Abstract
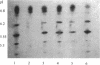
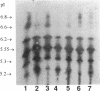
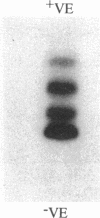
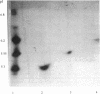
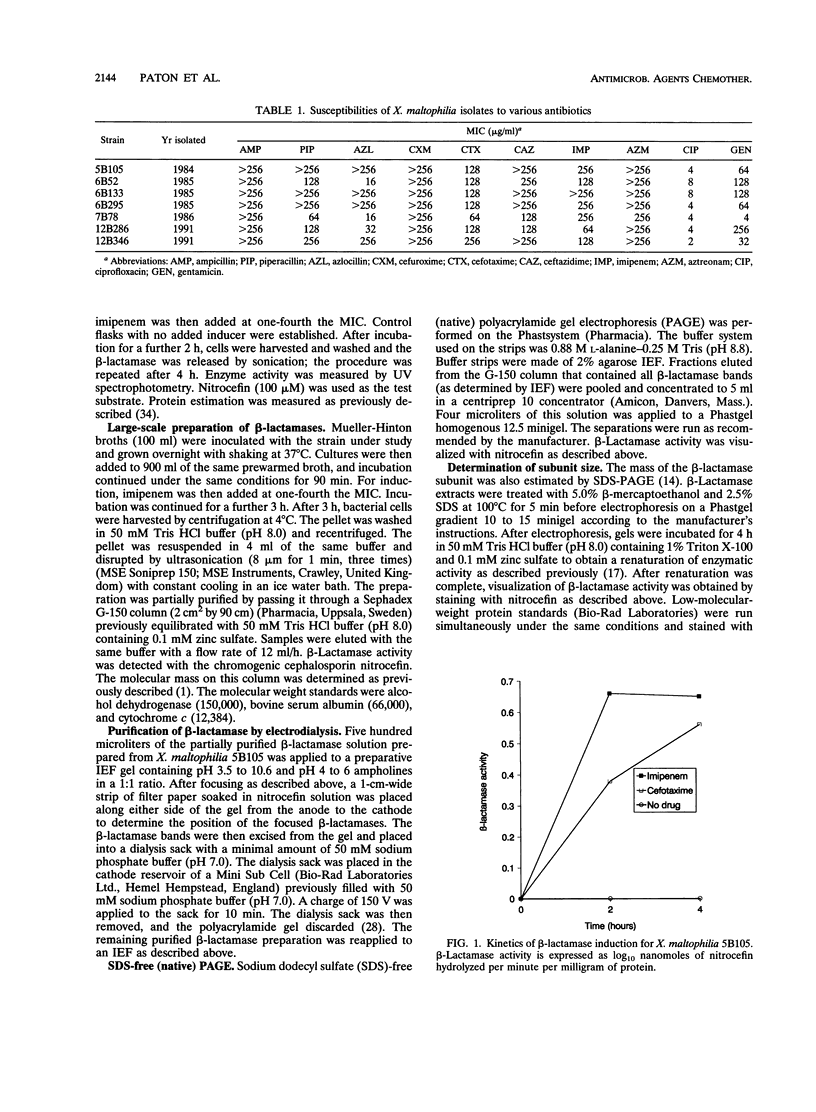
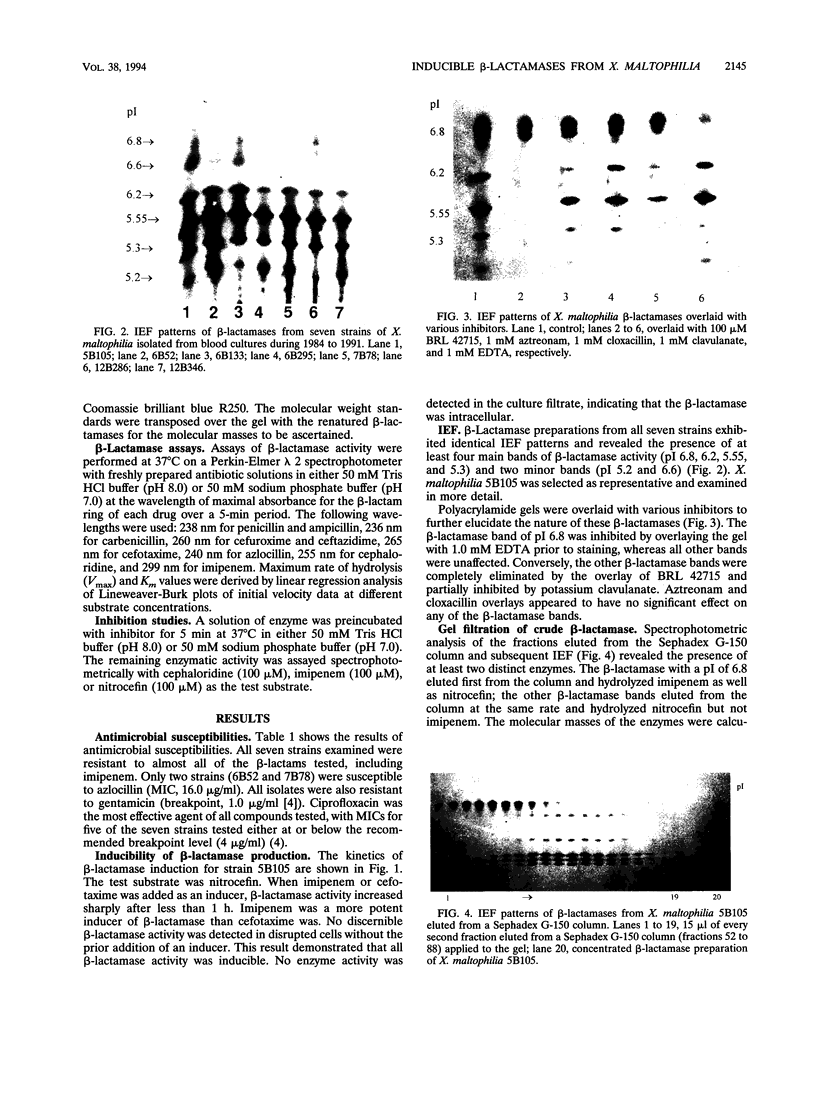
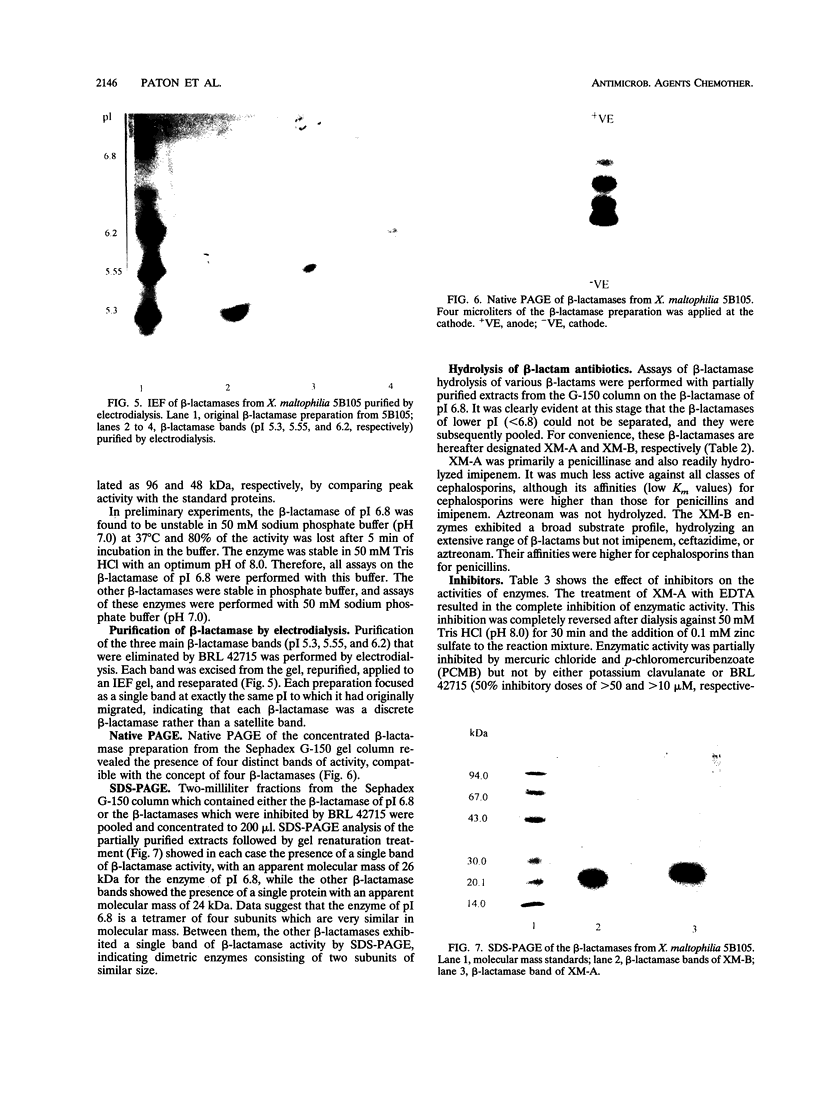
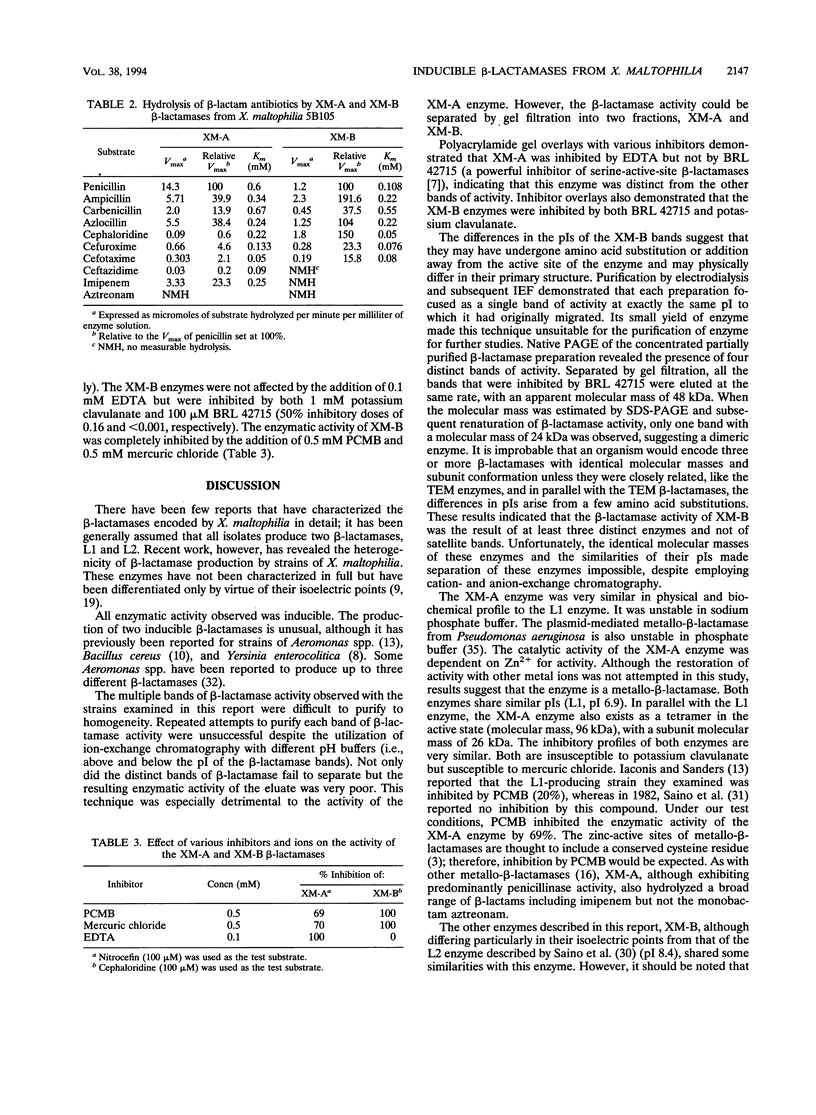
Four different beta-lactamases have been found in several strains of Xanthomonas maltophilia isolated from blood cultures during 1984 to 1991 at the Edinburgh Royal Infirmary. One was a metallo-beta-lactamase with predominantly penicillinase activity and an isoelectric point of 6.8. Its molecular size as determined by gel filtration was 96 kDa but was only 26 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), suggesting a tetramer of four equal subunits. The enzyme hydrolyzed all classes of beta-lactams except the monobactam aztreonam. This enzyme was not inhibited by potassium clavulanate or BRL 42715 but was inhibited by p-chloromercuribenzoate, mercuric chloride, and EDTA. The beta-lactamase was unstable in 50 mM sodium phosphate buffer (pH 8.0) but stable in 50 mM Tris HCl (pH 8.0). The other beta-lactamases focused as a series of different isoelectric points, ranging from pI 5.2 to 6.6. Together, these enzymes exhibited a broad spectrum of activity, hydrolyzing most classes of beta-lactams but not imipenem or aztreonam. Their molecular size was 48 kDa by Sephadex gel filtration and 24 kDa by SDS-PAGE, indicating that they were