1, and AhR activation upregulates secretion of the inflammatory cytokines IL17 and IL22 [55]. Regulatory T-cells (Treg) inhibit cell proliferation of effector cells and cytokine secretion to suppress immune responses after they have been mounted. Ligand-mediated AhR activation increases Treg proliferation while suppressing experimental autoimmune encephalitis [63] and preventing graft-versus-host disease [69, 70].
Thymic atrophy has been recognized as a hallmark of exposure to the xenobiotic AhR agonist TCDD. As such, much effort has been devoted to understanding the mechanisms responsible and the role of the AhR functioning as a transcription factor in the hematopoiesis, and lymphopoiesis in particular. Studies using AhR-chimeric mice have shown that thymocytes and/or thymocyte precursors need to express AhR in order for TCDD or other dioxin-like xenobiotics to elicit this particular toxic effect [71], suggesting that the persistent activation by TCDD is disrupting some normal role of the AhR in these cells. There is evidence supporting TCDD-elicited reduced seeding of the thymus by bone marrow-derived progenitors [72], reduced stromal-mediated proliferation of thymocytes [73, 74] and a skewing of the thymic output through direct effects on developing thymocytes [75]. Additionally, it has been shown that AhR activation during gestation leads to alterations of B lineage cells with possible consequences on autoimmunity [76].
Given a proposed, but not yet clearly defined, role of the AhR during hematopoietic development, it has been further postulated that this transcription factor may be a homeostatic regulator of HSCs. AhR activation by TCDD has been shown to alter the functional activity of lymphoid committed progenitors and early progenitors of hematopoiesis [6, 77], accompanied by increased numbers of LSK cells [8, 78]. This also suggests some important function of the AhR in the regulation of HSCs that TCDD is disrupting through its ability to produce prolonged AhR activation. However, the molecular mechanisms by which AhR regulates the functions and numbers of HSCs are not yet clear. The further study of the AhR and AhR-regulated signaling pathways in hematopoietic stem and progenitor cell populations needs to consider the particular functions of these cells, cycling status, commitment towards certain differentiation pathways, and interactions with other cell types.
THE AHR MAY BE INVOLVED IN THE PROGRESSION OF HEMATOPOIETIC DISEASE
Limited epidemiological data from humans accidentally exposed to dioxins suggest an association between TCDD exposure and the development of leukemias and other hematopoietic malignancies [79-82]. Fifteen years after an industrial accident that exposed thousands of people to TCDD, a follow-up study of this population reported increased incidence of lymphohematopoietic neoplasms, non-Hodgkin’s lymphoma and myeloid leukemia, even at relatively low levels of TCDD exposure [79].
At least at present, there are few Ahr gene polymorphisms known to be associated with human cancers. Several of these are associated with lung and upper intestinal tract cancer and appear to be related to the ability of the AhR to regulate the expression of enzymes responsible for the metabolism of several xenobiotics, in particular the PAHs [80-83]. One of the most studied Ahr polymorphisms in humans, G1661A, where a lysine residue at amino acid 544 replaces an arginine, has also been associated with DNA damage in peripheral lymphocytes of coke-oven workers occupationally exposed to PAHs [84]. In a recent study, a particular Ahr polymorphism, in conjunction with exposure to organochlorines, was also associated with increasing risk of non-Hodgkin lymphoma [85]. There is at present no known association between any Ahr polymorphism and increased incidence of any hematopoietic disease or cancers in the absence of known xenobiotic exposures. However, there have been few, if any, studies to examine for these associations.
