Abstract
Aims
Nociceptive behavior and its relationship with bladder dysfunction were investigated in two cystitis models, which were induced by intraperitoneal (ip) injection of cyclophosphamide (CYP) or intravesical instillation of acetone, using freely moving, non-catheterized conscious rats.
Methods
Female Sprague-Dawley rats were used. Cystitis was induced by ip injection of CYP (100 and 200mg/kg) or intravesical instillation of acetone (10, 30 and 50%) via a polyethylene catheter temporarily inserted into the bladder through the urethra. Then the incidence of nociceptive behavior (immobility with decreased breathing rates) was scored. Voided urine was collected simultaneously and continuously to measure bladder capacity. The plasma extravasation in the bladder was quantified by an evans blue (EB) dye leakage technique.
Results
CYP (100mg/kg, ip) induced nociceptive behavior without affecting bladder capacity or EB concentration in the bladder. A higher dose of CYP (200mg/kg, ip) decreased bladder capacity and increased EB levels as well as nociceptive behavior. In contrast, intravesical instillation of acetone (30%) decreased bladder capacity and increased EB levels, but evoked nociceptive behavior less frequently compared with CYP-treated animals. In capsaicin pretreated rats, nociceptive behavior induced by CYP or acetone was reduced; however, the overall effects of CYP or acetone on bladder capacity and bladder EB levels were unaffected.
Conclusions
These results suggest that there is a difference in the induction process of nociceptive behavior and small bladder capacity after two different types of bladder irritation and that C-fiber sensitization is more directly involved in pain sensation than reduced bladder capacity.
Keywords: rat, cystitis, nociceptive behavior, small bladder capacity
Introduction
Cyclophosphamide (CYP) is an anti-tumor agent, which is known to be metabolized to acrolein that is excreted in urine and accumulates in the bladder to produce toxic effects on the bladder wall, resulting in hemorrhagic cystitis 1. In animals, intraperitoneal (ip) administration of CYP induces plasma extravasation in the bladder, but not in other abdominal organs 2, and also induces pain behaviors such as decrease in breathing rate, closing of the eyes and specific immobile postures characterized by a rounded back.3,4 Thus, CYP-treated rats have been used as a bladder pain model. On the other hand, intravesical instillation of acetone, which is known to remove the glycosaminoglycan layer of the transitional uroepithelium,5 has also been used to induce chemical cystitis.6,7 However, there have been few studies of the connection between changes in micturition, nociceptive behavior and inflammation in the CYP or acetone induced cystitis models. Therefore, in this study, we examined behavioral responses to nociceptive bladder stimuli and their correlation with bladder dysfunction in these two chemical cystitis models with or without desensitization of capsaicin-sensitive C-fiber afferent pathways, using methodology developed in our laboratory to study freely moving, non-catheterized conscious rats.9
In addition, pelvic pain related to bladder filling coupled with urinary frequency due to small bladder capacity are the major symptoms in patients with interstitial cystitis/painful bladder syndrome (IC/PBS). Although the underlying etiology of IC/PBS is still unknown, sensory fiber sensitization associated with neurogenic inflammation has been proposed as one potential pathogenesis of the disease8. Thus, it would be interesting to examine how bladder pain and urinary frequency are correlated or affected by C-fiber desensitization following bladder inflammation even though chemical cystitis induced by CYP or acetone in rats would not directly represent the disease condition of IC/PBS.
Materials and Methods
Animals
Female Sprague-Dawley rats weighing 230-275 g from Hilltop Animal Care (Pittsburgh, PA) were used. Care and handling of animals were in accordance with institutional guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
C-fiber desensitization
In some rats, capsaicin (total 125mg/kg) was injected subcutaneously in divided doses in three consecutive days in order to desensitize capsaicin-sensitive C-fiber afferents.10 Experiments were performed one week after the first injection of capsaicin.