enzymes consisting of two equal subunits. They were inhibited by p-chloromercuribenzoate, mercuric chloride, potassium clavulanate, and BRL 42715 but not EDTA. This study demonstrated that X. maltophilia produces more than just the L1 and L2 beta-lactamases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken J. S., Sanders C. C., Clark R. B., Hori M. Beta-lactam resistance in Aeromonas spp. caused by inducible beta-lactamases active against penicillins, cephalosporins, and carbapenems. Antimicrob Agents Chemother. 1988 Sep;32(9):1314–1319. doi: 10.1128/aac.32.9.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin G. S., Galdes A., Hill H. A., Smith B. E., Waley S. G., Abraham E. P. Histidine residues of zinc ligands in beta-lactamase II. Biochem J. 1978 Nov 1;175(2):441–447. doi: 10.1042/bj1750441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 1, 2a, 2b, and 2b'. Antimicrob Agents Chemother. 1989 Mar;33(3):264–270. doi: 10.1128/aac.33.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K., Griffin D. R., Page J. W., Upshon P. A. In vitro evaluation of BRL 42715, a novel beta-lactamase inhibitor. Antimicrob Agents Chemother. 1989 Sep;33(9):1580–1587. doi: 10.1128/aac.33.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Abraham E. P. Beta-lactamases from Yersinia enterocolitica. J Gen Microbiol. 1975 Apr;87(2):273–284. doi: 10.1099/00221287-87-2-273. [DOI] [PubMed] [Google Scholar]