Based on the evidence for different mechanisms recognized to disrupt HSC functions and lead to hematopoietic diseases, along with evidence supporting a role of the AhAR in the regulation of HSCs, we propose that AhR involvement in these diseases may occur through (a) dysregulation of AhR signaling in HSCs/HPCs, (b) generation of leukemic stem cells, (c) depletion of the stem cell compartment, and/or (d) disruption of HSC-microenvironment interactions. This hypothesis is based on the following evidence as summarized in Table 2:
Dysregulation of AhR signaling and transcriptional regulation in HSCs/HPCs: Dysregulated AhR signaling in HSCs and HPCs may result in hematopoietic disease by molecular mechanisms in which AhR activation could lead to inappropriate expression of genes. Similarly, altered AhR levels and/or activity could lead to modified interactions of the AhR with other transcription factors, cofactors or regulators of the epigenome. The subsequent dysregulation of critical transcription factors in HSCs/HPCs may block differentiation and promote the accumulation of progenitors [11]. It is known that the co-existence of lineage-specific transcription factors in HSC allows lineage selection by narrowing the potential for differentiation. For example, the transcription factor PU.1 is essential for lymphoid commitment, but T-cell lineage specification relies on GATA-3 [86], and B-cell commitment on the sequential activation of E2A, EBF1, and Pax5 [87]. During lineage commitment, transcription factors need to activate certain genes and stabilize their expression while simultaneously downregulating other genes not necessary for the differentiation pathway taken. Given the large number of genes known to be directly or indirectly regulated by the AhR [88-90], there are several likely suspects for those that would affect these differentiation pathways. For example, the genes encoding c-Myc and HES-1, that are involved in determining HSC quiescence, proliferation, and function [91-93] are known to be directly regulated by the AhR [94, 95]. Although more research is clearly needed to define AhR regulation of these genes and the exact effect on HSCs/HPCs, there is some work to indicate that alterations in differentiation may occur. When AhR-expressing human non-terminally differentiated hematopoietic CD34+ cells were cultured and exposed to PAHs in vitro, proliferation and differentiation were affected in an AhR-dependent manner [62]. PAHs also inhibit in vitro differentiation of hematopoietic-derived osteoclasts [96]. AhR activation has been shown to alter differentiation of HPCs in mice exposed to TCDD resulting in an increase in the number of cells of the myeloid lineage at the expense of lymphoid lineage cells [6]. AhR expression has been reported to be up-regulated in human adult T-cell leukemia cell lines suggesting a possible link between AhR overexpression and leukemogenesis [97]. The gene for BMAL, a basic-loop-helix-PAS domain containing transcription factor and a sensor of circadian rhythms, is transcriptionally silenced by methylation of Cytosine-poly-Guanosine repeats (CpG islands) in diffuse large B-cell lymphoma and acute lymphocytic and myeloid leukemias [98]. A similar epigenetic mechanism of CpG island methylation results in the down-regulation of AhR expression in Acute Lymphoblastic Leukemia cells [99]. Together, these studies suggest an important role of AhR dysregulation in the progression towards malignancy of hematopoietic cells.
Generation of leukemic stem cells: Leukemias are believed to originate from multiple events of malignant transformations in HSCs or HPCs. Leukemic stem cells (LSCs) emerge as a phenotype in which the self-renewal function, usually limited to HSCs, is acquired by more differentiated populations. The self-renewing phenotypes then lead to clonogenic accumulation of progenitors instead of homeostatic differentiation [100]. It has been reported that increased cyclooxygenase-2 is associated with AhR-mediated resistance to apoptotic responses in human lymphoma cell lines, and with a lymphoproliferative disorder consistent with a pre-malignant state in the lymph nodes of TCDD-exposed animals [101]. The leukemogenic activity of the xenobiotic benzene has been recognized to be AhR dependent [102, 103], and AhR-regulated CYPs are responsible for metabolizing benzene into genotoxic compounds [104]. The latter observation is particularly relevant since recent data support the hypothesis that benzene-induced hematotoxicity results from the generation of ROS in HSCs/HPCs by benzene metabolites and subsequent oxidative stress. Furthermore, overexpression of the human thioredoxin gene, responsible for curbing oxidative stress, decreased clastogenic induction and lymphoid toxicity in mice exposed to benzene [105]. These observations suggest that AhR-mediated benzene genototoxicity results in the formation of LSCs responsible for benzene-induced leukemogenesis. Although it appears that the AhR has an important function in regulating benzene-induced leukemogenicity through its presence and activity in bone marrow cells, the specific role is not yet known. While this may be due to AhR-dependent regulation of enzymes responsible for the generation of active benzene metabolites, additional data suggest that the AhR may have other important functions in HSCs/HPCs leading to the formation of LSCs [102, 106] (see below).
Depletion of the stem cell compartment: Malignant transformations in HSCs can lead to stem cell exhaustion by mechanisms specific to the gene(s) being disrupted [107]. Early evidence for this was observed in p21 knock-out animals subjected to hematopoietic stress. Lack of this G1 checkpoint cell cycle regulator promoted cell cycling, but also depleted the pool of quiescent HSC, with fatal consequences [10]. Similar outcomes have been observed for stem cell leukemia (SCL) factor signaling [108]. SCL is a transcription factor of the basic helix-loop-helix family of proteins that is able to interact with regulatory elements of hematopoietic transcription factors such as GATA-1. SCL negatively regulates quiescence in long-term HSCs, and its dysregulation results in an impaired long-term repopulation activity of these cells [108]. SCL has been shown to be dysregulated in human T-cell Acute Lymphoblastic Leukemia [109]. Different reports also suggest that AhR is a negative regulator of proliferation of hematopoietic progenitor and/or stem cells. Murine HSCs/HPCs being forced to proliferate due to chemical stress or growth factors show a down-regulation of the Ahr gene [6, 66], and a similar outcome is observed following TCDD-induced AhR activation [49, 50]. The finding that TCDD exposure also results in impaired long-term repopulation activity of HSCs [6, 77] suggests that AhR activation results in a depletion of HSCs via increased entry of quiescent cells with long-term repopulation activity into cycling. TCDD has been shown to decrease the number of LSK cells in quiescence in a time-dependent manner [48]. Furthermore, recent data also show that a greater percentage of LSK cells from AhR-/- mice are in the G1/S phase of the cell cycle compared to cells from wild-type mice (Singh et al. unpublished observations). Together these data suggest that prolonged AhR down-regulation/inactivation, as may occur in Acute Lymphoblastic Leukemia [98], might be a step to develop malignancy in hematopoietic cells by depleting the pool of HSCs. While HSC exhaustion does not always result in leukemia, the accumulation of progenitors increases the possibility of these cells acquiring transforming mutations. Furthermore, because the pool of HSCs is decreased, the transformed progenitors are less likely to be replaced [106].