Chemical induction of experimental cystitis
After acclimation for at least two hours in a transparent metabolic cage (Nalgene, Rochester, NY), cystitis was induced by CYP or acetone. In the CYP-treated group, CYP (100 or 200 mg/kg) or vehicle (saline) was injected intraperitoneally. In acetone-treated group, rats were placed in a Bollman-type restraining device (KN-326, Natsume, Tokyo, Japan). Then, a polyethylene tube (PE-50, Clay Adams, NJ) was inserted into the bladder through the external urethral orifice, and residual urine was withdrawn. Acetone (10, 30 or 50%) or vehicle (saline) was then instilled into the bladder via the catheter in a volume of 0.3 ml and kept for one minute. Thereafter, the transurethral catheter was removed. After the CYP or acetone treatment, rats were placed back in a metabolic cage. These treatments (vehicle, CYP or acetone) were blinded to the observer of behavioral testing.
Simultaneous recordings of bladder activity and nociceptive behavior
In a previous behavioral study, ip injection of CYP mainly induced a decrease in breathing rate, closing of the eyes and specific immobile postures characterized by rounded back, indicative of CYP-induced nociceptive responses.4 From our preliminary observations (n=6), immobility with a decrease in breathing frequency was considered as a sufficiently reliable parameter. Therefore, breathing rate was continuously monitored along with the observation of animal behavior. The behavior was observed for the 0-5 min (early), 15-20 min (middle) and 60-65 min (late) periods after the CYP or acetone treatment. Each 5-min period was divided into 60 segments with 5-second duration, and the absence or presence of ‘nociceptive behavior’ was examined in every 5-second segment. If the immobility associated with reduced breathing frequency less than 5 times during a 5-second observation segment were observed, we scored as one positive event of ‘nociceptive behavior’. The number of positive events of nociceptive behavior was then counted in each rat for three 5-min periods (0-5 min, 15-20 min and 60-65 min after the CYP or acetone treatment). Thus, the maximal nociceptive score could be 60 during each 5-min observation period. At the same time, voided urine was collected continuously using a cup placed underneath the metabolic cage and specially fitted onto a force displacement transducer (Fort 100, World Precision Instruments, FL) connected to an amplifier (Transbridge TBM4M, World Precision Instruments, FL) in order to measure voided volume. The voiding data were collected using a data acquisition system equipped with an analog-to-digital converter (Power Lab, AD Instruments, CO) for 180 or 65 minutes after treatment of CYP or acetone, respectively. We observed CYP-treated rats for a longer period because it takes 3-4 hours for CYP to induce the maximal effects on bladder capacity.11 Distilled water (30 ml/kg) was administered 15 minutes before the treatment of CYP, acetone or vehicle (saline) by gavaging in order to increase urine production. This water hydration procedure increased the number of voiding, and reduced the variation of bladder capacity, which was determined by dividing the total voided volume by the number of micturitions.
Measurement of plasma extravasation in the bladder
The plasma extravasation in the bladder was quantified by an evans blue (EB) dye leakage technique.12 After physiological measurements, rats were anesthetized with pentobarbital (50mg/kg, ip), and EB (50mg/kg) was injected into the left femoral vein. Fifteen minutes after EB injection, the bladders were dissected and dried at 50°C for 24 hours. The dried bladder was weighed, and immersed in tubes containing 1 ml formamide for 72 hours. The content of dye in the formamide solution was then determined in duplicate using a Microplate Reader (ELx800, Bio-Tek instrument, VT) set at 620 nm wavelength, and the results are expressed as micrograms of EB per dry weight of the bladder.
Statistics
The values of bladder capacity and EB concentration are expressed as mean ± SEM (standard error of the mean). Behavioral scores are expressed as median and IQR (interquartile range) because they are nonparametric data. Statistical significance was determined with Student's t-test or ANOVA followed by Dunnett's multiple test for parametric data, and with Wilcoxon rank sum test or Steel's multiple test for nonparametric data. P-values less than 0.05 were considered to be significant. All data analyses were performed using the SAS statistical software (SAS Institute, Cary, NC).