- Cullmann W., Dick W. Heterogeneity of beta-lactamase production in Pseudomonas maltophilia, a nosocomial pathogen. Chemotherapy. 1990;36(2):117–126. doi: 10.1159/000238757. [DOI] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodríguez J. A., García Sánchez J. E., García García M. I., García Sánchez E., Muñoz Bellido J. L. Antibiotic susceptibility profile of Xanthomonas maltophilia. In vitro activity of beta-lactam/beta-lactamase inhibitor combinations. Diagn Microbiol Infect Dis. 1991 May-Jun;14(3):239–243. doi: 10.1016/0732-8893(91)90038-h. [DOI] [PubMed] [Google Scholar]
- Gilardi G. L. Pseudomonas maltophilia infections in man. Am J Clin Pathol. 1969 Jan;51(1):58–61. doi: 10.1093/ajcp/51.1.58. [DOI] [PubMed] [Google Scholar]
- Iaconis J. P., Sanders C. C. Purification and characterization of inducible beta-lactamases in Aeromonas spp. Antimicrob Agents Chemother. 1990 Jan;34(1):44–51. doi: 10.1128/aac.34.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecso-Bornet M., Pierre J., Sarkis-Karam D., Lubera S., Bergogne-Berezin E. Susceptibility of Xanthomonas maltophilia to six quinolones and study of outer membrane proteins in resistant mutants selected in vitro. Antimicrob Agents Chemother. 1992 Mar;36(3):669–671. doi: 10.1128/aac.36.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massidda O., Rossolini G. M., Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among class B metallo-beta-lactamases. J Bacteriol. 