Disruption of HSC-microenvironment interactions: HSCs/HPCs respond to chemokines and growth factors released by other cells in their microenvironment, and these in turn play a major role in regulating self-renewal, proliferation and differentiation. Even if intrinsic regulation of HSC is optimal, hematopoietic disease can have an extrinsic component through dysregulation of the microenvironment providing aberrant signaling to the HSC. It has been shown that mice lacking the expression of retinoic acid receptor (RAR) within cells constituting the microenvironment have increased extramedullary hematopoiesis and accumulation of granulocyte progenitors by mechanisms yet unclear [110]. Known interactions between the AhR and RAR signaling pathways [111, 112] may explain some of the extramedullary hematopoiesis observed in AhR-/- mice (Table 1). Additionally, mice lacking the expression of the cell cycle regulator Rb in hematopoietic cells showed uncoupled communication between hematopoietic cells and the bone marrow microenvironment resulting in mobilization of HSCs and aberrant extramedullary hematopoiesis [113]. Given that the AhR has been shown to directly interact with Rb protein [42, 44], it seems likely that any dysregulation of AhR levels and/or activity could result in altered responses of HSCs to the cues provided by their microenvironment to make cell fate decisions. Since 1) the AhR appears to modulate angiogenesis through a mechanism involving upregulation of VEGF in endothelial cells and downregulation of TGFβ in mesenchymal cells [36, 37, 52, 53], and 2) recruitment of vasculature-forming cells to the bone marrow is required for adult hematopoiesis [114], it is further possible that alterations in AhR function may disrupt hematopoiesis by modifying endothelial cells in the marrow environment. Alternatively, AhR could regulate the expression of proteins, such as TGF-β, that control the expression, activity, and/or secretion of other molecules governing the HSC-microenvironment [115,116]. Finally, because HSC-microenvironment interactions resemble interactions between lymphocytes and structural/stromal components in tissue sites undergoing an inflammatory reaction [117], the known involvement of the AhR in multiple inflammatory pathways [38-41] also provides a myriad of molecular targets for AhR-mediated HSC-microenvironment regulation.
Table 2.
Possible mechanisms for AhR-mediated leukemogenesis and hematopoietic disease.
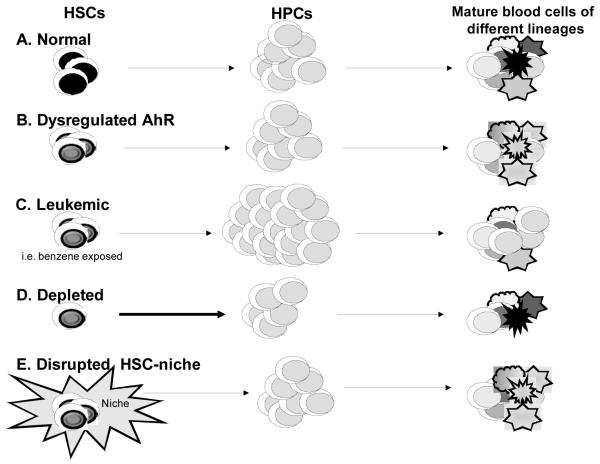
A simplified scheme highlighting these possible mechanisms for AhR involvement in HSC/HPC dysregulation that may result in leukemia or other hematopoietic diseases is depicted in Figure 2. During healthy hematopoiesis (A), HSCs provide HPCs that will differentiate and repopulate all the lineages present in blood. However, dysregulation of AhR signaling in HSCs (as in B) may be carried on to HPCs and mature lineages, thus altering differentiation and lineage commitment decisions. Alternatively (C), AhR dysregulation alone or in combination with exposure to particular xenobiotics, i.e. benzene, may result in the formation of leukemic stem cells with self-renewal properties resulting in the accumulation of progenitors and a block of differentiation into the different lineages. Alterations of AhR levels or activity may also cause (D) chronic changes in HSC cycling resulting in stem-cell exhaustion, reduced numbers of mature cells, and/or conditions that promote the development of hematopoietic cancers. Finally (E), AhR-elicited disruption of the interactions between HSCs and their microenvironment may alter the homeostatic mechanisms responsible of supporting hematopoiesis.
Figure 2.