Drugs
CYP, capsaicin and EB were purchased from Sigma (St. Louis, MO). Capsaicin (total 125mg/kg) was dissolved in a small amount of ethanol, diluted to concentrations in 10% ethanol, 10% Tween 80 and 80% saline, and given at a volume of 2 ml/kg. CYP and EB were dissolved in saline, and given at a volume of 5 and 1 ml/kg, respectively. Acetone was purchased from Fisher Scientific (Pittsburgh, PA) and diluted in saline.
Results
Effects of ip injection of CYP or intravesical instillation of acetone on bladder function, bladder EB concentration and nociceptive behavior in normal rats
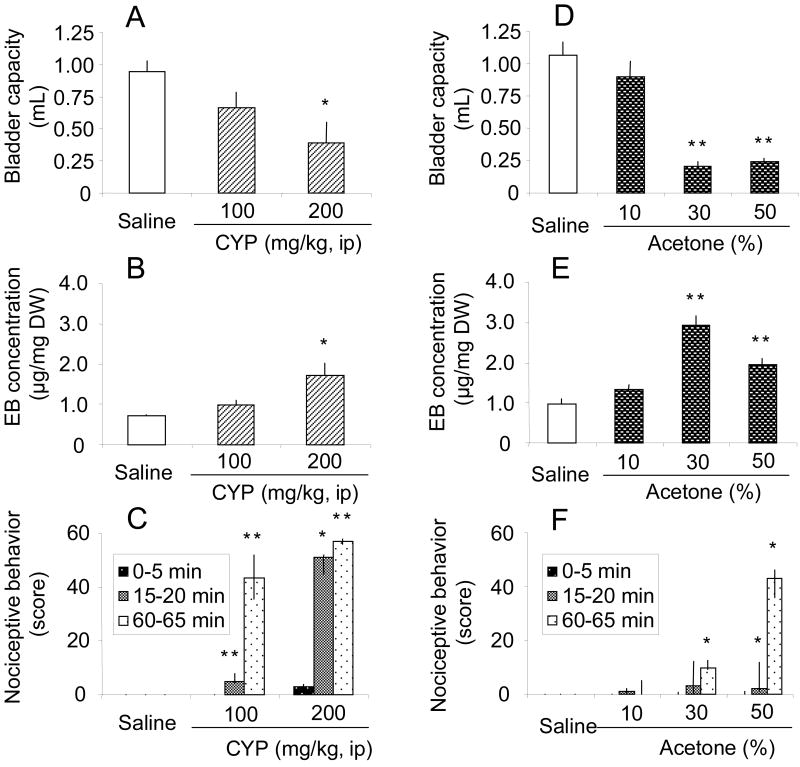
Ip injection of CYP (100 and 200 mg/kg) increased nociceptive behavior in a dose-dependent manner during the middle (15-20 min) and late (60-65 min) periods after CYP injection. However, a significant reduction in bladder capacity and an increase in bladder EB concentration were observed after ip injection of 200 mg/kg, but not 100 mg/kg, CYP (fig. 1A, B and C).
Fig. 1.
Effects of intraperitoneal administration of cyclophosphamide (CYP) (A, B and C) or intravesical instillation of acetone (D, E and F) on bladder capacity, evans blue (EB) concentration in the bladder and nociceptive behavior in normal rats. A, B, D and E, Bars represent mean ± SEM (n=5 or 6 rats/group). * p<0.05, ** p<0.01 compared with vehicle (saline)-treated rats (Dunnett's multiple comparison test). C and F, nociceptive behavior score, which represents the number of 5-second observation periods during which at least one nociceptive event was detected, during three 5-minute intervals (0-5, 15-20 or 60-65 min) following CYP treatment. The maximal nociceptive score could be 60 during each 5-minute observation period. Bars represent median and IQR (n=5 or 6 rats/group). * p<0.05, ** p<0.01 compared with vehicle (saline)-treated rats (Steel's multiple comparison test).