1991 Aug;173(15):4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Mett H., Rosta S., Schacher B., Frei R. Outer membrane permeability and beta-lactamase content in Pseudomonas maltophilia clinical isolates and laboratory mutants. Rev Infect Dis. 1988 Jul-Aug;10(4):765–769. doi: 10.1093/clinids/10.4.765. [DOI] [PubMed] [Google Scholar]
- Muder R. R., Yu V. L., Dummer J. S., Vinson C., Lumish R. M. Infections caused by Pseudomonas maltophilia. Expanding clinical spectrum. Arch Intern Med. 1987 Sep;147(9):1672–1674. [PubMed] [Google Scholar]
- Neu H. C., Saha G., Chin N. X. Resistance of Xanthomonas maltophilia to antibiotics and the effect of beta-lactamase inhibitors. Diagn Microbiol Infect Dis. 1989 May-Jun;12(3):283–285. doi: 10.1016/0732-8893(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Nordmann P., Mariotte S., Naas T., Labia R., Nicolas M. H. Biochemical properties of a carbapenem-hydrolyzing beta-lactamase from Enterobacter cloacae and cloning of the gene into Escherichia coli. Antimicrob Agents Chemother. 1993 May;37(5):939–946. doi: 10.1128/aac.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleroni N. J., Bradbury J. F. Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) Swings et al. 1983. Int J Syst Bacteriol. 1993 Jul;43(3):606–609. doi: 10.1099/00207713-43-3-606. [DOI] [PubMed] [Google Scholar]
- Payne D. J., Marriott M. S., Amyes S. G. Characterisation of a unique ceftazidime-hydrolysing beta-lactamase, TEM-E2. J Med Microbiol. 1990 Jun;32(2):131–134. doi: 10.1099/00222615-32-2-131. [DOI] [PubMed] [Google Scholar]