Summary of the HSC/HPC populations that might be regulated by AhR. (A) During healthy hematopoiesis, HSCs provide HPCs that will differentiate and repopulate all the lineages present in blood, (B) Dysregulation of AhR in HSCs may be carried on to HPCs and mature lineages, (C) Generation of leukemic stem cells with self-renewal properties may cause accumulation of progenitors and block differentiation into the different lineages, (D) Stem-cell exhaustion may result in reduced numbers of mature cells, (E) Disruption of the interactions between HSCs and their microenvironment may alter the homeostatic mechanisms responsible of supporting hematopoiesis. HSCs, hematopoietic stem cells; HPCs, hematopoietic progenitor cells; AhR, aryl hydrocarbon receptor.
FUTURE PERSPECTIVES
While there is sufficient evidence for a regulatory role of AhR in HSCs from both the exposure of humans to xenobioitic AhR ligands and work in experimental animals, the molecular pathways that are regulated by the AhR and which control HSC function and survival are, as yet, poorly understood. Multiple signaling pathways reported to crosstalk with the AhR may participate in the maintenance of homeostatic HSC function. Further studies will need to specifically address at a molecular level whether AhR has a direct and/or indirect role in maintaining HSC function. This information may in turn provide a clear understanding about the physiological role of AhR in the hematopoietic and immune system.
Given that the AhR is a well described ligand-activated transcription factor, it is likely to be a potentially important pharmacological target for prevention and/or treatment of diverse hematological diseases. Potential clinical applications may include regenerative and/or chemotherapeutic approaches to treat leukemia, autoimmune disease, and other hematological diseases.
ACKNOWLEDGEMENTS
The authors thank Dr. Ellen Henry for her critical reading of the manuscript. This work was supported by National Institutes of Health Grants ES04862, ES0166606, Training Grant ES07026, and Center Grant ES01247
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cells assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [2].Singh KP, Casado FL, Opanashuk LA, Gasiewicz TA. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem Pharmacol. 2009;77:577–587. doi: 10.1016/j.bcp.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Osawa M, Nakamura K, Nishi N, et al. In vivo self-renewal of ckit+Sca-1+Lin(low/-) HSCs. J Immunol. 1996;156:3207–3214. [PubMed] [Google Scholar]
- [4].Ito CY, Li CYJ, Bernstein A, et al. Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A–null mice. Blood. 2003;1001:517–523. doi: 10.1182/blood-2002-06-1918. [DOI] [PubMed] [Google Scholar]
- [5].Bowie MB, Kent DG, Copley MR, Eaves CJ. Steel factor responsiveness regulates the high self-renewal phenotype of fetal hematopoietic stem cells. Blood. 2007;109:5043–5048. doi: 10.1182/blood-2006-08-037770. [DOI] [PubMed] [Google Scholar]
- [6].Singh KP, Wyman A, Casado FL, et al. Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis. 2009;30:11–19. doi: 10.1093/carcin/bgn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- [9].Hock H, Hamblen MJ, Rooke HM, et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431:1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- [10].Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- [11].Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- [12].Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain hematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- [13].Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- [14].Arai F, Yoshihara H, Hosokawa K, et al. Niche regulation of hematopoietic stem cells in the endosteum. Ann N Y Acad Sci. 2009;1176:36–46. doi: 10.1111/j.1749-6632.2009.04561.x. [DOI] [PubMed] [Google Scholar]
- [15].Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- [16].Kuznetsov SA, Riminucci M, Ziran N, et al. The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. J Cell Biol. 2004;167:1113–1122. doi: 10.1083/jcb.200408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- [18].Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- [19].Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- [20].Zhou J, Brüne B. Cytokines and hormones in the regulation of hypoxia inducible factor-1alpha (HIF-1alpha) Cardiovasc Hematol Agents Med Chem. 2006;4:189–197. doi: 10.2174/187152506777698344. [DOI] [PubMed] [Google Scholar]
- [21].Hunt T, Sassone-Corsi P. Riding tandem: Circadian clocks and the cell cycle. Cell. 2007;129:461–464. doi: 10.1016/j.cell.2007.04.015. [DOI] [PubMed] [Google Scholar]