Intravesical instillation of acetone (30 and 50%) significantly reduced bladder capacity and increased EB levels in the bladder, indicating cystitis-induced tissue extravasation (fig. 1D and E). However, bladder EB levels after 50% acetone were lower than those after 30% acetone (fig. 1E). The higher percentage acetone treatment also induced whitish-colored changes in bladder tissues, suggesting that some tissue fixation or protein degradation may be induced by the treatment of acetone. Acetone-induced nociceptive behavior was observed during the late (60-65 min) period after 30% acetone and during the middle (15-20 min) and late (60-65 min) periods after 30 and 50% acetone (fig. 1F). However, the nociceptive behavior score after 30% acetone was lower than the score after 100 mg/kg CYP (fig. 1C), which did not significantly alter bladder capacity or EB concentration (fig. 1A and B).
Correlation between the CYP or acetone-induced reduction in bladder capacity, EB concentration in the bladder and nociceptive behavior
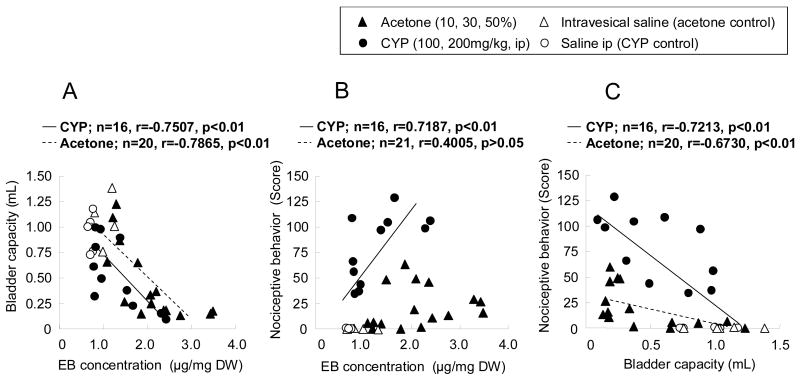
Fig. 2 shows the relationship of individual values of bladder capacity, nociceptive behavior and bladder EB concentration after CYP or acetone treatment. In both groups of rats, the reduction in bladder capacity was correlated with the EB levels in the bladder (CYP, r=-0.7507, p<0.01, n=16; acetone, r=-0.7865) (p<0.01, n=20) (fig. 2A). In addition, because the slope line of correlation in the CYP-treated group was shifted leftward to the acetone-treated group, suggesting that at the same level of tissue extravasation indicated by increased EB levels, more frequent voiding was induced in CYP rats than in acetone-treated rats. In terms of nociceptive behavior, a significant correlation with the bladder EB levels was found in the CYP-treated group while it was not clearly detected in the acetone-treated group (fig. 2B). However, in both groups, nociceptive behavior was correlated with the reduction in bladder capacity although more pronounced nociceptive behavior was observed in the CYP-treated group compared with the acetone-treated group (fig. 2C).
Fig. 2.
Relationship between individual values of bladder capacity and bladder evans blue (EB) concentration (A), nociceptive behavior and bladder EB concentration (B) and nociceptive behavior and bladder capacity (C) (n=16-21/group). The nociceptive behavior score (total number) was summed for each 5-minute period (0-5, 15-20 or 60-65 min) after vehicle (saline), cyclophosphamide (CYP) treatment (100 or 200 mg/kg) or acetone treatment (10, 30 or 50%).