- Rolston K. V., Messer M., Ho D. H. Comparative in vitro activities of newer quinolones against Pseudomonas species and Xanthomonas maltophilia isolated from patients with cancer. Antimicrob Agents Chemother. 1990 Sep;34(9):1812–1813. doi: 10.1128/aac.34.9.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Inoue M., Mitsuhashi S. Purification and properties of an inducible cephalosporinase from Pseudomonas maltophilia GN12873. Antimicrob Agents Chemother. 1984 Mar;25(3):362–365. doi: 10.1128/aac.25.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K., King A., Phillips I. Beta-lactamases with high activity against imipenem and Sch 34343 from Aeromonas hydrophila. J Antimicrob Chemother. 1986 Jan;17(1):45–50. doi: 10.1093/jac/17.1.45. [DOI] [PubMed] [Google Scholar]
- Toma E. C., Morisset R., Agbaba O., Phaneuf D. In vitro antagonism between N-formimidoyl thienamycin and aztreonam, ticarcillin and ticarcillin/clavulanic acid. Ann Microbiol (Paris) 1984 Jul-Aug;135B(1):111–115. doi: 10.1016/s0769-2609(84)80048-4. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Watanabe M., Iyobe S., Inoue M., Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Jan;35(1):147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. J., Wu P. J., Livermore D. M. Biochemical characterization of a beta-lactamase that hydrolyzes penems and carbapenems from two Serratia marcescens isolates. Antimicrob Agents Chemother. 1990 May;34(5):755–758. doi: 10.1128/aac.34.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]