- [22].Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pandini A, Soshilov AA, Song Y, et al. Detection of the TCDD binding-fingerprint within the Ah receptor ligand binding domain by structurally driven mutagenesis and functional analysis. Biochemistry. 2009;48:5972–5983. doi: 10.1021/bi900259z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yao EF, Denison MS. DNA sequence determinants for binding of transformed Ah receptor to a dioxin-responsive enhancer. Biochemistry. 1992;31:5060–5067. doi: 10.1021/bi00136a019. [DOI] [PubMed] [Google Scholar]
- [25].Wang F, Wang W, Safe S. Regulation of constitutive gene expression through interactions of Sp1 protein with the nuclear aryl hydrocarbon receptor complex. Biochemistry. 1999;38:11490–11500. doi: 10.1021/bi982578f. [DOI] [PubMed] [Google Scholar]
- [26].Furness SG, Whelan F. The pleiotropy of dioxin toxicity – Xenobiotic misappropriation of the aryl hydrocarbon receptor’s alternative physiological roles. Pharmacol Ther. 2009;124:336–353. doi: 10.1016/j.pharmthera.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [27].Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338:311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- [28].Nguyen LP, Hsu EL, Chowdhury G, et al. D-Amino acid oxidase generates agonists of the aryl hydrocarbon receptor from D-tryptophan. Chem Res Toxicol. 2009;22:1897–1904. doi: 10.1021/tx900043s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chiaro CR, Morales JL, Prabhu KS, Perdew GH. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry. 2008;47:8445–8455. doi: 10.1021/bi800712f. [DOI] [PubMed] [Google Scholar]
- [30].Oesch-Bartlomowicz B, Huelster A, Wiss O, et al. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proc Natl Acad Sci U S A. 2005;102:9218–9223. doi: 10.1073/pnas.0503488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture International Congress of Toxicology XI. Toxicol Sci. 2007;98:5–38. doi: 10.1093/toxsci/kfm096. [DOI] [PubMed] [Google Scholar]
- [32].Domingo JL, Bocio A. Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: a literature review. Environ Int. 2007;33:397–405. doi: 10.1016/j.envint.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [33].Theobald HM, Kimmel G.l., Peterson RE. Developmental and reproductive toxicity of dioxins and related chemicals. In: Schecter A, Gasiewicz TA, editors. Dioxins and Health. second ed John Wiley & Sons; Hoboken: 2003. pp. 329–432. [Google Scholar]
- [34].U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program Report on Carcinogens. (eleventh ed) 2005 http://ntp.niehs.nih.gov/go/16183.
- [35].Kerkvliet NI. AHR-mediated immunomodulation: the role of altered gene transcription. Biochem Pharmacol. 2009;77:746–760. doi: 10.1016/j.bcp.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ichihara S, Yamada Y, Gonzalez FJ, et al. Inhibition of ischemia-induced angiogenesis by benzo[a]pyrene in a manner dependent on the aryl hydrocarbon receptor. Biochem Biophys Res Commun. 2009;381:44–49. doi: 10.1016/j.bbrc.2009.01.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roman AC, Carvajal-Gonzalez JM, Rico-Leo EM, Fernandez-Salguero PM. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-β overexpression in the stroma. J Biol Chem. 2000;275:2943–2950. doi: 10.1074/jbc.M109.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim DW, Gazourian L, Quadri SA, et al. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- [39].Tian Y. Ah receptor and NF-kappaB interplay on the stage of epigenome. Biochem Pharmacol. 2009;77:670–680. doi: 10.1016/j.bcp.2008.10.023. [DOI] [PubMed] [Google Scholar]
- [40].Vogel CF, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem Biophys Res Commun. 2007;363:722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baglole CJ, Maggirwar SB, Gasiewicz TA, et al. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. J Biol Chem. 2008;283:28944–28957. doi: 10.1074/jbc.M800685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Puga A, Barnes SJ, Dalton TP, et al. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J Biol Chem. 2000;275:2943–2950. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- [43].Yang X, Liu D, Murray TJ, et al. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 2005;24:7869–7881. doi: 10.1038/sj.onc.1208938. [DOI] [PubMed] [Google Scholar]
- [44].Huang G, Elferink CJ. Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Mol Pharmacol. 2005;67:88–96. doi: 10.1124/mol.104.002410. [DOI] [PubMed] [Google Scholar]