Effects of ip injection of CYP or intravesical instillation of acetone on bladder function, bladder EB concentration and nociceptive behavior in capsaicin-pretreated rats
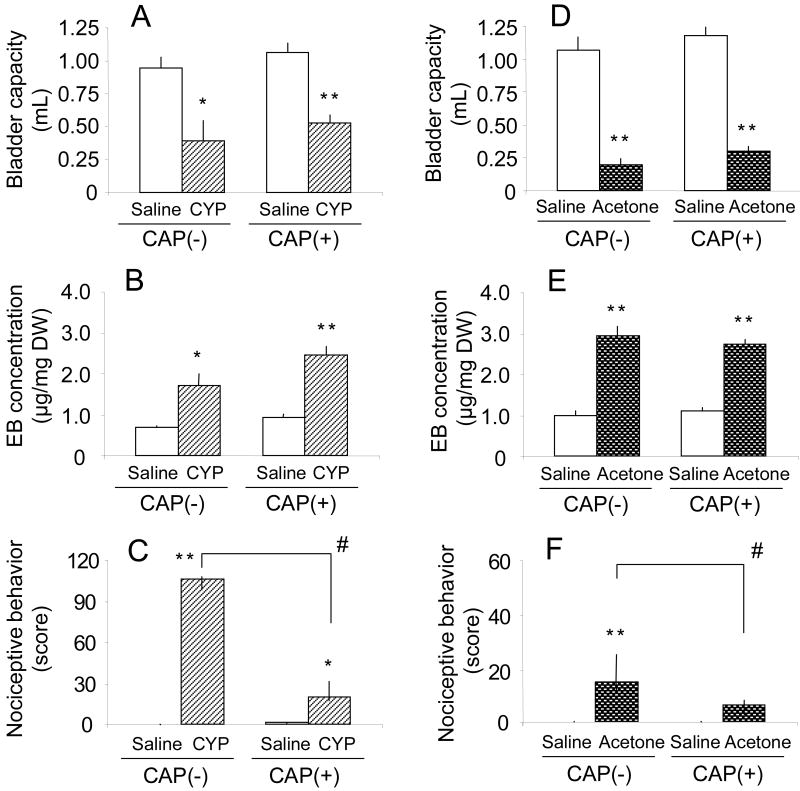
Ip injection of CYP (200mg/kg) or intravesical instillation of acetone (30%) also reduced bladder capacity and increased bladder EB levels as well as nociceptive behavior in rats with C-fiber desensitization induced by capsaicin pretreatment (CYP, fig. 3A-C; acetone, fig. 3D-F). However, CYP or acetone-induced nociceptive behavior was significantly reduced by capsaicin pretreatment (fig. 3C and 3F) even though the changes in bladder capacity and bladder EB levels induced by cystitis were not significantly affected by capsaicin pretreatment (fig. 3A-B and 3D-E) .
Fig. 3.
Effects of cyclophosphamide (CYP) (200mg/kg, ip) (A, B and C) or acetone (30%) (D, E and F) on bladder capacity, bladder evans blue (EB) concentration and nociceptive behavior in rats with [capsaicin (CAP) (+)] or without C-fiber desensitization [CAP (-)]. A, B, D and E, Bars represent mean ± SEM (n=5 rats/group). * p<0.05, ** P<0.01 compared with vehicle (saline)-treated rats (Student's t-test). C and F, the nociceptive behavior score (total number), which was summed for three 5-minute periods (0-5, 15-20 or 60-65 min) after CYP or acetone treatment. Bars represent median and IQR (n=5 rats/group). * p<0.05, ** p<0.01 compared with vehicle (saline)-treated rats. # p<0.05 compared with rats without capsaicin pretreatment (Wilcoxon rank sum test).
Correlation between the CYP or acetone induced reduction in bladder capacity and bladder EB concentration in capsaicin-untreated and -pretreated rats
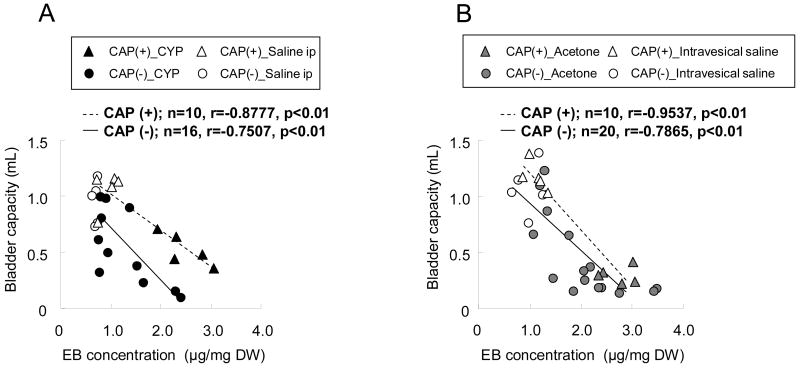
Although the overall effects of CYP or acetone on bladder capacity and EB levels were not significantly reduced by capsaicin pretreatment (fig. 3A-B and 3D-E), C-fiber desensitization appears to reduce the effects of bladder extravasation on bladder capacity. Fig. 4 shows the correlation of individual values of bladder capacity and EB concentration in rats with or without capsaicin pretreatment, and showed that there was a significant, negative correlation between changes in bladder capacity and EB levels in both capsaicin-untreated and -pretreated groups (r=-0.7507--0.9537, p<0.01, n=10-20) (fig. 4A and B). However, bladder capacity after capsaicin pretreatment was larger in both CYP and acetone groups when bladder capacity was compared at equivalent EB levels (fig. 4A and B) although this effect of capsaicin pretreatment on bladder capacity appeared to be smaller in the acetone group than in the CYP group.
Fig. 4.
Relationship between individual values of bladder capacity and bladder evans blue (EB) concentration (n=10-20/group). A, vehicle (saline) or cyclophosphamide (CYP)-treated rats with [capsaicin (CAP) (+)] or without CAP pretreatment [CAP (-)]. B, vehicle (saline) or acetone-treated rats with [CAP (+)] or without CAP pretreatment [CAP (-)].
Discussion
The present study which measured nociceptive behavior, bladder extravasation and changes in bladder capacity in CYP and acetone-induced cystitis revealed that different mechanisms may be involved in these two models of chemically induced cystitis. A low dose of CYP elicited nociceptive behavior without significant effects on bladder capacity or EB levels. In contrast, intravesical instillation of 30% acetone initially decreased bladder capacity and increased EB levels without inducing nociceptive behavior. Acetone then did increase nociceptive behavior to a lesser extent than CYP after a considerable delay of one hour. These results indicate that CYP is likely to induce pain sensation before affecting bladder function or inducing extravasation while acetone seems to induce extravasation in the bladder resulting in frequent voiding prior to pain induction. This is in line with our previous findings that CYP-induced frequent urination is maximized 3-4 hours after CYP treatment11 while CYP-induced nociceptive behavior was observed within an hour in this study. Pretreatment with capsaicin, which desensitizes capsaicin-sensitive C-fiber bladder afferents, reduced nociceptive behavior induced by CYP and acetone without significantly altering the changes in EB levels or bladder capacity, suggesting that nociceptive behavior is mediated by different bladder afferent pathways (C-fiber afferents) from those (Aδ-fiber afferents) inducing extravasation in the bladder and overactivity and that CYP and acetone differentially activated these afferent pathways during the early stage of chemically induced cystitis. In addition, in our previous study using rats with intravesical application of resiniferatoxin (RTx), lower abdominal licking was observed as major nociceptive behavior associated with reduced bladder capacity.9 However, licking behavior was not obviously found in the present study with CYP or acetone treatment. Because RTx-induced licking behavior was significantly reduced by pudendal nerve transection9, it seems likely that pudendal nerve afferents at the urethra or external genital region are stimulated when RTx solution is eliminated through the urethra during voiding while CYP or acetone treatment appears to predominantly stimulate bladder afferents to induce nociceptive behavior (immobility).
One of the advantages of our animal model system is that we can evaluate bladder function, extravasation and pain behavior in the same animals.9 Therefore, the score of nociceptive behavior and increased EB levels in bladder were individually plotted to elucidate their relationship. We found that, in the CYP-treated group, nociceptive behavior was correlated with the EB levels in the bladder; however, the correlation was not clear in the acetone-treated group (fig.2B). However, when the relationship between bladder capacity and increased EB levels in the bladder was examined by plotting data from individual rats, we found that the reduction in bladder capacity was correlated with the EB levels in both CYP and acetone-treated rats. These results indicate that extravasation in the bladder is closely related with small bladder capacity, but does not necessarily contribute to pain sensation, especially in acetone-treated rats. In addition, bladder capacity was reduced to a greater extent by CYP treatment than by acetone treatment at the same level of extravasation because the slope of the relationship between bladder capacity and EB concentration in the CYP-treated group was shifted leftward to the acetone groups (fig. 2A), suggesting that other mechanisms such as direct C-fiber stimulation in addition to extravasation might be involved in the induction of small bladder capacity in CYP-induced cystitis.