- [45].Fritsche E, Schafer C, Calles C, et al. Lightening up the UV response by identification of the aryl hydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Patel RD, Murray IA, Flaveny CA, et al. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest. 2009;89:695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mukai M, Lin TM, Peterson RE, et al. Behavioral rhythmicity of mice lacking AhR and attenuation of light-induced phase shift by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Rhythms. 2008;23:200–211. doi: 10.1177/0748730408316022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol Pharmacol. 2006;69:2076–2083. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- [49].Pollenz RS, Popat J, Dougherty EJ. Role of the carboxy-terminal transactivation domain and active transcription in the ligand-induced and ligand-dependent degradation of the mouse Ahb-1 receptor. Biochem Pharmacol. 2005;70:1623–1633. doi: 10.1016/j.bcp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- [50].Evans BR, Karchner SI, Allan LL, et al. Repression of aryl hydrocarbon receptor (AHR) signaling by AHR repressor: role of DNA binding and competition for AHR nuclear translocator. Mol Pharmacol. 2008;73:387–398. doi: 10.1124/mol.107.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- [52].Fernandez-Salguero P, Pineau T, Hilbert DM, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- [53].Schmidt JV, Su GH, Reddy JK, et al. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Negishi T, Kato Y, Ooneda O, et al. Effects of aryl hydrocarbon receptor signaling on the modulation of Th1/Th2 balance. J Immunol. 2005;175:7348–7356. doi: 10.4049/jimmunol.175.11.7348. [DOI] [PubMed] [Google Scholar]
- [55].Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links T(H)17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- [56].Shi LZ, Faith NG, Nakayama Y, et al. The aryl hydrocarbon receptor is required for optimal resistance to Listeria monocytogenes infection in mice. J Immunol. 2007;179:6952–6962. doi: 10.4049/jimmunol.179.10.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kimura A, Naka T, Nakahama T, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bunger MK, Glover E, Moran SM, et al. Abnormal liver development and resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in mice carrying a mutation in the DNA-binding domain of the aryl hydrocarbon receptor. Toxicol Sci. 2008;106:83–92. doi: 10.1093/toxsci/kfn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Brunnberg S, Andersson P, Lindstam M, et al. The constitutively active Ah receptor (CA-AhR) mouse as a potential model for dioxin exposure – effects in vital organs. Toxicology. 2006;224:191–201. doi: 10.1016/j.tox.2006.04.045. [DOI] [PubMed] [Google Scholar]
- [60].Allan LL, Sherr DH. Constitutive activation and environmental chemical induction of the aryl hydrocarbon receptor/transcription factor in activated human B lymphocytes. Mol Pharmacol. 2005;67:1740–1750. doi: 10.1124/mol.104.009100. [DOI] [PubMed] [Google Scholar]
- [61].Platzer B, Richter S, Kneidinger D, et al. Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J Immunol. 2009;183:66–74. doi: 10.4049/jimmunol.0802997. [DOI] [PubMed] [Google Scholar]
- [62].van Grevenynghe J, Bernard M, Langouet S, et al. Human CD34-positive hematopoietic stem cells constitute targets for carcinogenic polycyclic aromatic hydrocarbons. J Pharmacol Exp Ther. 2005;314:693–702. doi: 10.1124/jpet.105.084780. [DOI] [PubMed] [Google Scholar]
- [63].Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- [64].Lavin AL, Hahn DJ, Gasiewicz TA. Expression of functional aromatic hydrocarbon receptor and aromatic hydrocarbon nuclear translocator proteins in murine bone marrow stromal cells. Arch Biochem Biophys. 1998;352:9–18. doi: 10.1006/abbi.1998.0587. [DOI] [PubMed] [Google Scholar]
- [65].Hayashi S, Okabe-Kado J, Honma Y, Kawajiri K. Expression of AhR (TCDD receptor) during human monocytic differentiation. Carcinogenesis. 1995;16:1403–1409. doi: 10.1093/carcin/16.6.1403. [DOI] [PubMed] [Google Scholar]
- [66].Venezia TA, Merchant AA, Ramos CA, et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2:1640–1651. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Frericks M, Meissner M, Esser C. Microarray analysis of the AHR system: tissue-specific flexibility in signal and target genes. Toxicol Appl Pharmacol. 2007;220:320–332. doi: 10.1016/j.taap.2007.01.014. [DOI] [PubMed] [Google Scholar]
- [68].Lawrence BP, Denison MS, Novak H, et al. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Marshall NB, Vorachek WR, Steppan LB, et al. Functional characterization and gene expression analysis of CD4+CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hauben E, Gregori S, Draghici E, et al. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112:1214–1222. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- [71].Staples JE, Murante FG, Fiore NC, et al. Thymic alterations induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are strictly dependent on aryl hydrocarbon receptor activation in hemopoietic cells. J Immunol. 1998;160:3844–3854. [PubMed] [Google Scholar]
- [72].Fine JS, Silverstone AE, Gasiewicz TA. Impairment of prothymocyte activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 1990;144:1169–1176. [PubMed] [Google Scholar]
- [73].Kremer J, Gleichmann E, Esser C. Thymic stroma exposed to arylhydrocarbon receptor-binding xenobiotics fails to support proliferation of early thymocytes but induces differentiation. J Immunol. 1994;153:2778–2786. [PubMed] [Google Scholar]