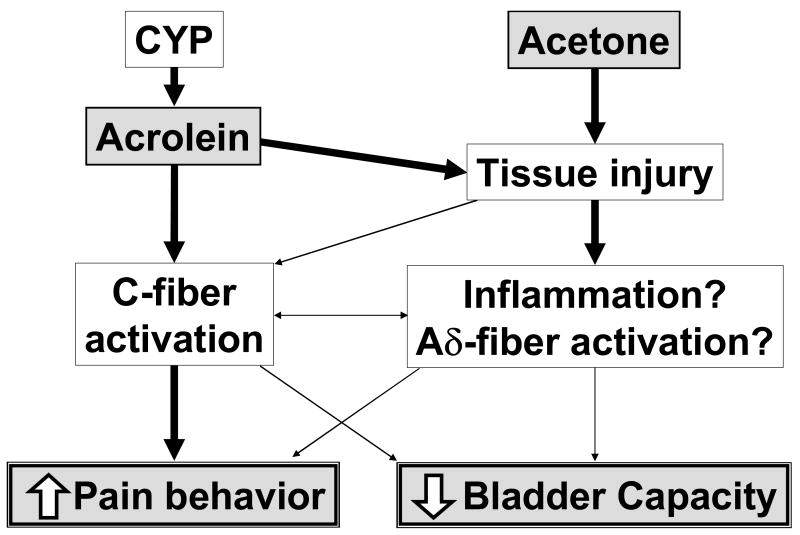
Acrolein, which is the major metabolite of CYP, has been reported to excite sensory nerve fibers though TRPA1 receptors13, which is shown to be expressed in a subset of unmyelinated peptidergic nociceptors14. We therefore investigated the involvement of C-fiber afferents in the responses to CYP or acetone, and found that nociceptive behavior induced by CYP (200mg/kg, ip) or acetone (30%) was reduced in rats with C-fiber desensitization induced by capsaicin pretreatment. Therefore, it is likely that CYP or acetone-induced nociceptive behavior is induced by activation of C-fiber afferents. The CYP or acetone-induced decrease in bladder capacity was also attenuated in capsaicin-pretreated rats when analyzed at the equivalent level of plasma extravasation (i.e., the same bladder EB concentration) in the plot showing the relationship of bladder capacity and EB levels (fig. 4AB) although the overall changes in bladder capacity and EB levels were not significantly altered by capsaicin-pretreatment (fig. 3AB and 3DE). In addition, the normalizing effects of C-fiber desensitization on bladder capacity appeared to be clearer in the CYP-group than in acetone-group. Dinis et al. also reported that desensitization of capsaicin-sensitive bladder afferents blocked both increased spinal c-fos expression and enhanced bladder activity in CYP-injected rats.15 Taken together, it is assumed that pain sensation is directly related to C-fiber activation whereas C-fiber afferents as well as capsaicin-insensitive factors such as direct tissue damage and/or activation of Aδ-fiber afferents could be involved in the development of small bladder capacity following cystitis (fig. 5).
Fig. 5.
Proposed mechanisms inducing a reduction in bladder capacity and nociceptive behavior after cyclophosphamide (CYP) or acetone treatment. The CYP metabolite, acrolein, which is excreted into urine, directly stimulates C-fiber afferents to induce pain behavior, and then elicit extravasation in the bladder and/or Aδ-fiber excitation to decrease bladder capacity. In contrast, acetone, which directly induces tissue damage, is more likely to induce extravasation in the bladder and/or Aδ-fiber stimulation to reduce bladder capacity before inducing pain behavior. Thick and thin arrows represent the high and low likelihood of the events to occur, respectively.
Conclusions
Systemic application of CYP, which is metabolized to acrolein that is then excreted into urine, can directly stimulate C-fiber afferents to induce nociceptive behavior prior to increasing plasma extravasation. In contrast, intravesical instillation of acetone is more likely to cause bladder tissue injury to induce extravasation, resulting in small bladder capacity and pain behavior. Thus, multiple factors such as C-fiber stimulation and extravasation in the bladder seem to be involved in the development of reduced bladder capacity and bladder pain in different models of chemical cystitis. Because different endpoints such as pain behavior and bladder activity in multiple animal models have been used in bladder pain research, we should take into account these differences in the mechanisms inducing bladder pain and bladder dysfunction when evaluating the effects of new drugs or elucidating the pathophysiology of bladder hypersensitive disorders such as IC/PBS.
Acknowledgments
This study was supported by grants from National Institutes of Health: DK057267, DK068557 and P01 DK044935
References
- 1.Cox PJ. Cyclophosphamide cystitis - identification of acrolein as the causative agent. Biochem Pharmacol. 1979;28:2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- 2.Olivar T, Laird JMA. Cyclophosphamide cystitis in mice: behavioural characterisation and correlation with bladder inflammation. Eur J Pain. 1999;3:141–149. doi: 10.1053/eujp.1998.0105. [DOI] [PubMed] [Google Scholar]
- 3.Lantéri-Minet M, Bon K, de Pommery J, Michiels JF, Menétrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1995;105:220–232. doi: 10.1007/BF00240958. [DOI] [PubMed] [Google Scholar]
- 4.Boucher M, Meen M, Codron JP, Coudore F, Kemeny JL, Eschalier A. Cyclophosphamide-induced cystitis in freely moving conscious rats: behavioral approach to a new model of visceral pain. J Urol. 2000;164:203–208. [PubMed] [Google Scholar]
- 5.Levin RM, Wein AJ, Whitmore K, Monson FC, Mc Kenna BAW, Ruggieri MR. Trypan blue as an indicator of urothelial integrity. Neurourol Urodyn. 1990;9:269–279. [Google Scholar]
- 6.Kato K, Kitada S, Longhurst PA, Wein AJ, Levin RM. Time-course of alterations of bladder function following acetone-induced cystitis. J Urol. 1990;144:1272–1276. doi: 10.1016/s0022-5347(17)39718-5. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu I, Kawashima K, Hosoki K. Urodynamics in acetone-induced cystitis of anesthetized rats. Neurourol Urodyn. 1999;18:115–127. doi: 10.1002/(sici)1520-6777(1999)18:2<115::aid-nau7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Chancellor MB, Yoshimura N. Treatment of interstitial cystitis. Urology. 2004;63:85–92. doi: 10.1016/j.urology.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Saitoh C, Chancellor MB, de Groat WC, Yoshimura N. Effects of intravesical instillation of resiniferatoxin on bladder function and nociceptive behavior in freely moving, conscious rats. J Urol. 2008;179:359–364. doi: 10.1016/j.juro.2007.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- 11.Smaldone MC, Vodovotz Y, Tyagi V, Barclay D, Philips BJ, Yoshimura N, Chancellor MB, Tyagi P. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology. 2009;73:421–426. doi: 10.1016/j.urology.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasmin L, Janni G, Manz HJ, Rabkin SD. Activation of CNS circuits producing a neurogenic cystitis: evidence for centrally induced peripheral inflammation. J Neurosci. 1998;18:10016–10029. doi: 10.1523/JNEUROSCI.18-23-10016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 15.Dinis P, Charrua A, Avelino A, Cruz F. Intravesical resiniferatoxin decreases spinal c-fos expression and increases bladder volume to reflex micturition in rats with chronic inflamed urinary bladders. BJU Int. 2004;94:153–157. doi: 10.1111/j.1464-4096.2004.04855.x. [DOI] [PubMed] [Google Scholar]