- [74].Frericks M, Burgoon LD, Zacharewski TR, Esser C. Promoter analysis of TCDD-inducible genes in a thymic epithelial cell line indicates the potential for cell-specific transcription factor crosstalk in the AhR response. Toxicol Appl Pharmacol. 2008;232:268–279. doi: 10.1016/j.taap.2008.07.009. [DOI] [PubMed] [Google Scholar]
- [75].Laiosa MD, Wyman A, Murante FG, et al. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol. 2003;171:4582–4591. doi: 10.4049/jimmunol.171.9.4582. [DOI] [PubMed] [Google Scholar]
- [76].Mustafa A, Holladay SD, Goff M, et al. An enhanced postnatal autoimmune profile in 24 week-old C57BL/6 mice developmentally exposed to TCDD. Toxicol App Pharmacol. 2008;232:51–59. doi: 10.1016/j.taap.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sakai R, Kajiume T, Inoue H, et al. TCDD treatment eliminates the long-term reconstitution activity of hematopoietic stem cell. Toxicol Sci. 2003;72:84–91. doi: 10.1093/toxsci/kfg002. [DOI] [PubMed] [Google Scholar]
- [78].Murante FG, Gasiewicz TA. Hematopoietic progenitor cells are sensitive targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J mice. Toxicol Sci. 2000;54:374–383. doi: 10.1093/toxsci/54.2.374. [DOI] [PubMed] [Google Scholar]
- [79].Bertazzi PA, Consonni D, Bachetti S, et al. Health effects of dioxin exposure: A 20-year mortality study. Am J Epidemiol. 2001;153:1031–1044. doi: 10.1093/aje/153.11.1031. [DOI] [PubMed] [Google Scholar]
- [80].Cauchi S, Stucker I, Laurent-Puig P, et al. Polymorphisms of human aryl hydrocarbon receptor (AhR) gene in a French population: relationship with CYP1A1 inducibility and lung cancer. Carcinogenesis. 2001;22:1819–1824. doi: 10.1093/carcin/22.11.1819. [DOI] [PubMed] [Google Scholar]
- [81].Bin P, Leng S, Cheng J, et al. Association of aryl hydrocarbon receptor gene polymorphisms and urinary 1-hydroxypyrene in polycyclic aromatic hydrocarbon-exposed workers. Cancer Epidemiol Biomarkers Prev. 2008;17:1702–1707. doi: 10.1158/1055-9965.EPI-07-2812. [DOI] [PubMed] [Google Scholar]
- [82].Chen D, Tian T, Wang H, et al. Association of human aryl hydrocarbon receptor gene polymorphisms with risk of lung cancer among cigarette smokers in a Chinese population. Pharmacogenet Genomics. 2009;19:25–34. doi: 10.1097/FPC.0b013e328316d8d8. [DOI] [PubMed] [Google Scholar]
- [83].Roth MJ, Wei WQ, Baer J, et al. Aryl hydrocarbon receptor expression is associated with a family history of upper gastrointestinal tract cancer in a high-risk population exposed to aromatic hydrocarbons. Cancer Epidemiol Biomarkers Prev. 2009;18:2391–2396. doi: 10.1158/1055-9965.EPI-08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chen Y, Bai Y, Yuan J, et al. Association of polymorphisms in AhR, CYP1A1, GSTM1, and GSTT1 genes with levels of DNA damage in peripheral blood lymphocytes among coke-oven workers. Cancer Epidemiol Biomarkers Prev. 2006;15:1703–1707. doi: 10.1158/1055-9965.EPI-06-0291. [DOI] [PubMed] [Google Scholar]
- [85].Ng CH, Janoo-Gilani R, Sipahimalani P, et al. Interaction between organochlorines and the AHR gene, and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9429-5. doi:10.1007/s10552-009-9429-5. [DOI] [PubMed] [Google Scholar]
- [86].Rothenberg EV, Anderson MK. Elements of transcription factor network design for T-lineage specification. Dev Biol. 2002;246:29–44. doi: 10.1006/dbio.2002.0667. [DOI] [PubMed] [Google Scholar]
- [87].Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;27:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- [88].Gasiewicz TA, Henry EC, Collins LL. Expression and activity of aryl hydrocarbon receptors in development and cancer. Crit Rev Eukaryot Gene Expr. 2008;18:279–321. doi: 10.1615/critreveukargeneexpr.v18.i4.10. [DOI] [PubMed] [Google Scholar]
- [89].Sartor MA, Schnekenburger M, Marlowe JL, et al. Genomewide analysis of aryl hydrocarbon receptor binding targets reveals an extensive array of gene clusters that control morphogenetic and developmental programs. Environ Health Perspect. 2009;117:1139–1146. doi: 10.1289/ehp.0800485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Terskikh AV, Miyamoto T, Chang C, et al. Gene expression analysis of purified hematopoietic stem cells and committed progenitors. Blood. 2003;102:94–101. doi: 10.1182/blood-2002-08-2509. [DOI] [PubMed] [Google Scholar]
- [92].Bruno L, Hoffmann R, McBlane F, et al. Molecular signatures of self-renewal, differentiation, and lineage choice in multipotential hematopoietic progenitor cells in vitro. Mol Cell Biol. 2004;24:741–756. doi: 10.1128/MCB.24.2.741-756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science. 2008;321:1095–1100. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kim DW, Gazourian L, Quadri SA, et al. The RelA NF-κB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- [95].Thomsen JS, Kietz S, Strom A, et al. HES-1, a novel target gene for the aryl hydrocarbon receptor. Mol Pharmacol. 2004;65:165–171. doi: 10.1124/mol.65.1.165. [DOI] [PubMed] [Google Scholar]
- [96].Voronov I, Heersche JN, Casper RF, et al. Inhibition of osteoclast differentiation by polycyclic aromatic hydrocarbons is dependent on cell density and RANKL concentration. Biochem Pharmacol. 2005;70:300–307. doi: 10.1016/j.bcp.2005.04.028. [DOI] [PubMed] [Google Scholar]
- [97].Hayashibara T, Yamada Y, Mori N, et al. Possible involvement of aryl hydrocarbon receptor (AhR) in adult T-cell leukemia (ATL) leukemogenesis: constitutive activation of AhR in ATL. Biochem Biophys Res Commun. 2003;300:128–134. doi: 10.1016/s0006-291x(02)02793-6. [DOI] [PubMed] [Google Scholar]
- [98].Taniguchi H, Fernandez AF, Setien F, et al. Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res. 2009;69:8447–8454. doi: 10.1158/0008-5472.CAN-09-0551. [DOI] [PubMed] [Google Scholar]
- [99].Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, et al. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27:1099–1104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- [100].Rossi DJ, Jamieson CH, Weissman IL. Stem cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- [101].Vogel CF, Li W, Sciullo E, et al. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am J Pathol. 2007;171:1538–1548. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hirabayashi Y, Inoue T. Aryl hydrocarbon receptor biology and xenobiotic responses in hematopoietic progenitor cells. Biochem Pharmacol. 2009;77:521–535. doi: 10.1016/j.bcp.2008.09.030. [DOI] [PubMed] [Google Scholar]
- [103].Gasiewicz TA, Singh KP, Casado FL. The aryl hydrocarbon receptor has an important role in the regulation of hematopoiesis: Implications for benzene-induced hematopoietic toxicity. Chem Biol Interact. 2009 doi: 10.1016/j.cbi.2009.10.019. doi:10.1016/j.cbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yoon BI, Hirabayashi Y, Kawasaki Y, et al. Aryl hydrocarbon receptor mediates benzene-induced hematoxicity. Toxicol. Sci. 2002;70:150–156. doi: 10.1093/toxsci/70.1.150. [DOI] [PubMed] [Google Scholar]
- [105].Li GX, Hirabayashi Y, Yoon BI, et al. Thioredoxin overexpression in mice, model of attenuation of oxidative stress, prevents benzene-induced hematolymphoid toxicity and thymic lymphoma. Exp Hematol. 2006;34:1687–1697. doi: 10.1016/j.exphem.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [106].Badham HJ, Winn LM. Investigating the role of the aryl hydrocarbon receptor in benzene-initiated toxicity in vitro. Toxicology. 2007:177–185. doi: 10.1016/j.tox.2006.10.021. [DOI] [PubMed] [Google Scholar]
- [107].Jacob B, Osato M. Stem cell exhaustion and leukemogenesis. J Cell Biochem. 2009;107:393–399. doi: 10.1002/jcb.22150. [DOI] [PubMed] [Google Scholar]
- [108].Lacombe J, Herblot S, Rojas-Sutterlin S, et al. Scl regulates the quiescence and the long-term competence of hematopoietic stem cells. Blood. 2009 doi: 10.1182/blood-2009-01-201384. doi:10.1182/blood-2009-01-201384. [DOI] [PubMed] [Google Scholar]
- [109].Lecuyer E, Hoang T. SCL: from the origin of hematopoiesis to stem cells and leukemia. Exp Hematol. 2004;32:11–24. doi: 10.1016/j.exphem.2003.10.010. [DOI] [PubMed] [Google Scholar]
- [110].Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor-gamma deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Murphy KA, Quadro L, White LA. The intersection between the aryl hydrocarbon receptor and retinoic acid signaling pathways. Vitam Horm. 2007;75:33–67. doi: 10.1016/S0083-6729(06)75002-6. [DOI] [PubMed] [Google Scholar]
- [112].Widerak M, Ghoneim C, Dumontier MF, et al. The aryl hydrocarbon receptor activates the retinoic acid receptor alpha through SMRT antagonism. Biochimie. 2006;88:387–397. doi: 10.1016/j.biochi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- [113].Walkley CR, Shea JM, Sims NA, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chan CK, Chen CC, Luppen CA, et al. Endochondreal ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gomez-Duran A, Carvajal-Gonzalez JM, Mulero-Navarro S, et al. Fitting a xenobiotic receptor into cell homeostasis: how the dioxin receptor interacts with TGFbeta signaling. Biochem Pharmacol. 2009;77:700–712. doi: 10.1016/j.bcp.2008.08.032. [DOI] [PubMed] [Google Scholar]
- [116].Soderberg SS, Karlsson G, Karlsson S. Complex and context dependent regulation of hematopoiesis by TGF-beta superfamily signaling. Ann N Y Acad Sci. 2009;1176:55–69. doi: 10.1111/j.1749-6632.2009.04569.x. [DOI] [PubMed] [Google Scholar]
- [117].Boehmler AM, Drost A, Jaggy L, et al. The CysLT1 ligand leukotriene D4 supports alpha4beta1- and alpha5beta1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. J Immunol. 2009;182:6789–6798. doi: 10.4049/jimmunol.0801525. [DOI] [PubMed] [Google Scholar]